Abstract
Acyclovir, penciclovir, and their prodrugs have been widely used during the past two decades for the treatment of herpesvirus infections. In spite of the distribution of over 2.3 × 106 kg of these nucleoside analogues, the prevalence of acyclovir resistance in herpes simplex virus isolates from immunocompetent hosts has remained stable at approximately 0.3%. In immuncompromised patients, in whom the risk for developing resistance is much greater, the prevalence of resistant virus has also remained stable but at a higher level, typically 4 to 7%. These observations are examined in the light of characteristics of the virus, the drugs, and host factors.
INTRODUCTION
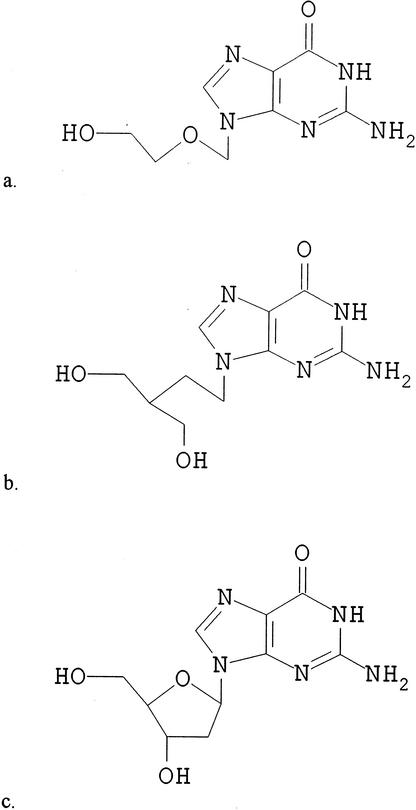
Research into antiviral chemotherapy was rudimentary prior to the discovery of acyclovir in 1974. The first report detailing the selective antiviral activity of acyclovir against herpesviruses was published in 1978 (25), marking the start of an exciting chapter in clinical medicine. Penciclovir, a structurally related compound identified in the 1980s (9), is also a potent and selective inhibitor of many human herpesviruses (Fig. 1). Both compounds are analogues of the natural nucleoside deoxyguanosine (Fig. 1). Oral prodrugs of penciclovir (famciclovir) and acyclovir (valaciclovir) were subsequently developed to improve their oral bioavailability (5, 97), although the oral formulation of acyclovir continues to be used widely.
FIG. 1.
Structures of antivirals. (a) Acyclovir. (b) Penciclovir. (c) Deoxyguanosine.
Antiviral treatment of herpes simplex virus (HSV) infections with nucleoside analogues has been well established for over two decades, but isolation of drug-resistant HSV from immunocompetent patients remains infrequent (0.1 to 0.7% with a mean of 0.3% [2, 8, 14, 17; M. J. Reyes, J. M. Graber, N. Weatherall, C. Hodges-Savola, W. C. Reeves, and the Task Force on Herpesvirus Resistance, Abstr. 11th Int. Conf. Antiviral Res., abstr. A44, 1998]) and, with very rare exceptions, resistant HSV is cleared normally with no adverse clinical outcome. The isolation of resistant HSV from immunocompromised patients is more common (4 to 7% [13, 14, 27, 98; J. W. Gnann, M. G. Davis, E. A. Harden, E. R. Kern, and Task Force on HSV Resistance, Abstr. 38th Int. Conf. Dis. Soc. Am., abstr. 59, 2000]) and more likely to be clinically significant. Nonetheless, even in this population there is no evidence of any increase in resistance despite the progressive increase in antiviral usage.
The aim of this article is to review data on antiviral resistance in HSV and to explain why the level of resistant HSV has remained stable. This outcome with nucleoside analogues contrasts sharply with the experience with many antibiotics, where misuse and overuse have contributed to the emergence and spread of antibiotic-resistant bacteria. Antiviral resistance of other viruses has emerged very rapidly in certain settings, for instance during zidovudine treatment of human immunodeficiency virus (HIV) infection, leading to treatment failure and transmission of resistant virus (51). Similarly, resistant mutants can be recovered from up to 30% of children and adults treated for acute influenza A with amantadine or rimantadine, and such mutants arise as early as 2 to 3 days after initiation of therapy (35).
MODE OF ACTION
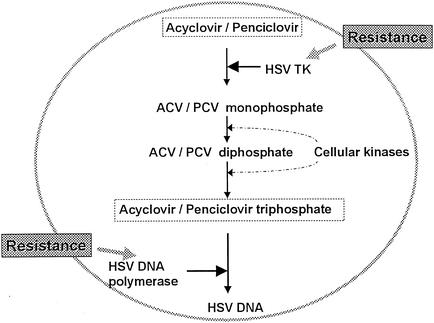
Acyclovir and penciclovir have a similar mechanism of antiviral action against HSV (Fig. 2). Both compounds are selectively phosphorylated only within virus-infected cells by viral thymidine kinase (TK). Further phosphorylation by cellular enzymes leads to the production of acyclovir or penciclovir triphosphate, both of which compete with the natural nucleotide, dGTP, resulting in the selective inhibition of viral DNA polymerase (24, 95). Incorporation of the analogue triphosphate into the growing DNA chain prevents continued extension of the DNA chain. Several differences in the mode of action of these compounds have been observed. (i) HSV TK is expressed in productively infected cells. Penciclovir has a higher affinity for HSV TK than acyclovir (20), and consequently the levels of penciclovir triphosphate in infected cells are much higher than the levels of acyclovir triphosphate (50, 95). (ii) Penciclovir triphosphate is more stable than acyclovir triphosphate in HSV-infected cells (23, 96), resulting in an intracellular half-life that is between 10- and 20-fold longer. (iii) HSV DNA polymerases have a higher affinity for acyclovir triphosphate than for penciclovir triphosphate (23). This distinction is counterbalanced by the difference in phosphorylation mentioned previously favoring penciclovir: the net effect is that the two compounds have similar antiviral potencies. (iv) Acyclovir triphosphate is an obligate DNA chain terminator (61), whereas penciclovir triphosphate allows limited DNA chain elongation (short-chain terminator) (95) by virtue of the 3′ hydroxyl group on its acyclic side chain (Fig. 1). Nonetheless, penciclovir triphosphate is more effective than acyclovir triphosphate in a DNA chain elongation assay under conditions designed to simulate HSV-infected cells (95).
FIG. 2.
Mode of action of acyclovir and penciclovir.
Not only is phosphorylation of acyclovir or penciclovir minimal in uninfected cells, but cellular DNA polymerases have much lower affinities for the antiviral triphosphates compared with HSV DNA polymerases (23, 40, 50). The excellent clinical safety record of acyclovir, penciclovir, and their prodrugs reflects the high selectivity of these antivirals for infected cells and their negligible activity in uninfected cells.
Mechanisms of Resistance
The viral TK and DNA polymerase (Fig. 2) are both intimately involved in mechanisms of resistance to acyclovir (16, 84) and penciclovir (10). Three distinct classes of acyclovir-resistant TK mutants have been identified: TK-negative (TKN), TK-partial (TKP), and TK-altered (TKA) mutants. TKN mutants lack TK activity, whereas TKP mutants express reduced levels of TK activity. TKA mutants are substrate specificity mutants, which phosphorylate thymidine but not acyclovir and/or penciclovir.
Approximately 95 to 96% of acyclovir-resistant HSV isolates are TK deficient (TKN or TKP), and the remaining isolates are usually TKA (59). Mutants with altered DNA polymerase have also been identified (16, 66), although these are infrequently reported.
Characteristics of Acyclovir-Resistant HSV
The pathogenicity of acyclovir-resistant HSV mutants has been evaluated in animal models. Typically, TKN mutants reveal the greatest reduction in virulence (15). With rare exceptions, TKN strains are unable to reactivate from latency following infection in mice. One TKN mutant, for example, was able to reactivate by using a cellular frameshifting mechanism to permit limited TK expression (39).
It has been suggested that certain genetic variations may compensate for the loss of TK (38). A TKN mutant was repeatedly isolated from the same patient following acyclovir treatment during two bone marrow transplantations separated by 2 years. Since the two mutants were genetically indistinguishable, it was concluded that the virus had established latency and subsequently reactivated. Another case has been described in a bone marrow transplant recipient, from whom an acyclovir-resistant, TK-deficient HSV-1 mutant was isolated 20 months after healing of the original HSV infection. This isolate also was identical to the original isolate (52).
Although it is difficult to identify TKP mutants based on biochemical assays alone, evaluation of pathogenicity in animal models can be helpful since TKP strains show some reduction in pathogenicity compared with wild-type virus but are generally able to reactivate from latency (15). Key characteristics of the different types of acyclovir-resistant mutants are summarized in Table 1.
TABLE 1.
Characterization of acyclovir-resistant HSV mutantsa
| Type of mutant | Thymidine phosphorylation (%) | Cross-resistant to penciclovir? | Pathogenicity in mice (% relative to wild-type virus)
|
||
|---|---|---|---|---|---|
| Neurovirulence after intracerebral inoculation | Replication at periphery | Ability to reactivate from latency | |||
| Wild type | 100 | NAb | 100 | 100 | 100 |
| TK negative (TKN) | <1 | Yes | 0.01-3 | 10-100 | No (rare exceptions) |
| TK partial (TKP) | 1-15 | Variable | 3-100 | 20-100 | 10-100 |
| TK altered (TKA) | Variablec | Variable | 10 | 100 | 50-100 |
| DNA polymerase | 100 | Variable | 1-10 | 20-100 | 50-100 |
Adapted from reference 15 with permission of the publisher.
NA, not applicable.
Ability to phosphorylate thymidine depends on the nature of the mutation in the TK gene.
In an analysis of 30 acyclovir-resistant HSV isolates from immunocompromised patients, 17 were TKN (the authors refer to these as TK deficient), 12 had decreased TK activity or expressed an altered TK, and 1 isolate was not defined (33). Approximately half of the resistant isolates had an insertion or a deletion of one or two nucleotides, usually within homopolymer runs of G's and C's in the TK gene that were previously identified as mutational hot spots (82), and a variety of other mutations were described. The analysis was difficult because of the heterogeneity of the isolates.
While all TKN viruses tested to date are cross-resistant to penciclovir and acyclovir, certain acyclovir-resistant TKA strains and certain acyclovir-resistant DNA polymerase mutants are sensitive or even hypersensitive to penciclovir (10). The clinical significance of these unusual mutants is uncertain (59).
In summary, clinical and laboratory experience has shown, with very rare exceptions, that acyclovir-resistant mutants do not appear to be capable of initiating a latent infection that can subsequently be reactivated. Indeed, it may be the failure to express functional TK, a characteristic that is typical of almost all resistant HSV mutants, that accounts for their inability to reactivate.
Characterization of Penciclovir-Resistant HSV in Cell Culture
Acyclovir-resistant mutants selected in cell culture or isolated from patients have been well studied over the past two decades. Famciclovir and penciclovir are relative newcomers to the antiviral armamentarium, and few penciclovir-resistant clinical isolates are available. For this reason, a series of HSV-1 (n = 13) and HSV-2 (n = 11) isolates was selected for resistance to penciclovir in cell culture (80). Acyclovir-resistant isolates were selected in parallel under similar conditions (HSV-1 [n = 15] and HSV-2 [n = 9]). A total of 21 HSV isolates were confirmed to be resistant to penciclovir in the plaque reduction assay following selection with penciclovir. All were cross-resistant to acyclovir, but none was resistant to foscarnet, indicating that these were likely to be TK deficient. Of 22 confirmed acyclovir-resistant HSV isolates derived by selection with acyclovir, all were cross-resistant to penciclovir but 1 (HSV-2 2P10) was also resistant to foscarnet and other DNA polymerase inhibitors. In plaque reduction assays with HSV-2 2P10, the 50% inhibitory concentrations (IC50s) of acyclovir, penciclovir, and foscarnet were >100, 61, and >400 μg/ml, although sensitivity to vidarabine and cidofovir was retained. This isolate carries a single nucleotide deletion within the TK gene that results in the expression of a truncated TK polypeptide. There was partial restoration of sensitivity to penciclovir when HSV-2 2P10 was tested in D21 cells expressing the HSV TK gene product constitutively. These data suggest that HSV-2 2P10 may be a double mutant, although this would need to be confirmed by sequence analysis of the DNA polymerase gene. In a functional TK assay, little or no TK activity (0.3 to 7% relative to wild-type virus) was detected in TK-negative human osteosarcoma cells infected with each of these drug-resistant mutants, consistent with a TKN phenotype; a control TKP strain produced 13 to 20% TK activity in this assay. However, as mentioned above, biochemical assays alone are not always able to distinguish TKN from TKP mutants. Sequence analysis of the TK genes of the HSV-1 mutants revealed that single or double point mutations leading to amino acid substitution were typical for the penciclovir-selected mutants. Frameshift mutations leading to formation of a truncated gene product were typical for the acyclovir-selected mutants of either type and also for penciclovir-selected HSV-2 mutants. As has been shown previously with clinical isolates, two putative homopolymeric nucleotide runs within the TK gene (G7 and C6) were identified as potential hot spots for frameshift mutations in the acyclovir-selected mutants (82). In contrast, TK mutations in the penciclovir-selected HSV mutants tended to be located upstream of the ATP-nucleoside binding site (80).
The dominant phenotype of penciclovir-selected resistant HSV mutants in this study was TKN (80), in accord with historical studies of acyclovir-resistant mutants whether selected in cell culture (31), animal models (30), or humans (12, 18). Preliminary studies with mice suggest that penciclovir-selected TKN HSV mutants derived in vitro have markedly reduced pathogenicity relative to wild-type virus (A. R. Awan, T. H. Bacon, R. T. Sarisky, J. J. Leary, D. Sutton, and H. J. Field, Abstr. 12th Int. Conf. Antiviral Res., abstr. A63, 1999), consistent with findings for acyclovir-selected TKN mutants.
SUSCEPTIBILITY ASSAYS
A variety of phenotypic methods have been used to determine the susceptibility of HSV isolates to antivirals, including dye uptake assays, viral DNA inhibition assays, enzyme immunoassays, and the plaque reduction assay (36). Efforts have been made to standardize these assays, since many variables can influence the final result (37, 43, 78). The plaque reduction assay is used most widely for routine susceptibility testing, in part because a correlation has been established between in vitro susceptibility to acyclovir measured by this assay and clinical response based on data from 243 HSV isolates from 115 HIV-infected patients (Table 2) (69). The predictive value of an IC50 of <2 μg/ml, measured by the plaque reduction assay in Vero cells, for complete healing of lesions in this study was 62%. Accordingly, an IC50 of ≥2 μg/ml is often used as a breakpoint in in vitro assays.
TABLE 2.
Association between in vitro susceptibility to acyclovir and clinical response to therapya
| In vitro susceptibilityb | Response to acyclovir therapy (no. of patients)
|
|||
|---|---|---|---|---|
| Complete healing | Partial healing | Failure to heal | Total | |
| Sensitive (IC50 <2 μg/ml) | 24 | 8 | 7 | 39 |
| Resistant (IC50 ≥2 μg/mlc) | 2 | 2 | 72 | 76 |
| Total | 26 | 10 | 79 | 115 |
Adapted from reference 69.
Plaque reduction assay in Vero cells.
Highly significant association between clinical response and IC50 (P < 0.001).
The lack of a complete correlation between in vitro susceptibility of viral isolates and clinical response implicates other factors in the clinical outcome. These include the antiviral medication (dose, dose frequency, route of administration, compliance), absorption and metabolism of the antiviral, immunological response of the patient, and heterogeneity of the virus population.
One drawback of the plaque reduction assay is that several days are required for completion, particularly if a viral stock has to be prepared and subjected to titer determination. For this reason, it cannot be used in a clinical virology laboratory as a rapid means of identifying resistant virus. A rapid assay for detecting acyclovir-resistant HSV isolates has been developed (93). This assay utilizes a genetically engineered CV19 cell line, which expresses β-galactosidase only after infection with HSV. To screen for resistant HSV, viruses are subjected to titer determination with and without acyclovir at 2 μg/ml and plaques are stained histochemically for β-galactosidase 2 days later. It seems likely that development of molecular probes for the rapid detection of resistant virus, targeted against conserved regions of the TK gene, would lead to more widespread testing of viral isolates.
Breakpoint for Defining Resistance
The breakpoint used to define in vitro resistance is of key importance, particularly for HSV isolates with borderline susceptibility to acyclovir or penciclovir. For acyclovir, a breakpoint of ≥2 μg/ml in the plaque reduction assay is widely accepted based on the report by Safrin et al (69). In addition, there appears to be a bimodal distribution of IC50s when the susceptibilities of large collections of viral isolates are examined, separating resistant from sensitive strains at concentrations in excess of approximately 1 to 3 μg/ml (14, 99). Other breakpoints have also been used. One of the breakpoints applied to define penciclovir resistance in a survey of recurrent herpes labialis isolates, for example, was an IC50 that was ≥3-fold higher than the mean IC50 for all isolates tested (8). The mean IC50 of penciclovir against all isolates tested in this survey was 0.64 μg/ml. Antiviral resistance to acyclovir or penciclovir has also been proposed based on the IC50 of an internal standard, whereby resistance is defined as being present when the IC50 for the test virus is >10-fold higher than that for the sensitive control strain (78).
Assay sensitivity varies between laboratories even when identical viruses are tested by the same assay in the same cell line. For example, when a set of standard virus strains (sensitive and resistant) was tested by the plaque reduction assay in MRC-5 cells in two laboratories, the IC50s differed by up to approximately 20-fold (P. B. Crosson, Viridae Clinical Sciences, Inc., and C. Hodges-Savola, ViroMed, Inc., personal communication). For this reason, it may be misleading to rely on a single antiviral concentration as the sole criterion for defining resistance. Including an additional breakpoint based on the IC50 of an internal standard as mentioned above has been shown to help identify unusual isolates with borderline susceptibility. For example, an HSV-1 isolate from a patient with herpes keratitis showed an acyclovir IC50 of 0.89 μg/ml in the plaque reduction assay in MRC-5 cells. In the same assay, the IC50 for the sensitive control strain was 0.06 μg/ml. The isolate was concluded to be acyclovir resistant since the acyclovir IC50 was >10-fold higher than that for the control standard virus. Molecular characterization of this isolate confirmed the resistant phenotype (77); furthermore, the patient did not respond to acyclovir treatment (78). For the vast majority of HSV isolates, the 2-μg/ml breakpoint is acceptable. However, the plaque reduction assay can be made more rigorous by including an additional breakpoint based on the susceptibility of a sensitive control strain, which is run as an internal standard in each assay.
Viral Heterogeneity
It has long been recognized that laboratory strains and clinical isolates of HSV contain mixtures of wild-type and acyclovir-resistant virus (26, 58, 66, 86). In the plating efficiency assay, the infectivity titer (plaque number) of a virus preparation in the presence of a high concentration of an antiviral is compared with the titer in the absence of the antiviral. The antiviral concentration used must be sufficiently high to ensure that only preexisting mutants form plaques; that is, the concentration should be in the plateau portion of the dose-response curve. Concentrations used have ranged from 3 to 10 μg/ml (34, 58, 79, 86). Cell line and other laboratory variables are likely to affect the choice of concentration, as with the plaque reduction assay. In the plaque reduction assay, the IC90 represents that antiviral concentration which allows 10% of the infectious virus population to form plaques. Although this end point can readily be calculated, it cannot be measured with the same accuracy as an IC50 because it occurs close to the asymptote for the sigmoidal dose-response curve. Furthermore, virus able to grow under these conditions may not necessarily be resistant to the antiviral. In contrast, the plating efficiency assay measures the actual proportion of resistant virus within an isolate.
It has been shown that the proportion of naturally occurring acyclovir-resistant mutants contained within a virus preparation is very similar to that for penciclovir-resistant mutants, as measured by a plating efficiency assay (79). The mean frequency of resistant mutants for a series of HSV-1 strains was approximately 3 × 10−4 infectious virus particle (0.03%) with either compound. Results for acyclovir and penciclovir were again similar for HSV-2 strains, although the frequency of resistant mutants was about 9 × 10−3 infectious virus particle (0.9%), suggesting that HSV-2 may have a greater propensity to generate drug-resistant mutants than HSV-1 does. Similarly, a 30-fold type-specific difference in the proportion of acyclovir-resistant HSV was identified in another study (34). In a third study, frequencies of acyclovir-resistant HSV strains were reported to be similar regardless of HSV type, with values reported as 7.5 × 10−4 (0.08%) and 15 × 10−4 (0.15%) for HSV-1 and HSV-2, respectively (86). Differences in the methods used, such as cell line, concentration of acyclovir, and preparation of the virus prior to analysis, may account for this apparent inconsistency.
The natural heterogeneity of virus populations has important implications. (i) The plaque reduction assay, which is considered to be the “gold standard”, measures the overall sensitivity of the viral population. Thus, while an isolate may be classified as sensitive based on the IC50 measured by this assay, it may contain a significant proportion of resistant virus. In vitro reconstruction experiments indicate that the proportion of acyclovir-resistant virus in a mixture must exceed 20% in order to shift the IC50 to >2 μg/ml (86). The plating efficiency assay may be a useful tool to detect small shifts in the proportion of resistant virus over time. However, the clinical significance of such changes would need to be established. (ii) In an immunocompromised host, antiviral therapy provides an ideal scenario for the selection of resistant virus from a mixed viral population. In the absence of an effective immune response, HSV can replicate to a higher titer and cause a more prolonged infection (41, 87). Both of these features increase the chance that a resistant mutant will be selected. Moreover, the persistence of wild-type virus within a predominantly resistant population may complement resistant virus to facilitate the reactivation of TK-deficient virus from latency and increase virulence (81).
CLINICAL USAGE
Clinical indications for oral acyclovir, valaciclovir, and famciclovir include treatment of first episodes of genital HSV infection (11, 49), recurrent genital HSV infection (63, 65), herpes zoster (6, 21), and suppressive therapy to prevent recurrences of genital HSV (22, 91). Since mucocutaneous herpesvirus infections in immunocompromised patients can be severe and prolonged, oral therapy with acyclovir, valaciclovir, or famciclovir is usually indicated. An intravenous formulation of acyclovir is used for severe HSV or varicella-zoster virus (VZV) infections (including encephalitis and neonatal herpes), as well as for HSV or VZV infections in immunocompromised patients.
Topical formulations of penciclovir (90) and acyclovir (29, 88a) are effective in patients with recurrent herpes labialis. An ocular formulation of acyclovir is also available. Acyclovir ointment, approved over 15 years ago in the United States, is indicated in the management of initial genital herpes and of limited mucocutaneous HSV infections in immunocompromised patients.
As the clinical utility of acyclovir became apparent, one of the key features of the compound was its safety in clinical use (19). Although clinical experience with famciclovir is shorter, the safety profile is similar to that of placebo in clinical studies (70). Acyclovir, penciclovir, and their respective prodrugs are widely used because they are recognized as safe and effective treatments for the management of herpesvirus infections in immunocompetent and immunocompromised populations.
Extent of Use
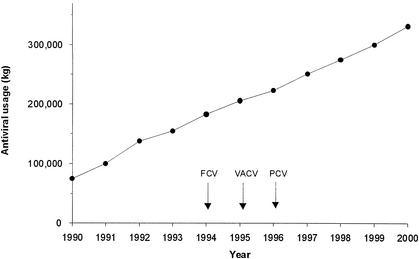
Since increasing clinical use of acyclovir and penciclovir might be expected to facilitate the emergence of resistance, an assessment of the extent of use of these antivirals and their prodrugs over the past 20 years is relevant. Acyclovir was first approved in 1981, while famciclovir, valaciclovir, and penciclovir were approved in the mid-1990s. Many millions of people have been treated with these antivirals. Worldwide use of nucleoside analogues for HSV and VZV infections has risen rapidly over the past decade from 75,000 kg in 1990 to 332,000 kg in 2000 (Fig. 3); total cumulative sales exceed 2.3 × 106 kg. Sales in the United States alone accounted for about 54% of the total volume for 2000. The high prevalence of genital herpes, medical advances in transplant medicine, and the magnitude of the HIV epidemic have contributed to this marked growth. Although not considered further in this review, acyclovir and valaciclovir are used at high dose to prevent cytomegalovirus infections, for example in bone marrow transplant recipients (47); therefore, the total use of these antivirals is even higher.
FIG. 3.
Worldwide antiviral use for HSV and VZV infections. Annual sales of acyclovir, famciclovir, penciclovir, and valaciclovir for HSV and VZV infections (1990 to 2000). First launch dates for famciclovir, valaciclovir, and topical penciclovir are shown by the arrows. Data are from Intercontinental Medical Statistics.
Acyclovir cream is available over the counter for the treatment of recurrent herpes labialis in 28 countries including Germany, France, the United Kingdom, and Australia. Topical penciclovir cream was approved as a prescription product for recurrent herpes labialis in the United States in 1996 and is also available without prescription in many countries, for example the Netherlands and Sweden.
PREVALENCE OF RESISTANT HERPES SIMPLEX VIRUS
Thousands of HSV isolates collected from worldwide clinical trials and surveillance programs for the last 20 years have been tested for susceptibility to acyclovir or penciclovir. Two consistent findings have emerged from this work. (i) The prevalence of acyclovir-resistant HSV is higher in severely immunocompromised patients than in immunocompetent patients. (ii) There is no evidence of any increase in the prevalence of resistant HSV in either immunocompromised or immunocompetent populations during this period.
Surveillance in the Immunocompetent Population
A dye uptake assay was initially used to determine the prevalence of acyclovir-resistant HSV among isolates collected between 1979 and 1984 (4, 55). This assay, which is a modification of the neutral red cell viability test, measures the ability of an antiviral drug to block HSV replication and thereby protect cells from virus-induced damage. Although amenable to rapid throughput, the assay can have a high false-positive rate of approximately 3% for isolates from immunocompetent patients (17). Nonetheless, results from this early work showed that acyclovir treatment in immunocompetent patients was not associated with the emergence of resistant virus.
The historical prevalence of acyclovir-resistant HSV isolates from untreated, immunocompetent patients as detected by the plaque reduction assay is 0.3% (71). Furthermore, there has been no detectable change over time in this prevalence based on data for isolates collected during clinical trials, from patients who had not responded well to acyclovir, and from population-based surveys (Table 3), (2, 8, 14, 17; Reyes et al., Abstr. 11th Int. Conf. Antiviral Res.). The prevalence of acyclovir-resistant HSV in these studies ranged from 0.1 to 0.7%, with a mean of 0.3%; isolates were from patients with genital herpes (14; Reyes et al., Abstr. 11th Int. Conf. Antiviral Res.), untreated recurrent herpes labialis (2, 8), or unspecified HSV infections (17).
TABLE 3.
Surveillance for acyclovir-resistant HSV in the immunocompetent population
| Variable tested | Results in reference:
|
||||
|---|---|---|---|---|---|
| 17 | 14 | Abstracta | 8 | 2 | |
| Prevalence of ACV-R HSV (%)b | 0.7 | 0.3 | 0.1 | 0.1 | 0.2 |
| No. of patients with ACV-R HSV/no. of patients testedc | 2/287 | 5/1,775 | 1/861 | 1/924d | 2/1,002e |
| Period of surveillance | 1980-1992 | 1991-1993 | 1996-1998 | 1998 | 1998-1999 |
| Location of survey | Europe | United Kingdom | United States | United Kingdom | United States |
Reyes et al., Abstr. 11th Int. Conf. Antiviral Res.
Acyclovir-resistant HSV. Studies included untreated patients and patients who had been treated with the antiviral, except in reference 17, where the data are from treated patients only.
Viral susceptibility to acyclovir was measured by the plaque reduction assay.
Isolates from 915 subjects were tested for susceptibility to penciclovir by the plaque reduction assay. One was resistant to penciclovir.
Isolates from 1,004 subjects were tested for susceptibility to penciclovir by the plaque reduction assay. None was resistant to penciclovir.
Until recently, little attention had been paid to surveillance for resistant HSV isolates in patients with recurrent herpes labialis despite the widespread availability of antiviral treatments for this indication in some markets. Isolates from the two surveys of herpes labialis conducted in the United Kingdom and the United States were tested for susceptibility to penciclovir in addition to acyclovir. HSV isolates from 924 and 1,004 subjects, respectively, were tested in these surveys (Table 3). One acyclovir-resistant isolate was identified in the 1998 United Kingdom survey which was cross-resistant to penciclovir; no other resistant isolates were identified (8). Two isolates identified in the U.S. survey as acyclovir resistant had showed acyclovir IC50s of 2.4 and 3.2 μg/ml, respectively, but were classified as sensitive to penciclovir (penciclovir IC50s, 0.34 and 0.38 μg/ml, respectively) (2). Further analysis of these isolates has shown that they are sensitive to acyclovir (IC50s in the plaque reduction assay in Vero cells were 1.24 and 0.72 μg/ml, respectively [M. Davis, personal communication, October 2001]). Based on these two surveys, it appears that widespread availability of topical acyclovir in the United Kingdom (acyclovir became available without prescription in 1993) has had no measurable impact on the prevalence of resistant HSV to date.
There is no evidence from these studies that isolates from patients who had received antiviral treatment were any less susceptible to acyclovir than isolates from untreated patients (2, 8, 14, 17; Reyes et al., Abstr. 11th Internat. Conf. Antiviral Res.).
Analysis of serial HSV isolates.
The data in the previous section described isolates collected from a cross-section of the population over a defined period. However, an alternative approach to examine the prevalence of resistant virus entails collecting serial isolates from a set of patients before, during, and after antiviral treatment. The latter approach is a powerful means of assessing whether exposure to antiviral treatment alters the susceptibility of viral isolates, although it is not practical to survey large numbers of patients in this way.
A collection of 2,143 HSV isolates obtained during clinical trials with famciclovir or penciclovir was tested for susceptibility to penciclovir (75; R. Sarisky, unpublished data [for preliminary information, see R. Sarisky, K. Esser, R. Saltzman, L. Locke, R. Boon, T. Bacon, and J. Leary, Program Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr H-10, 1998]). One of the objectives of this program was to evaluate whether antiviral treatment was associated with the development of resistance by comparing the sensitivities of the first and last isolates obtained from patients during the course of their participation in the clinical trial. A total of 1,446 isolates were obtained from 913 immunocompetent patients, and 697 were obtained from 272 immunocompromised patients (see below). Depending on the study design, the isolates may have been obtained from patients prior to treatment, during treatment (with famciclovir, penciclovir, acyclovir, or placebo), or after treatment. Trend analysis of the data for paired isolates (first and last isolates) failed to show any change in IC50 over time when isolates from antiviral treatment groups were compared with those from the appropriate placebo group. Overall, the prevalence of confirmed penciclovir-resistant HSV was 0.2% (2 of 913 immunocompetent patients), comparable to the results for acyclovir-resistant HSV. This observation is as expected, given that the spontaneous mutation frequency for penciclovir approximates to that for acyclovir (79).
Fife et al. concluded that 6 years of suppressive acyclovir therapy in immunocompetent patients with recurrent genital herpes did not lead to the selection of acyclovir-resistant virus (32). Sequential isolates from 13 patients showed no evidence of reduced antiviral susceptibility after cessation of suppressive therapy.
HSV isolates were collected from recurrent mucocutaneous lesions in a group of infants who had previously developed neonatal HSV infection in the first few weeks of life and had been treated systemically with acyclovir (n = 16) or vidarabine (n = 6) (60). There was no increase in the drug IC50s for the recurrent isolates compared with the isolates collected during primary infection prior to therapy.
A clinical study to investigate whether HSV-1 converts from a “susceptible” to a “resistant” phenotype (acquired resistance) has recently been completed (Y. K. Shin et al., personal communication). Sequential isolates recovered from a group of immunocompetent patients with frequent episodes of recurrent herpes labialis treated with topical penciclovir cream were collected and monitored for susceptibility to penciclovir by the plaque reduction assay. There was no significant change in IC50 when the first isolates, obtained before initiation of therapy, were compared with the last isolates, obtained during treatment. Fourteen patients had a pretreatment isolate from the first recurrence and at least one on-therapy isolate from the fourth treated episode. Mean IC50s were 0.34 and 0.26 μg/ml (P = 0.497). Furthermore, there was no evidence of a shift toward higher IC50s either within treated episodes or with each treated episode. No resistant isolates were detected in the study, which involved 110 patients and evaluation of a total of 360 isolates; therefore, the prevalence of resistant HSV was <0.3%.
Clinical resistance.
Although the acyclovir-resistant mutants that exist naturally in any virus population probably account for the very low background prevalence of resistant isolates in the immunocompetent population, clinical resistance to acyclovir is exceptionally rare in this group. Isolated cases of clinical resistance have been reported in patients with genital herpes (26, 42, 53, 92) or herpes keratitis (54, 78; A. B. Kressel, A. Wald, R. K. Hutchins, and A. H. Kaufman, Abstr. 38th Int. Conf. Dis. Soc. Am., abstr. 187, 2000). In all instances, acyclovir-resistant HSV was identified, thus providing an explanation for the lack of clinical response to acyclovir.
(i) Genital herpes.
All four HIV-negative patients described below were receiving suppressive acyclovir therapy for genital herpes. In one case, a homosexual man had increasingly frequent episodes of recurrent genital herpes despite receiving maximum doses of oral acyclovir (42). A TKA HSV-2 mutant was obtained repeatedly during these outbreaks. The infection may have been transmitted from a recent homosexual partner, two of whom were HIV positive. A female patient with genital herpes, who had been treated with oral acyclovir, developed chronic and recurrent disease that failed to respond to acyclovir, and a TK-deficient strain was isolated (92). No immunological abnormalities were detected, although the patient may also have had lichen planus in the area of the poorly healing HSV infection. Application of topical foscarnet cream (1%) led to complete resolution of the disease, and no further recurrence was noted during a 24-month follow-up. Apparent clinical resistance to acyclovir also developed in an immunocompetent male with recurrent genital herpes associated with a TKA HSV-2 strain (26) and a female with chronic recurrent genital herpes (53).
(ii) Herpes keratitis.
Three immunocompetent patients have been described with herpes keratitis that became clinically resistant to acyclovir. TK-deficient HSV-1 mutants were isolated from two of these patients (54; Kressel et al., Abstr. 38th Int. Conf. Dis. Soc. Am.). The third case involved a patient who responded to topical acyclovir during several episodes of keratitis but then became clinically resistant. HSV-1 isolated from this episode showed an acyclovir IC50 of 0.89 μg/ml in MRC-5 cells, below the standard breakpoint for defining in vitro resistance to acyclovir (≥2.0 μg/ml) (78). This isolate was judged to be resistant because the acyclovir IC50 was >10-fold higher than the IC50 for the control sensitive strain. The isolate had a reduced ability to phosphorylate acyclovir compared with a standard, acyclovir-sensitive strain, and it contained a mixture of wild-type virus and a TKA mutant (77). It may be that difficulty in maintaining adequate concentrations of antiviral at the site of an ocular infection increases the opportunity for ascent of resistant HSV.
In summary, clinical resistance to acyclovir is exceptionally rare in immunocompetent patients even though resistant HSV is detectable, albeit at a low frequency, in this population. This is because the normal immune response leads to the rapid resolution of the infection. Clinical resistance in an apparently immunocompetent individual should raise suspicion of some unappreciated immune deficit.
Surveillance in the Immunocompromised Population
Surveys in North America and Europe of HSV isolates from immunocompromised patients treated with acyclovir indicate that the prevalence of resistant HSV is generally between 4 and 7% (Table 4) (13, 14, 27, 98; Gnann et al., Abstr. 38th Int. Conf. Dis. Soc. Am.). Results from a continuing survey of HIV-positive patients within the United States and Canada in 1998 to 2000 (Task Force on Herpes Simplex Virus Resistance) are very similar (5.6%; Gnann et al., Abstr. 38th Int. Conf. Dis. Soc. Am.). Thus, despite widespread and increasing use of antivirals to treat HSV infections (Fig. 3), the frequency of resistant HSV even in high-risk, immunocompromised patients has remained stable for almost 20 years.
TABLE 4.
Surveillance for acyclovir-resistant HSV in the immunocompromised population
| Variable tested | Results in reference:
|
||||
|---|---|---|---|---|---|
| 98 | 27 | 14 | 13 | Abstracta | |
| Prevalence of resistant HSV (%)b | 4.1 | 4.7 | 6.3 | 7.1 | 5.6 |
| No. of patients with resistant HSV/no. of patients tested | 3/74c | 7/148 | 6/95 | 14/196 | 12/216d |
| Period of surveillance | Not specified | Not specified | 1991-1993 | 1996-1999 | 1998-2000 |
| Location of survey | United States | United States | United Kingdom | France | North America |
| Reason for immunodeficiency | BMTe | BMT, HIV, organ transplant, malignancy, high-dose steroids, neonate | BMT, HIV, organ transplant, malignancy | BMT | HIV |
| Assay method | Dye uptake | Viral DNA inhibition | Plaque reduction assay | Dye uptake | Plaque reduction assay |
Gnann et al., Abstr. 38th Int. Conf. Dis. Soc. Am.
Acyclovir-resistant HSV. With the exception of reference 27 and Gnann et al., data are from acyclovir-treated patients only.
Acyclovir-resistant HSV was recovered from 1 of 52 patients during the initial treatment course and from 2 of 22 patients during treatment for second recurrences.
Number of acyclovir-resistant HSV isolates/number of isolates tested. Multiple isolates from some patients were tested.
BMT, bone marrow transplant recipient.
Prevalence data obtained by the dye uptake assay for the immunocompromised population are consistent with data generated by the plaque reduction and DNA inhibition assays (Table 4). The relatively high false-positive rate for the dye uptake assay noted during surveys of immunocompetent populations becomes less significant when isolates from immunocompromised patients are surveyed, because the overall prevalence of resistant virus is higher.
In patients with defective T-cell-mediated immunity, the virus is cleared very slowly from the lesions (85, 100). Consequently, the lesions tend to be more prolonged and more severe than in immunocompetent individuals (27). Extensive viral replication occurring in the setting of prolonged antiviral therapy and immunosuppression provides an ideal scenario for the selection of resistant virus, analogous to selection in cell culture. Moreover, multiple courses of treatment may be required to manage recurrent episodes (18, 98). Consequently, patients with profound immunosuppression are more likely to carry acyclovir-resistant HSV than are patients with moderate immunosuppression (27).
For comparison, the prevalence of penciclovir-resistant HSV has been assessed in 697 HSV isolates obtained from 272 immunocompromised patients during the clinical trials with penciclovir and famciclovir. Twelve resistant HSV isolates were obtained from six patients; therefore, as with acyclovir, resistant HSV is more common in immunocompromised patients (2.2%; 6 of 272) than immunocompetent patients (0.2%) (Sarisky, unpublished [for preliminary information, see Sarisky et al., Program], Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother).
Analysis of serial HSV isolates.
Acyclovir-resistant HSV can emerge rapidly during the course of antiviral therapy in immunocompromised patients (12, 18, 66, 98). For example, Crumpacker et al. described a child with a congenital immune deficiency who received three courses of intravenous acyclovir for the treatment of recurrent mucocutanous HSV-1 infection (18). An isolate obtained at the start of the third course was sensitive to acyclovir, but within 9 days of starting the third course, an acyclovir-resistant HSV isolate was collected.
Recurrent HSV lesions developing after resolution of a clinically resistant lesion in immunocompromised patients often respond to acyclovir therapy. In one study, 7 of 10 first recurrences at the site of a healed lesion previously associated with acyclovir-resistant HSV were acyclovir sensitive (68). This observation is consistent with the view that HSV latent in sensory ganglia represents virus that reaches the ganglion early during primary infection (and thus is unlikely to have been influenced by therapy). Reactivation of the latent virus leads to renewed replication of sensitive HSV within the lesion, despite the development of resistant virus at the periphery in a prior episode. However, in the same study, all eight second recurrences were resistant to acyclovir therapy (68), suggesting that resistant virus became latent and reactivated. Based on the reported IC50s, isolates from these eight patients appeared to be sensitive to foscarnet but showed some resistance to acyclovir. An alternate interpretation is that acyclovir-resistant HSV may not have been completely cleared from the periphery during the previous recurrence.
Clinical resistance.
The immunocompromised host faces an increased risk of developing severe HSV infections and the emergence of acquired resistance compared with an individual with a fully functional immune system. Indeed, the probability of developing unresponsive lesions appears to be related to the severity of immunosuppression. Of 184 cases of clinical resistance reported between 1982 and 1994, 160 occurred in patients with AIDS and 24 occurred in patients who were otherwise immunocompromised, usually because of bone marrow transplantation (59). Typically, these patients present with chronic, nonhealing lesions that are unresponsive to high-dose acyclovir therapy. Rare cases of neonatal herpes associated with clinical resistance have also occurred and are described in the following section (45, 56, 57). It should be noted that there has been no unequivocal evidence of transmission of resistant HSV from these patients to other individuals.
HERPES SIMPLEX VIRUS INFECTION IN THE IMMUNOCOMPROMISED HOST
Clinical Features
Information about the clinical presentation of HSV infections in patients with immunodeficiency is presented in this section, together with guidance on therapeutic management strategies.
The failure to limit HSV replication in a timely fashion, which is a feature of infection in the immunocompromised host, can result in atypical lesions. These lesions are often larger than normal with deeper and more extensive ulceration, may have a heaped-up hyperkeratotic or verrucous appearance, may develop in atypical areas (e.g., sacral genital herpes confused with decubitus ulceration), and may persist for long periods (41, 87, 88, 94, 100). Lesions also remain culture positive for HSV for extended periods (85, 100), in contrast to infections in immunocompetent patients, where HSV is cleared within a few days (3, 89). The recurrence of severe mucosal lesions (oral or genital) is also much more common in immunocompromised patients, and unusual manifestations such as glossitis and papillitis occur, affecting the tongue and papillae of the gums, respectively. For these reasons, it is important to test any indolent mucocutaeous lesions in immunocompromised patients for the presence of HSV. Extensive cutaneous HSV infection can develop when local skin defenses are compromised, as in eczema or burns. Similarly, severe cutaneous herpes infections may complicate cases of mycosis fungoides. HSV may spread to other target sites to cause pneumonitis, esophagitis, hepatitis, retinal necrosis, and disseminated infection when the infection becomes widespread. Such visceral involvement is most often seen in patients with iatrogenic and acquired immunosuppression, but neonates, malnourished children, and pregnant women also have an increased risk of disseminated infection.
In contrast, atypical healing of HSV, with or without therapy, is very rare in the immunocompetent host. Its occurrence should signal the possibility of a defect in T-cell-mediated immunity, since normal immunity alone, without antiviral therapy, can be relied upon to clear HSV rapidly from infected tissues.
Neonatal herpes.
Neonates have an increased risk of developing severe, disseminated herpes because some elements of the immune response function less well than in adults (83). Three cases of clinical resistance to acyclovir are described.
A 10 day-old-term infant with a laryngeal HSV-2 infection was treated with intravenous acyclovir for 18 days without resolution (56). Neither parent had had genital herpes or recent herpes labialis or prior acyclovir therapy. Treatment with foscarnet resulted in complete recovery. Isolates obtained during antiviral therapy were resistant to acyclovir and susceptible to foscarnet.
Two other case reports, both involving premature neonates, have recently been published. An infant, born at 26 weeks gestation to a mother without genital herpes or prior treatment with acyclovir, developed neurocutaneous HSV-2 infection and was treated with acyclovir for 21 days (57). Eight days later, the infant developed disseminated disease. Although acyclovir therapy was resumed, the infant died of neonatal herpes 6 days later. HSV-2 from the primary infection was sensitive to acyclovir, but virus isolated 2 days after initiation of the second course of acyclovir therapy was resistant. The mutant was TKN and had reduced pathogenicity in mice. The second case involved an infant of 27 weeks gestation born to a mother subsequently diagnosed with disseminated HSV-2 infection (45). The infant received hydrocortisone for 6 days to treat blood pressure instability. Acyclovir was added on day 4 of life after the maternal HSV infection was detected. Cerebrospinal fluid collected on day 4 was HSV DNA positive, and HSV was isolated on day 11 from ascites. An isolate was recovered at autopsy 2 days later. DNA analysis of samples from the mother and the infant showed that the viruses were closely related since all carried an uncommon G420T mutation in the TK gene. This marker is not associated with acyclovir resistance. A new mutation in the TK gene was detected in the day 11 ascites sample that correlated with phenotypic resistance. The authors concluded that resistant HSV was selected de novo during the first 7 days of acyclovir treatment and suggested that in addition to the immaturity of the immune system in preterm infants, steroid treatment may have contributed to the rapid emergence of resistance.
Management Strategies
Prophylaxis.
Prophylactic antiviral therapy is highly effective in lowering the risk of HSV infection in patients with severe immunosuppression, e.g., following bone marrow transplantation or intensive chemotherapy (1, 72, 73, 74). Typically, the incidence of symptomatic HSV infection is reduced from about 70% to between 5 and 20% (102). Consequently prophylactic antiviral therapy lowers the potential for the development of resistance compared with acute therapy. A history of HSV infection or pretreatment serologic evidence of prior infection should lead to antiviral prophylaxis during the period of immunosuppression. For severely ill patients unable to take oral medication, intravenous acyclovir is effective (72, 73) and is administered at 5 mg/kg every 12 h. The risk of HSV infection is also reduced very well by administration of oral acyclovir (1, 74). For oral antivirals, appropriate doses are as follows: acyclovir, 400 mg administered three times a day; valaciclovir, 500 mg administered twice a day; famciclovir, 500 mg administered twice or three times a day. (No prophylactic therapy is approved by the Food and Drug Administration for immunocompromised patients.) This prophylaxis should not be necessary in patients receiving ganciclovir or valaciclovir to prevent cytomegalovirus infection.
Acute treatment.
Intravenous acyclovir (5 [or 10] mg/kg [or 250 mg/m2] three times a day) is indicated for patients with extensive disease, including all systemic infections (74), and treatment should be continued until there is good evidence that the infection is resolving. Additonal oral therapy may be considered until complete healing occurs. For patients with less severe HSV infections, oral antiviral therapy is effective (74, 85). The prodrugs valaciclovir and famciclovir have the advantage of improved pharmacokinetic properties compared with the parent drugs, although acyclovir may be the cheapest option. The mucositis that accompanies chemotherapy is often associated with HSV infection (62). Patients not receiving prophylaxis who develop significant mucositis should be tested for HSV infection and treated accordingly. The presence of resistant HSV in this setting has not been studied.
The healing period may be prolonged even when the infecting HSV strain is sensitive to the administered antiviral, reflecting delayed clearance of virus by residual host defenses and delayed tissue healing in severely ill patients.
Treatment Failure
The possibility of resistant HSV should be considered whenever lesions persist for more than 1 week without appreciable decrease in size; when they develop an atypical appearance (see above); or when new satellite lesions develop after 3 to 4 days of therapy. The history of prior antiviral therapy or prior resistance will help with this determination, although resistant HSV can occur after many prior episodes associated with sensitive HSV. If resistant HSV is suspected, virus should be isolated for susceptibility testing; analysis of serial isolates will facilitate the early identification of the emergence of resistant virus. Decisions about altering therapy without laboratory confirmation of resistance should be based on the clinical urgency.
In general, increasing the dose of antiviral administered is of little benefit in cases of clinical resistance, even when the route of treatment is changed from oral to intravenous. A possible exception is when the patient has not been compliant with oral treatment. Similarly, it is very unlikely that a patient failing to respond to therapy with acyclovir or valaciclovir will respond to famciclovir, since resistance to acyclovir and penciclovir almost always maps to mutations in the HSV TK gene with almost inevitable cross-resistance between acyclovir and penciclovir (10). In this setting, it is necessary to use a drug whose mechanism does not depend on activation by HSV TK such as foscarnet, which is a pyrophosphate analog that inhibits HSV DNA polymerase. Foscarnet is administered intravenously (40 mg/kg over 1 h every 8 to 12 h, with careful monitoring of renal function and adjustment for decreased renal function). Since foscarnet is effective against most acyclovir-resistant HSV mutants, it is the substitute of choice (13, 28, 67). Topical foscarnet is effective in treating cutaneous lesions but is not commercially available. Cidofovir, a monophosphate of an acyclic nucleoside analog and therefore a TK-independent inhibitor of HSV, may be used to treat cases of combined resistance to acyclovir and foscarnet (13) and is a possible alternative to foscarnet, although it is more toxic. It is administered intravenously at 5 mg/kg over 1 h once weekly for 2 weeks and then biweekly. Cidofovir is approved only for patients with normal renal function (probenicid and pretreatment hydration are indicated in the package insert). In the unlikely situation that HSV is resistant because of a mutation in the HSV DNA polymerase gene, with or without a coexisting mutation in the TK gene, either of these drugs may be ineffective.
Prophylaxis of severely immunocompromised patients with acyclovir, valaciclovir, or famciclovir should be considered after resolution of an acute lesion which was clinically resistant. This is a reasonable strategy because the virus that is latent in these patients is often acyclovir sensitive, even after recovery from an acyclovir-resistant outbreak, as mentioned above. Moreover, suppressing virus replication reduces the opportunity for antiviral resistant strains to reemerge.
POSSIBLE LIMITATIONS OF CURRENT SURVEILLANCE ACTIVITIES
While it is important to continue to monitor at-risk communities for resistant HSV, e.g., bone marrow transplant recipients (48) and HIV-infected patients (Gnann et al., Abstr. 38th Int. Conf. Dis. Soc. Am.) by using the plaque reduction assay, additional approaches to surveillance for resistance should also be considered in order to enhance information on antiviral resistance.
Historical Isolates
It could be argued that the prevalence of resistant HSV has remained stable because isolates surveyed to date have generally been obtained from recurrent infections occurring in adult patients. The virus initiating these episodes may have established latency at the time of a primary infection many years before the advent of antiviral therapy and may not have been exposed to antiviral selection. Hence, virus isolated at the periphery during recurrences could be considered “historical.” This may be an especially valid concern for surveys of adults with recurrent herpes labialis, since primary HSV-1 infections tend to be acquired in childhood (101). However, these reactivated viruses may also cause new primary infections in children and therefore should be representative of future isolates from adults. In contrast, primary genital herpes is typically observed in the adult population. Thus, unlike herpes labialis, these infections are more likely to have been acquired recently and to have been transmitted from a host undergoing antiviral therapy.
Acquired Resistance
As described above, a study to investigate the emergence of acquired resistance has been conducted with immunocompetent patients repeatedly treated with topical penciclovir cream for frequent episodes of recurrent herpes labialis. If cases of acquired resistance were detected, this would raise the possibility that resistant virus could be transmitted from patients receiving topical treatment to susceptible contacts. However, analyses of IC50 data failed to reveal any trend indicative of reduced susceptibility to penciclovir after multiple episodes of topical treatment and no resistant isolates were found in the entire study (Shin et al., personal communication). This suggests that the risk of acquired resistance in the immunocompetent host treated for HSV infection is low.
Proportion of Resistant Virus Within Mixed Populations
The plaque reduction assay is able to identify an HSV isolate as acyclovir resistant provided that a substantial proportion of the virus population (>20%) is resistant. While the proportion of resistant virus detected may vary between clinical isolates and over time as an infection progresses, the clinical implications of such changes are unknown. Regardless of this variability, the prevalence of resistant HSV as measured by the plaque reduction assay appears to be stable even in the immunocompromised population.
The effect of serial passage of HSV in immunocompetent mice treated with suboptimal doses of oral famciclovir or valaciclovir has been studied by using the plaque reduction and plating efficiency assays in parallel to monitor the emergence of resistance (76). Mice were infected in the ear pinnae with 105 PFU of HSV-1 or HSV-2 and treated with the antivirals for 4 days via the drinking water (0.2 mg/ml, providing an estimated daily dose of 15 mg/kg). Only one virus isolate of 140 isolated from mice treated with the antivirals was drug resistant by the plaque reduction assay during the seven sequential passes of either HSV-1 or HSV-2. The resistant isolate was obtained after four serial passages of HSV-1 in mice treated with valaciclovir. It showed mean acyclovir and penciclovir IC50s of 9.5 and 8.3 μg/ml, respectively, and contained a relatively large proportion of resistant virus (47 and 44%, respectively). Molecular analysis of clones derived from the original isolate revealed a frameshift mutation in the TK gene, leading to the expression of a truncated TK polypeptide. Although the preceding isolate in the series was susceptible to acyclovir and penciclovir (IC50s, 0.2 and 0.5 μg/ml, respectively, as determined by the plaque reduction assay), the proportion of resistant virus in the preparation was elevated (approximately 2% resistant to penciclovir or to acyclovir compared with 0.006 to 0.007% for the parental virus). Curiously, the resistant phenotype was lost on further passage in mice despite continued treatment with valaciclovir. These results suggest that enrichment of resistant HSV occurred rapidly under conditions favoring the selection of resistance. Equally, there was a rapid loss of the resistant mutant during the next sequential passage, as judged by both in vitro assays, suggesting that there was a fitness advantage for wild-type virus despite suboptimal antiviral therapy.
Further work is needed to establish whether the plating efficiency assay can provide a useful adjunct to the plaque reduction assay for monitoring the antiviral susceptibility of clinical isolates. The plaque reduction assay has proved to be an accurate and reliable method for determining antiviral resistance, even though up to 20% of virus within a “sensitive” (IC50, <2 μg/ml) HSV strain may be resistant (79). However, information to date suggests that the proportion of resistant virus present within sensitive HSV strains is in almost all cases far lower than 20% (34, 58, 79, 86). Additionally, the correlation between IC50 and clinical response to acyclovir therapy (69) provides important validation of the plaque reduction assay.
FACTORS INFLUENCING THE EMERGENCE AND SPREAD OF RESISTANCE
Resistant HSV can develop spontaneously, reflecting the natural variability of the HSV population, as evidenced by the detection of acyclovir-resistant HSV in patients who had not been treated with acyclovir (14, 71). Nonetheless, acquired resistance to acyclovir is extremely unusual in the immunocompetent population and almost all cases have occurred in severely immunocompromised patients. Cases of primary infection with resistant HSV appear to be very rare since there has been only one report to date of possible transmission of acyclovir-resistant HSV (42).
The extensive use of acyclovir over the past two decades and the introduction of penciclovir, valaciclovir, and famciclovir have had minimal impact on the overall prevalence of resistant HSV in the population. Properties of the virus, host, and these antivirals may explain the apparent rarity of acquired and primary resistance to acyclovir or penciclovir.
HSV-Related Factors
(i) Acyclovir-resistant HSV mutants are generally less fit than wild-type virus in terms of virulence and ability to reactivate from latency and replicate at the periphery (15), all of which will reduce the likelihood of transmission. (ii) HSV infections, particularly HSV-1 infections, have a relatively long generation time (time between initiation of infection in a person and subsequent transmission to another person); therefore, the dynamics of phenotypic change for HSV within the population are slower than for viruses which are more readily transmissible, e.g., influenza virus. (iii) HSV infection is lifelong, and infection with multiple strains of either HSV-1 or HSV-2 appears to be unusual (101). Accordingly, the likelihood of superinfection with an exogenous resistant strain in a host previously infected with a sensitive HSV strain appears to be low, at least in immunocompetent hosts. Likewise, if resistant virus does emerge during a recurrence, this virus is unlikely to become latent. Thus, it is the historical virus that caused the primary infection and established latency and will typically reactivate to cause a recurrence. Latency thus presents a formidable barrier to the accumulation of resistant HSV in the population. (iv) HSV has much lower chance than RNA viruses for errors to occur and accumulate during viral replication.
Host-Related Factors
The integrity of the host immune response has a critical effect on the severity of infection and the risk of clinical resistance. (i) Primary infection or recurrences of genital herpes or herpes labialis in the immunocompetent host typically last for only a few days and remain localized (102). Because HSV is cleared rapidly by the immune system, there is a limited time when selection of resistant virus can occur in the treated host. In patients with recurrent herpes labialis, for example, virus was cleared from the lesions within 4 or 5 days (3, 89). (ii) The immune system would clear resistant virus just as efficiently as it would clear sensitive virus, ensuring that resistant HSV is typically transient in immunocompetent patients (26, 55).
Drug-Related Factors
(i) The majority of mutants selected for resistance to acyclovir or penciclovir have reduced pathogenicity due to TK deficiency. Mutants selected in response to treatment with a compound with a different mode of action could be as pathogenic as wild-type virus. (ii) The selective pressure resulting from treatment with acyclovir or penciclovir (or their prodrugs) is another important consideration. In the absence of antiviral treatment, selection for resistant virus does not occur, but when antiviral activity is completely effective, such that there is no viral replication, there can be no selection for antiviral resistance (64). Selection for resistant virus can therefore occur only when there is sufficient viral replication despite the presence of the antiviral. Treatment with acyclovir or penciclovir reduces but does not completely prevent virus shedding in patients with acute genital or oral HSV infection (44, 90), as is also the case with suppressive therapy (44). Reasons for this may include poor absorption of antiviral, lack of compliance with therapy, and the occurrence of suboptimal antiviral concentrations between doses. The selective pressure for resistance arising from the use of these antivirals does not appear to be high since their effects on virus replication in vivo are relatively modest.
MATHEMATICAL MODELING
While surveillance provides information about the current prevalence of resistant virus, mathematical models are being used to make predictions about the future. A model has been developed to evaluate the effect that an increase in the proportion of immunocompetent patients treated episodically will have on the epidemiology of genital herpes and the risk of emergence of acyclovir-resistant HSV-2 (7). The model assumes a low probability that resistance will emerge, that resistant strains have low transmissibility, and that increased use of oral acyclovir will have a beneficial effect on the spread of HSV-2 by reducing the occurrence of infection with sensitive virus at the cost of generating a low prevalence of drug resistance. The model concludes that as a consequence of controlling the HSV-2 epidemic with treatment, genital herpes infections in immunocompromised patients would be expected to be less common and therefore the prevalence of resistant virus in this community would be lower than is the case today.
Another model was developed to predict the effect of topical antiviral use by individuals with recurrent herpes labialis on the transmission and prevalence of resistant HSV-1 (46). Even a substantial increase in the antiviral treatment of recurrent herpes labialis (episodic), such that 30% of all recurrences were treated with penciclovir, was calculated to have a minimal effect on the prevalence of HSV-1 infection in the community. The probability of acquired resistance becoming permanent was identified as the most important factor in influencing the predicted prevalence of resistance in the population; this was also the most uncertain parameter in the model, underlining the need for further investigation. Assuming that the probability of acquired resistance is low (optimistic scenario: probability of acquired resistance is less than 1 case per 2,500 treated episodes), the prevalence of resistant HSV-1 is predicted to remain below 0.5% for >50 years. Assuming that this probability is high (pessimistic scenario: probability of acquired resistance is 1 case per 625 treated episodes), the prevalence of resistant HSV-1 could increase from about 0.2% to between 1.5 and 3% within 50 years. Current evidence indicates that acquired resistance to acyclovir is rare for HSV, suggesting that the optimistic scenario is realistic.
CONCLUSION
There has been a dramatic increase in the use of acyclovir, penciclovir, and their prodrugs over the past two decades, but this has not been accompanied by a detectable increase in the prevalence of antiviral resistant HSV in immunocompetent or immunocompromised populations. The ability of HSV to establish a lifelong latent infection, together with the finding that the vast majority of resistant HSV isolates studied to date have reduced pathogenicity relative to wild-type virus, help to explain this observation, which is contrary to experience gained with many other anti-infective agents. While there is a need for continued surveillance for resistant HSV in the immunocompromised population, the significance of viral heterogeneity warrants further investigation by techniques such as the plating efficiency assay. Additional insights into antiviral resistance may also be gained by studying sequential isolates from patients receiving episodic or suppressive antiviral therapy.
Acknowledgments
We thank R. J. Boon, K. Esser (GlaxoSmithKline), and M. Lipsitch (Harvard School of Public Health) for helpful discussion and A. J. Fleetwood (GlaxoSmithKline) for providing global antiviral sales data.
REFERENCES
- 1.Anderson, H., J. H. Scarffe, R. N. P. Sutton, E. Hickmott, D. Bridgen, and C. Burke. 1984. Oral acyclovir prophylaxis against herpes simplex virus in non-Hodgkin lymphoma and acute lymphoblastic leukaemia patients receiving remission induction chemotherapy. A randomised double blind, placebo controlled trial. Br. J. Cancer 50:45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, T. H., R. J. Boon, M. Schultz, and C. Hodges-Savola. 2002. Surveillance for antiviral-agent-resistant herpes simplex virus in the general population with recurrent herpes labialis. Antimicrob. Agents Chemother. 46:3042-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader C., C. S. Crumpacker, L. E. Schnipper, B. Ransil, J. E. Clark, K. Arndt, and I. M. Freedberg. 1978. The natural history of recurrent facial-oral infection with herpes simplex virus. J. Infect. Dis. 138:897-905. [DOI] [PubMed] [Google Scholar]
- 4.Barry, D. W., S. Nusinoff-Lehrman, M. N. Ellis, K. K. Biron, and P. A. Furman. 1985. Viral resistance, clinical experience. Scand. J. Infect. Dis. 47(Suppl):155-164. [PubMed] [Google Scholar]
- 5.Beauchamp L. M., G. F. Orr, P. de Miranda, T. Burnette, and T. A. Krenitsky. 1992. Amino acid ester prodrugs of acyclovir. Antiviral Chem. Chemother. 3:157-164. [Google Scholar]
- 6.Beutner K. R., D. J. Friedman, C. Forszpaniak, P. L. Andersen, and M. J. Wood. 1995. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob. Agents Chemother. 30:1546-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blower, S. M., T. C. Porco, and G. Darby. 1998. Predicting and preventing the emergence of antiviral drug resistance in HSV-2. Nat. Med. 4:673-678. [DOI] [PubMed] [Google Scholar]
- 8.Boon, R. J., T. H. Bacon, H. L. Robey, T. J. Coleman, A. Connolly, P. Crosson, and S. L. Sacks. 2000. Antiviral susceptibilities of herpes simplex virus from immunocompetent subjects with recurrent herpes labialis: a UK-based study. J. Antimicrob. Chemother. 46:324-325. (Erratum, 46:1051.) [DOI] [PubMed] [Google Scholar]
- 9.Boyd M. R., T. H. Bacon, D. Sutton, and M. Cole. 1987. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob. Agents Chemother. 31:1238-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd M. R., S. Safrin, and E. R. Kern. 1993. Penciclovir: a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antiviral Chem. Chemother. 4(Suppl. 1):3-11. [Google Scholar]
- 11.Bryson Y. J., M. Dillon, M. Lovett, G. Acuna, S. Taylor, J. D. Cherry, B. L. Johnson, E. Weismeier, W. Growdon, T. Creagh-Kirk, and R. Keeney. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind trial in normal subjects. N Engl. J. Med. 308:916-921. [DOI] [PubMed] [Google Scholar]
- 12.Burns W. H., R. Saral, G. W. Santos, O. L. Laskin, and P. S. Lietman. 1982. Isolation and characterisation of resistant herpes simplex virus after acyclovir therapy. Lancet i:421-423. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y., C. Scieux, V. Garrait, G. Socie, V. Rocha, J.-M. Molina, D. Thouvenot, F. Morfin, L. Hocqueloux, L. Gareret, H. Esperou, F. Selimi, A. Devergie, G. Leleu, M. Aymard, F. Morinet, E. Gluckman, and P. Ribaud. 2000. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31:927-935. [DOI] [PubMed] [Google Scholar]
- 14.Christophers J., J. Clayton, J. Craske, R. Ward, P. Collins, M. Trowbridge, and G. Darby. 1998. Survey of resistance of herpes simplex virus to acyclovir in Northwest England. Antimicrob. Agents Chemother. 42:868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coen D. M. 1994. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 2:481-485. [DOI] [PubMed] [Google Scholar]
- 16.Coen, D. M., and P. A. Schaffer. 1980. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 77:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins, P., and M. N. Ellis. 1993. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J. Med. Virol. (Suppl. 1):58-66. [DOI] [PubMed]
- 18.Crumpacker, C. S., L. E. Schnipper, S. I. Marlowe, P. N. Kowalsky, B. J. Hershey, and M. J. Levin. 1982. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N. Engl. J. Med. 306:343-346. [DOI] [PubMed] [Google Scholar]
- 19.Darby, G. 1995. In search of the perfect antiviral. Antiviral Chem. Chemother. 6(Suppl. 1):54-63. [Google Scholar]
- 20.Datema, R., A.-C. Ericson, H. J. Field, A. Larsson, and K. Stenberg. 1987. Critical determinants of antiherpes efficacy of buciclovir and related acyclovir guanosine analogs. Antiviral Res. 7:303-316. [DOI] [PubMed] [Google Scholar]
- 21.Degreef, H., and the Famciclovir Herpes Zoster Clinical Study Group. 1994. Famciclovir, a new oral antiherpes drug: results of the first controlled clinical study demonstrating its efficacy and safety in the treatment of uncomplicated herpes zoster in immunocompetent patients. Int. J. Antimicrob. Agents 4:241-246. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Mitoma F., R. G. Sibbald, S. D. Shafran, R. Boon, and R. L. Saltzman. 1998. Oral famciclovir for the suppression of recurrent genital herpes. A randomized controlled trial. JAMA 280:887-892. [DOI] [PubMed] [Google Scholar]
- 23.Earnshaw, D. L., T. H. Bacon, S. J. Darlison, K. Edmonds, R. M. Perkins, and R. A. Vere Hodge. 1992. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob. Agents Chemother. 36:2747-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elion, G. B. 1993. Acyclovir: discovery, mechanism of action and selectivity. J. Med. Virol. (Suppl. 1):2-6. [DOI] [PubMed]
- 25.Elion, G. B., P. A. Furman, J. A. Fyfe, P. de Miranda, L. Beauchamp, and H. J. Shaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc. Natl. Acad. Sci. USA 74:5716-5720.202961 [Google Scholar]
- 26.Ellis, M. N., P. M. Keller, J. A. Fyfe, J. L. Martin, J. F. Rooney, S. E. Straus, S. Nusinoff Lehrman, and D. W. Barry. 1987. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob. Agents Chemother. 32:1117-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englund, J. A., M. E. Zimmerman, E. M. Swierkosz, J. L. Goodman, D. R. Scholl, and H. H. Balfour. 1990. Herpes simplex virus resistant to acyclovir: a study in a tertiary care center. Ann. Intern. Med. 112:416-422. [DOI] [PubMed] [Google Scholar]
- 28.Erlich, K. S., M. A. Jacobson, J. E. Koehler, S. E. Follansbee, D. P. Drennan, L. Gooze, S. Safrin, and J. Mills. 1989. Foscarnet therapy for severe acyclovir-resistant herpes simplex virus type-2 infections in patients with the acquired immunodeficiency syndrome (AIDS). An uncontrolled trial. Ann. Intern. Med. 110:710-713. [DOI] [PubMed] [Google Scholar]
- 29.Fiddian A. P., J. M. Yeo, R. Stubbings, and D. Dean. 1983. Successful treatment of herpes labialis with topical acyclovir. Br. Med. J. 286:1699-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field, H. J. 1982. Development of clinical resistance to acyclovir in herpes simplex virus-infected mice receiving oral therapy. Antimicrob. Agents Chemother. 21:744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Field, H. J., and G. Darby. 1980. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob. Agents Chemother. 17:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fife, K. H., C. S. Crumpacker, G. J. Mertz, E. L. Hill, G. S. Boone, and the Acyclovir Study Group. 1994. Recurrence and resistance patterns of herpes simplex virus following cessation of ≥6 years of chronic suppression with acyclovir. J. Infect. Dis. 169:1338-1341. [DOI] [PubMed] [Google Scholar]
- 33.Gaudreau, A., E. Hill, H. H. Balfour, A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa, T. H., T. Kawana, T. Okuda, M. Horii, T. Tsukuda, and K. Shiraki. 2001. Susceptibility to acyclovir of herpes simplex virus isolates obtained between 1977 and 1996 in Japan. J. Med. Virol. 63:57-63. [PubMed] [Google Scholar]
- 35.Hayden, F. G. 1996. Amantadine and rimantadine—clinical aspects, p. 59-77. In D. D. Richman, (ed), Antiviral drug resistance. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 36.Hodinka, R. L. 1997. What clinicians need to know about antiviral drugs and viral resistance. Infect. Dis. Clin. North Am. 11:945-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodinka, R., E. Swierkosz, D. Lancaster, B. M. Moore, S. Sacks, D. Scholl, and D. K. Wright. 2000. Antiviral susceptibility testing: proposed standard M33-P, i-34. National Committee for Clinical Laboratory Standards. Wayne, Pa.
- 38.Horsburgh, B. C., S.-H. Chen, A. Hu, G. Mulamba, W. H. Burns, and D. M. Coen. 1998. Recurrent acyclovir-resistant HSV infection in an immunocompromised patient: can strain differences compensate for loss of thymidine kinase in pathogenesis? J. Infect. Dis. 178:618-625. [DOI] [PubMed] [Google Scholar]
- 39.Hwang, C. B. C., B. Horsburgh, E. Pelosi, S. Roberts, P. Digard, D. M. Coen. 1994. A net +1 frameshift permits synthesis of thymidine kinase from a drug-resistant herpes simplex virus mutant. Proc. Natl. Acad. Sci. USA 91:5461-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilsley, D. D., S.-H. Lee, W. H. Miller, and R. D. Kuchta. 1995. Acyclic guanosine analogs inhibit DNA polymerases α, δ, and ɛ with very different potencies and have unique mechanisms of action. Biochemistry 34:2504-2510. [DOI] [PubMed] [Google Scholar]
- 41.Kalb, R. E., and M. Grossman. 1986. Chronic perianal herpes simplex in immunocompromised hosts. Am. J. Med. 80:486-490. [DOI] [PubMed] [Google Scholar]
- 42.Kost, R. G., E. L. Hill, M. Tigges, and S. E. Straus. 1994. Brief report: recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N. Engl. J. Med. 329:1777-1793. [DOI] [PubMed] [Google Scholar]
- 43.Leary, J. J., R. Wittrock, R. T. Sarisky, A. Weinberg, and M. J. Levin. 2002. Susceptibilities of herpes simplex viruses to penciclovir and acyclovir in eight cell lines. Antimicrob. Agents Chemother. 46:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung, D. T., and S. L. Sacks. 2000. Current recommendations for the treatment of genital herpes. Drugs 60:1329-1352. [DOI] [PubMed] [Google Scholar]
- 45.Levin, M. J., A. Weinberg, J. J. Leary, and R. T. Sarisky. 2001. Development of acyclovir-resistant herpes simplex virus early during the treatment of herpes neonatorum. Pediatr. Infect. Dis. J. 20:1094-1097. [DOI] [PubMed] [Google Scholar]
- 46.Lipsitch, M., T. H. Bacon, J. J. Leary, R. Antia, and B. R. Levin. 2000. Effects of antiviral usage on transmission dynamics of herpes simplex virus type 1 and on antiviral resistance: predictions of mathematical models. Antimicrob. Agents Chemother. 44:2824-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ljungman, P. 2001. Prophylaxis against herpesvirus infections in transplant recipients. Drugs 61:187-196. [DOI] [PubMed] [Google Scholar]
- 48.Ljungman P., M. N. Ellis, R. C. Hackman, D. H. Shepp, and J. D. Myers. 1990. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J. Infect. Dis. 162:244-288. [DOI] [PubMed] [Google Scholar]
- 49.Loveless, M., S. L. Sacks, and J. R. W. Harris. 1997. Famciclovir in the management of first episode genital herpes. Infect. Dis. Clin. Pract. 6(Suppl):S12-S16. [Google Scholar]
- 50.Lowe, D. M., W. K. Alderton, M. R. Ellis, V. Parmar, W. H. Miller, G. B. Roberts, J. A. Fyfe, R. Gaillard, P. Ertl, W. Snowden, and E. Littler. 1995. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob. Agents Chemother. 39:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayers, D. 1996. Rational approaches to resistance: nucleoside analogues. AIDS 10(Suppl. 1):S9-S13. [PubMed] [Google Scholar]
- 52.Morfin, F., D. Thouvenot, M. Aymard, and G. Souillet. 2000. Reactivation of acyclovir-resistant thymidine kinase-deficient herpes simplex virus harbouring single base insertion within a 7 Gs homopolymer repeat of the thymidine kinase gene. J. Med. Virol. 62:247-250. [PubMed] [Google Scholar]
- 53.Mouly, F., M. Baccard, C. Scieux, L. Schnell, S. Locq-Ebner, F. Morinet, P. Morel. 1995. Chronic recurrent acyclovir-resistant genital herpes in an immunocompetent patient. Dermatology 190:177. [DOI] [PubMed] [Google Scholar]
- 54.Nugier F., J. N. Colin, M. Aymard, and M. Langlois. 1992. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J. Med. Virol. 36:1-12. [DOI] [PubMed] [Google Scholar]
- 55.Nusinoff Lehrman, S., J. M. Douglas, L. Corey, and D. W. Barry. 1986. Recurrent genital herpes and suppressive oral acyclovir therapy. Ann. Intern. Med. 104:786-790. [DOI] [PubMed] [Google Scholar]
- 56.Nyquist, A-C., H. A. Rotbart, M. Cotton, C. Robinson, A. Weinberg, A. R. Hayward, R. L. Berens, and M. J. Levin. 1994. Acyclovir-resistant neonatal herpes simplex virus infection of the larynx. J. Pediatr. 124:967-971. [DOI] [PubMed] [Google Scholar]
- 57.Oram, R. J., D. Marcellino, D. Strauss, E. Gustafson, C. L. Talarico, A. K. Root, P. L. Sharma, K. Thompson, J. D. Fingeroth, C. Crumpacker, and B. C. Herold. 2000. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature infant. J. Infect. Dis. 181:1458-1461. [DOI] [PubMed] [Google Scholar]
- 58.Parris, D. S., and J. E. Harrington. 1982. Herpes simplex virus variants resistant to high concentrations of acyclovir exist in clinical isolates. Antimicrob. Agents Chemother. 22:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pottage, J. C., and H. A. Kessler. 1995. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect. Agents Dis. 4:115-124. [PubMed] [Google Scholar]
- 60.Rabalais, G. P., S. Nusinoff-Lehrman, A. M. Arvin, and M. J. Levin. 1989. Antiviral susceptibilities of herpes simplex virus isolates from infants with recurrent mucocutaneous lesions after neonatal infection. Pediatr. Infect. Dis. J. 8:221-223. [PubMed] [Google Scholar]
- 61.Reardon, J. E., and T. Spector. 1989. Herpes simplex virus type 1 DNA polymerase. J. Biol. Chem. 264:7405-7411. [PubMed] [Google Scholar]
- 62.Redding, S. W. 1990. Role of herpes simplex virus reactivation in chemotherapy-induced oral mucositis. NCI Monogr. 9:103-105. [PubMed] [Google Scholar]
- 63.Reichman R. C., G. J. Badger, G. J. Mertz, L. Corey, D. D. Richman, J. D. Connor, D. Redfield, M. C. Savoia, M. N. Oxman, Y. Bryson, D. L. Tyrrell, J. Portnoy, T. Creigh-Kirk, R. E. Keeney, T. Ashikaga, and R. Dolin. 1984. Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA 251:2103-2107. [PubMed] [Google Scholar]
- 64.Richman D. D. 1996. Antiviral drug resistance: issues and challenges, p. 1-9. In D. D. Richman (ed.), Antiviral drug resistance. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 65.Sacks S. L., F. Y. Aoki, F. Diaz-Mitoma, J. Sellors, and S. D. Shafran. 1996. Patient-initiated, twice-daily oral famciclovir for early recurrent genital herpes. A randomized, double-blind multicenter trial. JAMA 276:44-49. [PubMed] [Google Scholar]
- 66.Sacks, S. L., R. J. Wanklin, D. E. Reece, K. A. Hicks, K. L. Tyler, and D. M. Coen. 1989. Progressive esophagitis from acyclovir-resistant herpes simplex virus. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann. Intern. Med. 111:893-899. [DOI] [PubMed] [Google Scholar]
- 67.Safrin, S., T. Assaykeen, S. Follansbee S, and J. Mills. 1990. Foscarnet therapy for acyclovir-resistant mucocutaneous herpes simplex virus infection in 26 AIDS patients: preliminary data. J. Infect. Dis. 161:1078-1084. [DOI] [PubMed] [Google Scholar]
- 68.Safrin, S., C. Crumpacker, P. Chatis, R. Davis, R. Hafner, J. Rush, H. A. Kessler, B. Landry, J. Mills, and other members of the AIDS Clinical Trials Group. 1991. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. N. Engl. J. Med. 325:551-555. [DOI] [PubMed] [Google Scholar]
- 69.Safrin, S., T. Elbeik, L. Phan, D. Robinson, J. Rush, A. Elbaggari, and J. Mills. 1994. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 38:1246-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saltzman, R., R. Jurewicz, and R. Boon. 1994. Safety of famciclovir in patients with herpes zoster and genital herpes. Antimicrob. Agents Chemother. 38:2454-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sande, M., D. Armstrong, L. Corey, W. L. Drew, D. Gilbert, R. C. Moellering, Jr., and L. G. Smith. 1998. Perspectives on a switch of oral acyclovir from prescription to over-the-counter status: report of a consensus panel. Clin. Infect. Dis. 26:659-663. [DOI] [PubMed] [Google Scholar]
- 72.Saral, R., W. H. Burns, O. L. Laskin, G. W. Santos, and P. S. Lietman. 1981. Acyclovir prophylaxis of herpes-simplex-virus infections: A randomized, double-blind, controlled trial in bone-marrow-transplant recipients. N. Engl. J. Med. 305:63-67. [DOI] [PubMed] [Google Scholar]
- 73.Saral, R., R. F. Ambinder, W. H. Burns, C. M. Angelopulos, D. E. Griffin, P. J. Burke, and P. S. Lietman. 1983. Acyclovir prophylaxis against herpes simplex virus infection in patients with leukemia. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 99:773-776. [DOI] [PubMed] [Google Scholar]
- 74.Saral, R. 1990. Management of acute viral infections. NCI Monogr. 9:107-110. [PubMed] [Google Scholar]
- 75.Sarisky, R. T., T. Bacon, R. Boon, L. Locke, T. T. Nguyen, J. Leary, K. Esser, and R. Saltzman. 2002. Penciclovir susceptibilities of herpes simplex virus isolates from patients using penciclovir cream for the treatment of recurrent herpes labialis. Antimicrob. Agents Chemother. 46:2848-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarisky, R. T., H. R. Bartus, S. A. Dennis, M. R. Quail, T. T. Nguyen, R. J. Wittrock, W. S. Halsey, T. H. Bacon, J. J. Leary, and D. Sutton. 2001. Absence of rapid selection for acyclovir or penciclovir resistance following suboptimal oral prodrug therapy of HSV-infected mice. BMC Infect. Dis. 1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarisky, R. T., R. Cano, T. T. Nguyen, R. J. Wittrock, K. E. Duffy, P. Clark, J. O. Bartus, T. H. Bacon, L. Caspers-Velu, R. L. Hodinka, and J. J. Leary. 2001. Biochemical characterization of a virus isolate, recovered from a patient with herpes keratitis, that was clinically resistant to acyclovir. Clin. Infect. Dis. 33:2034-2039. [DOI] [PubMed] [Google Scholar]
- 78.Sarisky, R. T., P. Crosson, R. Cano, M. R. Quail, T. T. Nguyen, R. J. Wittrock, T. H. Bacon, S. L. Sacks, L. Caspers-Velu, R. L. Hodinka, and J. J. Leary. 2002. Comparison of methods for identifying resistant herpes simplex virus and measuring antiviral susceptibility. J. Clin. Virol. 23:191-200. [DOI] [PubMed] [Google Scholar]
- 79.Sarisky, R. T., T. T. Nguyen, K. E. Duffy, R. J. Wittrock, and J. J. Leary. 2000. Difference in incidence of spontaneous mutations between herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 44:1524-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarisky, R. T., M. R. Quail, P. E. Clark, T. T. Nguyen, W. S. Halsey, R. J. Wittrock, J. O'Leary Bartus, M. M. Van Horn, G. M. Sathe, S. Van Horn, M. D. Kelly, T. H. Bacon, and J. J. Leary. 2001. Characterization of herpes simplex viruses selected for resistance to penciclovir or acyclovir. J. Virol. 75:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasadeusz, J. J., and S. L. Sacks. 1996. Spontaneous reactivation of thymidine kinase-deficient, acyclovir-resistant type 2 herpes simplex virus: masked heterogeneity or reversion? J. Infect. Dis. 174:476-482. [DOI] [PubMed] [Google Scholar]
- 82.Sasadeusz, J. J., F. Tufaro, S, Safrin, K. Schubert, M. M. Hubinette, P. K. Cheung, and S. L. Sacks. 1997. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 71:3872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schelonka, R. L., and A. J. Infante. 1998. Neonatal immunology. Semin. Perinatol. 22:2-14. [DOI] [PubMed] [Google Scholar]
- 84.Schnipper, L. E., and C. S. Crumpacker. 1980. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc. Natl. Acad. Sci. USA 77:2270-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shepp, D. H., B. A. Newton, P. S. Dandliker, N. Flournoy, and J. D. Meyers. 1985. Oral acyclovir therapy for mucocutaneous herpes simplex infections in immunocompromised marrow transplant recipients. Ann. Intern. Med. 102:783-785. [DOI] [PubMed] [Google Scholar]
- 86.Shin Y. K., G.-Y. Cai, A. Weinberg, J. J. Leary, and M. J. Levin. 2001. Frequency of acyclovir-resistant herpes simplex virus in clinical specimens and laboratory isolates. J. Clin. Microbiol. 39:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siegal, F. P., C. Lopez, G. S. Hammer, A. E. Brown, S. J. Kornfeld, J. Gold, J. Hassett, S. Z. Hirschman, C. Cunningham-Rundles, B. R. Adelsberg, et al. 1981. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N. Engl. J. Med. 305:1439-1444. [DOI] [PubMed] [Google Scholar]
- 88.Smith, K. J., H. G. Skelton, D. M. Frissman, and P. Angritt. 1992. Verrucous lesions secondary to DNA viruses in patients infected with the human immunodeficiency virus in association with increased factor XIIIa-positive dermal dendritic cells. J. Am. Acad. Dermatol. 26:943-950. [DOI] [PubMed] [Google Scholar]
- 88a.Spruance, S. L., R. Nett, T. Marbury, R. Wolff, J. Johnson, and T. Spaulding. 2002. Acyclovir cream for treatment of herpes labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob. Agents Chemother. 46:2238-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spruance, S. L., J. C. Overall, E. R. Kern, G. G. Krueger, V. Pliam, and W. Miller. 1977. The natural history of recurrent herpes simplex labialis. Implications for antiviral therapy. N. Engl. J. Med. 297:69-75. [DOI] [PubMed] [Google Scholar]
- 90.Spruance, S. L., T. L. Rea, C. Thoming, R. Tucker, R. Saltzman, and R. Boon. 1997. Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. JAMA 277:1374-1379. [PubMed] [Google Scholar]
- 91.Straus, S. E., H. E. Takiff, M. Seidlin, S. Bachrach, L. Lininger, J. J. DiGiovanna, K. A. Western, H. A. Smith, S. Nusinoff Lehrman, T. Creagh-Kirk, and D. A. Alling. 1984. Suppression of frequently recurring genital herpes. A placebo-controlled double-blind trial of oral acyclovir. N. Engl. J. Med. 310:1545-1550. [DOI] [PubMed] [Google Scholar]
- 92.Swetter, S. M., E. L. Hill, E. R. Kern, D. M. Koelle, C. M. Posavad, W. Lawrence, and S. Safrin. 1998. Chronic vulvar ulceration in an immunocompetent woman due to acyclovir-resistant, thymidine kinase-deficient herpes simplex virus. J. Infect. Dis. 177:543-550. [DOI] [PubMed] [Google Scholar]
- 93.Tebas, P., D. Scholl, J. Jollick, K. McHarg, M. Arens, and P. D. Olivo. 1998. A rapid assay to screen for drug-resistant herpes simplex virus. J. Infect. Dis. 177:217-220. [DOI] [PubMed] [Google Scholar]
- 94.Tong, P., and D. F. Mutasim. 1996. Herpes simplex virus infection masquerading as condyloma acuminata in a patient with HIV disease. Br. J. Dermatol. 134:797-800. [PubMed] [Google Scholar]
- 95.Vere Hodge, R. A., and Y.-C. Chen. 1993. The mode of action of penciclovir. Antiviral Chem. Chemother. 4(Suppl. 1):13-24. [Google Scholar]
- 96.Vere Hodge, R. A., and R. M. Perkins. 1989. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob. Agents Chemother. 33:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vere Hodge, R. A., D. Sutton, M. R. Boyd, M. R. Harnden, and R. L. Jarvest. 1989. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine; penciclovir]. Antimicrob. Agents Chemother. 33:1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wade, J. C., C. McLaren, and J. D. Meyers. 1983. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J. Infect. Dis. 148:1077-1082. [DOI] [PubMed] [Google Scholar]
- 99.Weinberg, A., B. J. Bate, H. B. Masters, S. A. Schneider, J. C. Clark, C. G. Wren, J. A. Allaman, and M. J. Levin. 1992. In vitro activities of penciclovir and acyclovir against herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 36:2037-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whitley, R. J., M. Levin, N. Barton, B. J. Hershey, G. Davis, R. E. Keeney, J. Whelchel, A. G. Diethelm, P. Kartus, and S. J. Soong. 1984. Infections caused by herpes simplex virus in the immunocompromised host: natural history and topical acyclovir therapy. J. Infect. Dis. 150:323-329. [DOI] [PubMed] [Google Scholar]
- 101.Whitley, R. J., and J. W. Gnann. 1993. The epidemiology and clinical manifestations of herpes simplex virus infections, p. 69-105. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, New York, N.Y.
- 102.Whitley, R. J., D. W. Kimberlin, and B. Roizman. 1998. Herpes simplex viruses. Clin. Infect. Dis. 26:541-555. [DOI] [PubMed] [Google Scholar]