Abstract
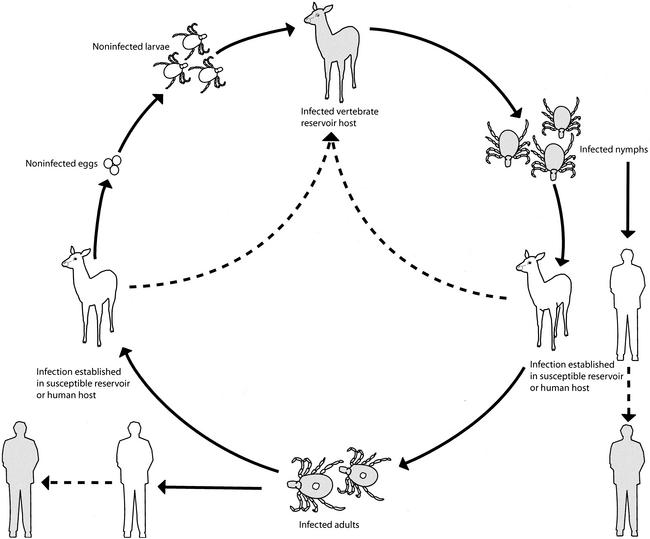
Ehrlichia chaffeensis is an obligately intracellular, tick-transmitted bacterium that is maintained in nature in a cycle involving at least one and perhaps several vertebrate reservoir hosts. The moderate to severe disease caused by E. chaffeensis in humans, first identified in 1986 and reported for more than 1,000 patients through 2000, represents a prototypical “emerging infection.” Knowledge of the biology and natural history of E. chaffeensis, and of the epidemiology, clinical features, and laboratory diagnosis of the zoonotic disease it causes (commonly referred to as human monocytic ehrlichiosis [HME]) has expanded considerably in the period since its discovery. In this review, we summarize briefly the current understanding of the microbiology, pathogenesis, and clinical manifestations associated with this pathogen but focus primarily on discussing various ecological factors responsible for the recent recognition of this important and potentially life-threatening tick-borne disease. Perhaps the most pivotal element in the emergence of HME has been the staggering increases in white-tailed deer populations in the eastern United States during the 20th century. This animal serves as a keystone host for all life stages of the principal tick vector (Amblyomma americanum) and is perhaps the most important vertebrate reservoir host for E. chaffeensis. The contributions of other components, including expansion of susceptible human populations, growth and broadening geographical distributions of other potential reservoir species and A. americanum, and improvements in confirmatory diagnostic methods, are also explored.
INTRODUCTION
In April 1986, a medical intern scanning the peripheral blood smear of a severely ill man with an unexplained illness observed peculiar intracytoplasmic inclusions in several of the patient's monocytes. The patient described multiple tick bites sustained approximately 2 weeks earlier during a visit to a rural area in northern Arkansas, and a presumptive diagnosis of Rocky Mountain spotted fever had been made (104, 174). Clinicians and scientists subsequently identified these inclusions as clusters of bacteria belonging to the genus Ehrlichia, previously known in the United States solely as veterinary pathogens (174). Within the next 5 years, the organism was isolated in cell culture, characterized by molecular techniques, and formally named Ehrlichia chaffeensis (9, 73). During this interval, surveillance efforts identified several hundred cases of moderate to severe, and occasionally fatal, ehrlichiosis in patients with unexplained illnesses following tick exposures (97, 106, 107, 125, 233, 263). These findings indicated that ehrlichiosis was a widespread and significant public health problem of increasing but undefined magnitude.
During the 1990s, two additional Ehrlichia spp., Anaplasma (formerly Ehrlichia) phagocytophila (the agent of human granulocytic ehrlichiosis [HGE]) and E. ewingii (a cause of granulocytic ehrlichiosis in dogs), were identified as human pathogens, and these reports greatly expanded the geographic region and the size of the human population at risk for acquiring one of these potentially lethal infections (19, 42). While most of the cases of ehrlichiosis caused by E. chaffeensis were being identified in the southeastern and south central United States, within a relatively few years of the initial recognition of HGE the number of cases of human ehrlichiosis identified in the northeastern and north cental states surpassed other regional totals (188).
Although the term “emerging infection” has become almost hackneyed, the Ehrlichia spp. that cause human disease in the United States epitomize the intended application of this designation (156). Not only are these pathogens new to science, but their maintenance in nature requires the complex interactions of tick vectors and vertebrate hosts that are sensitive to environmental influences that can drive epidemics (6). Changes in host susceptibility within a population can be a critical factor in disease emergence (193). Ehrlichiosis caused by E. chaffeensis has increasingly been identified in population segments immunosuppressed through aging, infectious causes, malignancy, or medical therapy (206). Reports of severe and fatal ehrlichioses in these population segments will increase as an unavoidable consequence of environmental forces that increase the risk of exposure to these pathogens, coupled with dramatic changes in human demography and the geographic distribution of AIDS cases (63, 154). This entire process has been fueled by technical developments and the application of sensitive and versatile diagnostic methods, particularly PCR, and a renewed interest in tick-borne and other zoonotic diseases (156, 212, 272).
Several reviews have been written on the microbiology and molecular biology of ehrlichiae and the clinical characteristics of the human ehrlichioses (86, 87, 93, 111, 186, 205, 227, 228). This review of E. chaffeensis summarizes much of this material but focuses primarily on the ecological and epidemiological factors that have contributed to its recognition as an agent of human disease. Although disease caused by E. chaffeensis has been termed human monocytic ehrlichiosis or human monocytotropic ehrlichiosis (i.e., HME), designations of ehrlichioses based on cell tropism may become less useful monikers as additional ehrlichial pathogens are recognized. However, this nomenclature is firmly established in the literature, and to avoid confusion in this review, the acronym HME is used to designate disease caused by E. chaffeensis.
MICROBIOLOGY
Taxonomy and Phylogenetic Placement
E. chaffeensis is an obligately intracellular bacterium in the family Anaplasmataceae and is a member of the α subdivision of the Proteobacteria. Until 2001, the genus Ehrlichia was composed of a heterogeneous collection of several recognized species (e.g., E. canis, E. phagocytophila, E. sennetsu, E. equi, E. risticii, E. chaffeensis, E. ewingii, and E. muris) and various other taxa that do not have current standing in bacterial nomenclature. This assemblage of species demonstrates considerable molecular diversity based on phylogenetic analyses of 16S rRNA genes, surface protein genes, and groESL heat shock protein operon sequences. On the basis of these differences, Ehrlichia spp. were until recently segregated into three informal “genogroups” (86). A contemporary taxonomic revision reassigned several of these species to other genera (E. sennetsu and E. risticii to Neorickettsia and E. phagocytophila and E. equi to Anaplasma) and emended the genus Ehrlichia to include Cowdria ruminantium, a closely related tick-borne pathogen that causes severe disease (“heartwater”) in ruminants in Africa and the Caribbean. In this classification, all members of the tribe Ehrlichieae were reassigned to the family Anaplasmataceae (88). The bacteria that cause human “ehrlichioses” are now represented by three genera rather than the single genus Ehrlichia; they include Neorickettsia sennetsu (the agent of sennetsu fever) Anaplasma phagocytophila, E. ewingii, and E. chaffeensis.
The emended genus Ehrlichia includes E. canis, E. chaffeensis, E. ewingii, E. muris, and E. ruminantium. These ehrlichiae share various genetic, morphologic, clinical, and ecological features: all are at least 97.7% similar in 16S rRNA gene sequences, all reside and multiply in cytoplasmic vacuoles of host cells (the principal cell types include mononuclear and polymorphonuclear leukocytes and endothelial cells, depending on the particular species); all cause disease in animals, humans, or both; and all are transmitted by hard-tick vectors (88).
Morphology
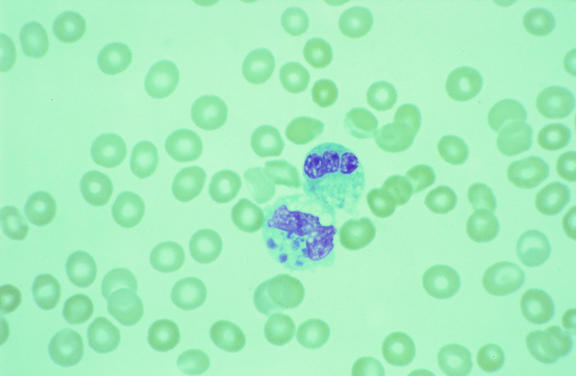
Light microscopic and ultrastructural descriptions of E. chaffeensis have been based on observations of the pathogen in human leukocytes and tissues and in various cell lines of mammalian origin. In these habitats, these small, nonmotile bacteria reside and grow in cytoplasmic vacuoles derived from an early endosome, forming loose to condensed aggregates of bacteria termed morulae. By light microscopy, these morulae appear as mulberry-like, bosselated intracytoplasmic inclusions that stain dark blue to purple with Romanovsky-type stains (Fig. 1) (227).
FIG. 1.
Peripheral blood smear from a patient with HME, demonstrating variably sized basophilic inclusions (morulae) within the cytoplasm of a monocyte (lower cell). Each morula consists of a cluster of E. chaffeensis contained with a vacuole. Modified Wright's stain. Magnification, ×1,000.
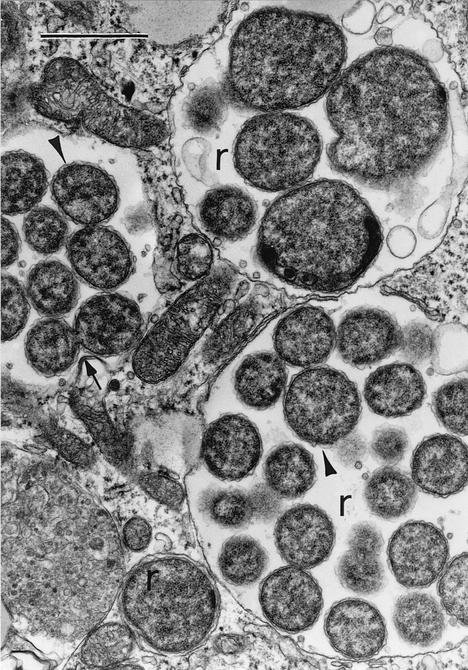
By electron microscopy, two distinct morphologic cell types are identified: coccoid and coccobacillary forms with ribosomes and nucleoid DNA fibrils uniformly dispersed throughout the cytoplasm (reticulate cells) (Fig. 2), and predominantly coccoid bacteria with centrally condensed nucleoid DNA and ribosomes (dense-cored cells). Reticulate cells measure 0.4 to 0.6 μm by 0.7 to 1.9 μm, and dense-cored cells measure 0.4 to 0.6 μm in diameter. Both cell types replicate by binary fission, and both demonstrate a gram-negative-type cell wall, characterized by a smooth-contoured cytoplasmic membrane and a generally ruffled outer membrane, separated by a periplasmic space. Members of the genus Ehrlichia do not appear to contain significant amounts of peptidoglycan (227). Both cell types have been demonstrated in clinical samples (209), although the microbiological significance of these distinct morphological forms is unknown. Morulae range from 1.0 to 6.0 μm in width and contain 1 to >40 organisms of uniform or mixed cell types (218, 228). The intramorular space may contain a fine, striated fibrillar matrix and intramorular tubules 25 nm in diameter and as long as 1.5 μm, which originate from the outer membrane of reticulate cells. In cell culture and infected human cells, host cell mitochondria are frequently apposed to the margins of morulae (209, 218).
FIG. 2.
Electron photomicrograph of intracytoplasmic vacuoles containing reticulate forms of E. chaffeensis (r) in a DH82 cell (continuous canine histiocytoma cell line) (267). Reticulate cells demonstrate prominent ruffled outer cell membranes (arrowheads) and divide by binary fision (arrow). Lead citrate-uranyl acetate stain. Magnification, ×18,000; bar, 1 μm. Reproduced with permission from V. Popov, University of Texas Medical Branch at Galveston.
Isolates of E. chaffeensis
At least 21 isolates of E. chaffeensis have been obtained from patients with HME, infected in Arkansas (73, 90), Oklahoma (59), Florida and Georgia (209, 259), Tennessee (206, 255), and Maryland (262). Isolates of E. chaffeensis from sources other than human tissues are few and include five from white-tailed deer (169) and one from a domestic goat (85), each obtained in Georgia.
Described isolates have been obtained in primary culture by using a continuous canine histiocytoma cell line (DH82 cells) and less frequently, human embryonic lung fibroblasts (HEL 299 cells) (59, 73, 85, 90, 169, 206, 209, 255).
In vitro, E. chaffeensis has been adapted to grow in various other cell lines, including human microvascular endothelial cells (HMEC-1 cells), African green monkey kidney cells (Vero cells), human cervical epithelioid carcinoma cells (HeLa cells), human monocytic leukemia cells (THP-1 cells), HEL 299 cells, mouse embryo cells, buffalo green monkey cells, and murine fibroblasts (25, 38, 58, 128, 187).
Genetic, Antigenic, and Phenotypic Characteristics
The genome size of E. chaffeensis is approximately 1250 kb (239). Among the nucleotide sequences that have been characterized are the 16S rRNA gene (9), various genes coding for immunoreactive proteins including the variable-length PCR target (VLPT) (259) and the 120-kDa (289), 106-kDa, and 37-kDa (290) protein genes, the groESL heat shock operon (260), a quinolate synthetase A gene (292), and a locus that contains 22 homologous but not identical genes (the p28 multigene family) (201, 286).
Two antigen-expressing genes that contain repetitive elements have been identified. The 120-kDa protein gene contains a series of 240-bp serine-rich tandem repeat units; the number of repeat units varies among isolates. To date, three variants of the gene (represented by two, three, or four repeats) have been identified in DNA extracts of E. chaffeensis obtained from patients with HME and from infected ticks (255, 259, 287, 289). The 120-kDa gene encodes a heavily glycosylated, immunodominant surface protein that is preferentially expressed on dense-cored forms of E. chaffeensis and as a component of the intramorular fibrillary matrix (183, 219). This gene demonstrates interstrain variation, and p120 proteins expressed by different isolates of E. chaffeensis vary in molecular weight; however, immune sera from patients with HME react with p120 antigens from various strains regardless of variations in the number of repeat units (290). The VLPT gene demonstrates even greater interstrain diversity (209, 259). This gene is also characterized by a series of direct tandem repeats, whose number may vary among isolates. DNAs of VLPT genes amplified from cultured isolates of E. chaffeensis or from ticks or patient blood samples infected with this pathogen have shown two to six repeats. Qualitative differences in the nucleotide sequences of the imperfect 90-bp repeats results in at least seven different types of repeat units. Additional genetic diversity is produced by differences in the linear order of the individual repeats and by various deletions and substitutions along the length of the gene. Based on a relatively small number of DNAs evaluated, VLPT patterns of E. chaffeensis in the southeastern United States are most frequently represented by three or four repeats, and the six-repeat variant appears to be the rarest version of this gene (206, 255, 257, 259). The biological function of this gene has not yet been elucidated; however, VLPT sequences code for immunoreactive proteins with apparent molecular masses of 30 to 60 kDa (259). Collectively, the occurrence of genetic heterogeneity among several of the recognized genes of E. chaffeensis suggests that considerable molecular diversity exists within this bacterium: evaluation of 18 patient isolates by using genetic composites created by polymorphisms in the VLPT gene and the 120-kDa protein gene reveal eight distinct genotypes (255, 259). No distinct biological, clinical, or epidemiological correlates have been associated with a particular genotype, although future studies may be more revealing.
Isolate-dependent sequence polymorphisms have also been described for a locus of E. chaffeensis genes that encodes major outer membrane proteins (OMP), described as the omp cluster or the p28 multigene family (286). Detailed analysis of this locus in the Arkansas isolate of E. chaffeensis reveals 22 complete, paralogous genes from 813 to 900 bp distributed along a 27-kb segment of the genome (201). The p28 genes code for mature proteins with predicted molecular sizes of approximately 26 to 32 kDa; none of the proteins are identical, and the amino acid sequence identity varies from approximately 20 to 80% (286). Sequences of individual p28 genes also vary among different isolates of E. chaffeensis (171, 286, 291). At least 16 p28 alleles are actively transcribed, and it is likely that the antigenic diversity of E. chaffeensis results from differential expression within this gene family (171). Homologous immunodominant proteins encoded by multigene families have been identified in closely related bacteria, including E. canis, E. ruminantium, A. phagocytophila, and A. marginale (183, 201, 226).
Several major immunoreactive proteins of the Arkansas isolate of E. chaffeensis have been identified by using human antisera in immunoblot analyses. These include polypeptides with relative molecular masses of approximately 120, 66, 58, 55, 44, 29, 28, and 22- kDa (57, 59). Genetic correlates have been established for several of these antigens, including the 120-kDa protein, the GroEL protein (58 kDa), and the p28 proteins.
Variations in reactivity among different isolates of E. chaffeensis have been demonstrated by using monoclonal antibody (MAb) analyses. MAb 1A9 reacts with epitopes of various p28 proteins with different molecular sizes; however, it does not react with all isolates of E. chaffeensis (59, 285), reflecting heterogeneity in the antigenic composition among isolates created by the diversity of p28 proteins (286, 291). Isolate-specific reactivity is also demonstrated by MAb 6A1, which reacts with a surface-exposed, 30-kDa antigen of the Arkansas isolate but does not react with the 91HE17 isolate (56). Variation in sizes of apparently homologous proteins have also been detected by using MAbs (59) and immunoblot analyses demonstrating isolate-specific expression of the 120-kDa protein and VLPT repetitive-element gene products (56, 259). Biological correlates for these variably sized proteins to pathogen virulence or clinical disease in humans are incompletely characterized, although MAbs directed against specific epitopes of p28 OMPs can mediate the clearance of E. chaffeensis in a SCID mouse model (160).
Other than descriptions of the antigenic composition and immunolocalization of these proteins, relatively few phenotypic characteristics of E. chaffeensis have been identified. Experiments with a low-passage, culture-adapted isolate show that this strain can survive for at least 11 days in anticoagulated human whole blood and for as long as 21 days in cell culture media at 4 to 6°C (187).
PATHOGENESIS
Factors Relating to Disease Severity
The pathogenesis and determinants of disease severity for HME are incompletely understood. Soon after the recognition of this disease, it became apparent that a wide range of clinical outcomes were possible in persons infected with E. chaffeensis (263). In a study of 149 patients diagnosed during 1985 to 1990, logistic regression was used to demonstrate that age (≥60 years) operated as an independent risk factor for severe or fatal illness (105). However, many cases of severe or fatal disease have been described in apparently healthy children (32, 103, 109, 246) and young adults (155, 179, 181). In this context, disease severity may ultimately depend on a complex interaction of several components relating to the host, the pathogen, and perhaps therapeutic interventions.
Severe or fatal HME has been described in persons with compromised immunity from various causes including human immunodeficiency virus (HIV) disease (23, 179, 206, 208), immunosuppresive therapies (12, 14, 177, 180, 241, 242, 262), monoclonal gammopathy (79), asplenia (98, 103), sickle β-thalassemia (246), and Down's syndrome (96).
For some patients, the severity of clinical manifestations appears directly correlated with the level of bactermia, particularly among severely immunocompromised patients infected with HIV. In these individuals, morulae are often detected in peripheral blood leukocytes in relatively large numbers and the organism is detected in cell culture relatively rapidly (206). However, this correlate does not apply to all patients, since peripheral blood smears and bone marrow aspirates of some critically ill patients fail to reveal morulae (32, 91, 209, 262). There are no data to specifically associate distinct molecular or antigenic features among strains of E. chaffeensis with variations in disease severity or particular disease manifestations; however, it is possible that intrinsic markers for these outcomes will emerge as additional isolates are obtained and a broader repertoire of genetic identifiers are evaluated (90, 209, 255).
Among published descriptions of patients with particularly severe or fatal HME are reports of individuals who received long-term sulfa drug therapy for ulcerative colitis (194, 211) or as prophylaxis for opportunistic infections (14, 206, 242, 262) and reports of patients for whom trimethoprim-sulfamethoxazole was administered for several days or weeks before ehrlichiosis was correctly diagnosed (1, 27, 28, 84, 94, 103, 119, 236, 237, 243). An association between the use of sulfa-containing antibiotics and exacerbation of disease severity has been described for other rickettsial infections, and the frequency of similar reports of this association among patients with HME warrants further investigation (215).
Pathology
In vertebrate hosts, E. chaffeensis infects predominantly mononuclear phagocytic cells. The most frequently infected blood cells are monocytes; however, infections in other cell types have been described, including lymphocytes, atypical lymphocytes, promyelocytes, metamyelocytes, and band and segmented neutrophils (1, 91, 174, 209). Although E. chaffeensis appears capable of inhabiting other phagocytic cells (e.g., granulocytes), it is likely that mononuclear phagocytes maintain the productive infection (91). Infected cells typically contain only 1 or 2 morulae, although as many as 15 have been observed in leukocytes of immunosuppressed patients (23, 179, 208).
There are relatively few histopathologic data describing lesions in tissues and organs of persons with HME. The most extensively sampled and described tissue has been bone marrow. However, no consistent histopathologic patterns have emerged from these examinations, possibly because the biopsy specimens have been obtained during different stages in the course of the illnesses. The most frequently reported finding is a normocellular or hypercellular marrow with myeloid hyperplasia, megakaryocytosis, or both (91, 121, 253). Bone marrow biopsy specimens may reveal aggregates of foamy histiocytes or small noncaseating granulomas (91, 99, 121) or may show hemophagocytosis (1, 84, 91, 180) or may be unremarkable or normal (91, 99, 127, 138). Morulae have been detected in fewer than half of the described bone marrow biopsy specimens but are frequently visualized in marrow of patients infected with HIV (23, 208, 209). Hypocellular bone marrow is seldom observed in patients with acute disease, and diminished peripheral blood cell counts are characteristically far out of proportion to the absolute numbers infected leukocytes, implying that cytopenias associated with HME result from peripheral events that may include sequestration, consumption, or destruction of infected and noninfected cells (91, 125).
Pathologic findings in other tissues have been described most frequently in patients with fatal disease (79, 89, 91, 180, 208, 209). Because persons who die of HME often represent specialized patient cohorts (e.g., immunocompromised patients), quantitative and qualitative features of the histopathologic findings in these patients may not be directly comparable to features of disease in the general patient population. Findings in the lungs of these patients may include intra-alveolar hemorrhage, diffuse alveolar damage, and interstitial pneumonitis and edema (89, 109, 180, 208, 209). Perivascular, predominantly lymphohistiocytic infiltrates without evidence of endothelial damage or thrombosis can occur in many organs, including the meninges (180, 209, 275). Other findings may include hemophagocytosis and microvesicular steatosis in the liver (89, 208, 209), focal necroses in spleen, liver, and lymph nodes (89, 208), and diffuse hemorrhages involving soft tissues, kidneys, urinary bladder, diaphragm, and meninges (180, 209).
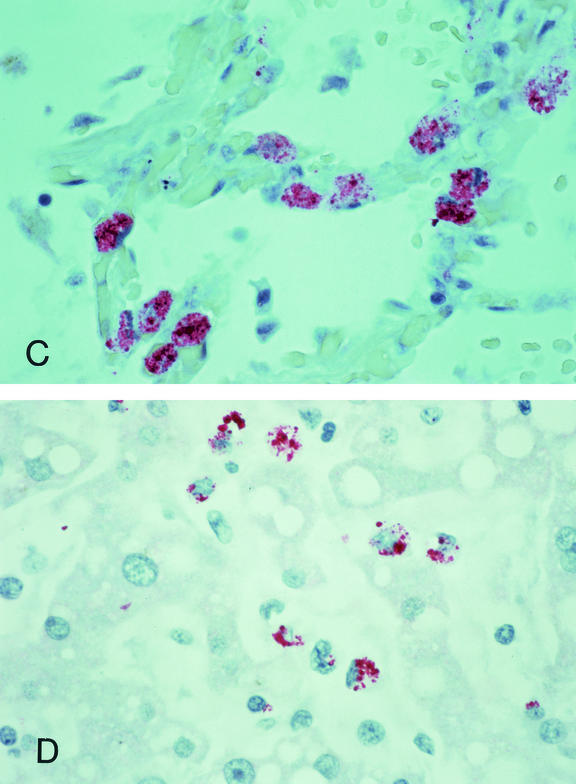
Localization of ehrlichiae and ehrlichial antigens by immunohistochemical and in situ hybridization techniques reveal systemic, multiorgan involvement in patients with fatal HME. The greatest distribution of bacteria occurs in tissues containing abundant mononuclear phagocytic cells, including splenic cords and periarteriolar sheaths, lymph nodes, and bone marrow (23, 79, 180, 208). Morulae are less frequently observed in macrophages in the pulmonary microvasculature and in the liver within Kupffer cells (79, 89, 208) (Fig. 3C and D). E. chaffeensis is detected occasionally and in lower abundance in mononuclear cell aggregates or perivascular infiltrates in the brain, heart, pancreas, adrenals, kidneys, gastrointestinal tract, omentum, ovaries, and connective tissue (23, 79, 180).
FIG. 3.
Immunohistochemical localization of E. chaffeensis in mononuclear cells of the spleen (A) and bone marrow (B), in pulmonary macrophages (C), and in hepatic Kupffer cells (D) in patients with fatal HME; tissues represented in panels A through C were obtained from patients coinfected with HIV. Bacterial burdens in severely immunocompromised individuals are generally far greater than those observed in immunologically intact patients. Ehrlichiae and ehrlichial antigens appear as red inclusions within the cytoplasms of infected cells. Immunoalkaline phosphatase stain with naphthol phosphate-fast red substrate and hematoxylin counterstain. Magnifications, ×630. Reproduced with permission from S. Zaki, CDC.
Immunology
As with many aspects of the pathogenesis of HME, there is only a nascent understanding of the immune mechanisms that follow infection with E. chaffeensis in a human host. The relative contributions of humoral and cell-mediated immunity have not been definitively established, although both appear to play important roles in host defenses against this pathogen. Because ehrlichiae are intracellular pathogens, it is intuitive that cellular immunity is an important component of successful clearance of E. chaffeensis. This paradigm is suggested directly by descriptions of particularly severe disease in HIV-infected patients (206) and indirectly by observations of the profound lymphoproliferative responses described for patients recovering from HME (45, 99, 105).
Various inbred mouse strains have been used to dissect the impact of cellular and humoral processes following infection. Wild-type mouse strains infected with E. chaffeensis clear the bacteria within 16 days, while mice with defective macrophage and T-cell functions maintain infections that may persist for one to several months (112). Mice lacking functional toll-like receptor 4 (tlr4) alleles, whose gene product is responsible for macrophage stimulation following exposure to lipopolysaccharide of gram-negative bacteria, produce significantly decreased levels of nitric oxide and interleukin-6 (IL-6) and develop infections with E. chaffeensis that persist for at least 2 weeks beyond the duration of infection observed in wild-type mice. However, macrophage activation alone does not appear to be sufficient for successful clearance of this pathogen. The role of major histocompatibility complex class II (MHC-II) genes appear to be even more profound, and mice lacking functional MHC-II genes are unable to clear E. chaffeensis following infection. These findings suggest that CD4+ T lymphocytes are essential for complete clearance of this intracellular pathogen (112). These observations are supported by the results obtained with other murine models using immunodeficient animals. In contrast to tlr4 and MHC-II mutants which do not become ill or die following infection with E. chaffeensis, SCID mice deficient in T and B lymphocytes develop persistent, overwhelming infections and become moribund within 24 days postinfection (280). However, animals with functional B cells but deficient for α/β T cells or both α/β and γ/δ T cells remain persistently infected but do not become ill. Similarly, immune serum from immunocompetent mice or MAbs recognizing an immunodominant outer membrane protein (p28) of E. chaffeensis, administered passively to SCID mice prior to or during active infection, results in protection from disease but does not effect complete bacterial clearance (160, 281). Collectively, observations in murine systems suggest that antibodies contribute to the elimination of this pathogen during active infection and may ameliorate disease and that intact cellular immunity, particularly involving processes coordinated by CD4+ T cells, appears to be the crucial determinant of complete recovery following infection with this agent.
Paradoxically, the relative paucity of bacteria detected in the blood and tissues of most patients infected with E. chaffeensis, even those with severe illnesses, suggests that clinical manifestations of HME may also be mediated by host immune responses, and possibly amplified by specific cytokine production (228). In vitro studies have shown that human monocytes infected with E. chaffeensis produce only two proinflammatory interleukins, IL-1β and IL-8, and an immunosuppresive cytokine, IL-10 (157). However, when infected cells are exposed to hyperimmune serum containing anti-E. chaffeensis IgG antibodies, additional proinflammatory cytokines, including tumor necrosis factor alpha and IL-6, are generated by the cells (158). Binding of the E. chaffeensis-antibody complex to human monocytes via the Fcγ receptor is required for expression of TNF-α and IL-6 mRNAs and enhances the expression of IL-1β mRNA. The presence of immune complexes also activates nuclear factor kappa-B, further stimulating secretion of these cytokines. In concert, these processes generate levels of major proinflammatory cytokines as high as the levels observed in cells stimulated with Escherichia coli lipopolysaccharide. These findings suggest that the generation of antibodies to E. chaffeensis may trigger pathophysiologic responses detrimental to the host through a mechanism similar to endotoxic shock (158). In this context, cytokine production and modulation by anti-E. chaffeensis antibodies may play critical roles in processes involving both elimination of the pathogen and generation of systemic disease (228).
Patients with HME typically develop a lymphocytosis during recovery that is disproportionately represented by CD3+ CD4− CD8− T cells expressing a T-cell receptor composed of γ and δ chains. Expansion of lymphocytes with this relatively unusual phenotype has been associated with immune responses to various intracellular pathogens, including Mycobacterium, Listeria, and Leishmania spp. However, patients with HME display the most profound γ/δ T-cell lymphocytosis reported, with levels as high as 97%. Because this response is temporally associated with resolution of infection, it is uncertain if this peculiar lymphocytosis is directly involved in host defense against ehrlichiae or represents an epiphenomenon of the infection (45). Resolution of the γ/δ T-cell lymphoproliferation involves apoptotic cell death of the lymphocytes, which may represent an important mechanism for modulating the T-cell immune response during recovery from the infection (46).
Extensive genetic variability exhibited by the p28 multigene locus of E. chaffeensis and in expressed surface antigen proteins has been proposed as a mechanism of immune evasion (225, 226, 286). Although quantitative transcriptional analyses remain to be performed, it is possible that E. chaffeensis may differentially and sequentially express the p28 multigene family to rapidly alter the composition of one or more of its immunodomminant surface proteins and thereby escape immune surveillance. Only 12 (38%) of 32 convalescent-phase serum samples from patients with HME demonstrated reactivity with a recombinant p28 cloned from the Arkansas isolate (p28-19) of E. chaffeensis, supporting the concept that differential expression of p28 genes results in proteins with substantially different antigenic properties (291). Inoculation with recombinant p28 protects mice from E. chaffeensis infection (202), raising hopes that this antigen will have uses as vaccines for ehrlichial pathogens. MAbs directed against epitopes within the amino terminus of a hypervariable region of the OMP-1g protect SCID mice from otherwise fatal E. chaffeensis infection, and humans with HME produce antibodies reactive with the same OMP-1g hypervariable region (160).
There are few data available that evaluate long-term immunity to E. chaffeensis in persons infected with this pathogen. A single case of sequential infections with two genetically distinct strains of E. chaffeensis has been described. The patient was a liver transplant recipient receiving immunosuppressive therapy, who developed illnesses characteristic of HME during each episode (161). However, the susceptibility of previously infected, immune-intact individuals to reinfection with different strains or even the identical strain remains undetermined. Similarly, a single case of persistent infection with E. chaffeensis has been documented in a 68-year-old debilitated patient in whom ehrlichiae were detected in intrasinusoidal histiocytes of the liver at the time of death, 68 days following the onset of illness (92). The prevalence or clinical significance of persistent infection with this pathogen in human hosts is unknown.
Asymptomatic infection with E. chaffeensis has not been conclusively demonstrated; however, isolation of an ehrlichia closely related or identical to E. canis from the blood of an asymptomatic human from Venezuela suggests that infections with some Ehrlichia spp. may remain clinically silent (214).
Entry and Survival of E. chaffeensis in the Cell
Because E. chaffeensis lacks pili or a capsule, it may bind to its host cell via its outer membrane (229). In vitro studies showing attachment and invasion of HeLa cells by E. coli containing a plasmid expressing the 120-kDa OMP of E. chaffeensis suggest that the p120 is an adhesin that might also enhance the internalization of ehrlichiae (219). Internalized ehrlichiae are invested by the host cell membrane, forming endosomes that maintain distinct cytoplasmic compartments that do not fuse with lysosomes (229).
Survival of ehrlichiae within the cell may be influenced by complex molecular and biochemical pathways involving iron acquisition. Iron is essential for cytochromes and other iron-containing enzymes of E. chaffeensis (229). The iron chelator deferoxamine completely inhibits the growth of E. chaffeensis, indicating that this bacterium is sensitive to cytoplasmic iron depletion (25). Early endosomes containing E. chaffeensis selectively and progressively accumulate transferrin and transferrin receptors (26, 195). Because these endosomes are slightly acidic, ehrlichiae may acquire iron directly from transferrin-iron complexes present in the endosome. Infection of cells by E. chaffeensis further modulates iron uptake by activating a cytoplasmic protein (iron-responsive protein 1), which increases host cell transferrin receptor mRNA levels (24). In vitro studies using recombinant gamma interferon show that this cytokine activates the intracellular killing of E. chaffeensis in human monocytes early in the course of infection by markedly diminishing the number of host cell transferrin receptors, thereby reducing the intracellular labile iron pool (25); however, E. chaffeensis appears to rapidly block the ehrlichiacidal activity of gamma interferon by increasing protein kinase A activity in host cells within 30 min following infection (159). E. chaffeensis also expresses a 37-kDa protein homologous to iron binding proteins of gram-negative bacteria; however, the exact role of this protein remains to be determined (290).
Animal Models of Disease
Investigations of the pathogenesis and immunology of HME have been hampered by the lack of a convenient, reproducible, and generalizable animal model of disease. White-tailed deer, which serve as important natural reservoirs of E. chaffeensis, maintain persistent bacteremias capable of infecting lone star ticks but do not demonstrate clinical manifestations of disease (70, 76, 100).
E. chaffeensis causes naturally occurring disease among dogs that is indistinguishable clinically from diseases caused by E. canis and E. ewingii (37). Experimental infection of dogs also suggests that these animals may have E. chaffeensis circulating in blood for over 3 weeks (77) and may develop characteristic antibody responses (230). However, needle-inoculated animals appear to develop only mild febrile responses without hematologic abnormalities (77). Other factors limit the utility and versatility of canines as experimental hosts, including the absence of inbred syngeneic dogs (particularly animals with genetically defined immune defects) and commercially available canine-specific markers for immune effectors (252).
Several strains of inbred immunocompetent mice (Mus musculus) have been inoculated with E. chaffeensis. These animals appear to rapidly clear the infection and seldom develop illness consistent with HME (165, 264, 280); however, neutrophil infiltrates, hepatocyte apoptosis, and granuloma formation have been observed in the livers of some infected mice (112). Although relatively restricted in their applicability as models of pathogenesis of HME in immune-intact patients, various murine strains with defined immunological deficiencies have proved useful for exploring cellular and antibody-mediated host defenses to E. chaffeensis (112, 280). Small-scale studies using other rodents including white-footed mice, hamsters, and red-backed voles have been unsuccessful in reproducing disease (264).
Animal models of HME using closely related Ehrlichia spp. as surrogates for E. chaffeensis include infection of BALB/c mice with E. muris (144, 145) and infection of C57BL/6 or BALB/c mice with an as yet unnamed Ehrlichia sp. (Ixodes ovatus ehrlichia [IOE]) that is >98% similar to E. chaffeensis by 16S rRNA sequence analysis (203, 249, 252). Mice infected with E. muris develop a transient, mild illness and almost always recover from infection, decreasing the utility of this model as an instrument to study severe HME. However, animals infected with appropriate inocula of IOE consistently die within 9 days and demonstrate histopathologic lesions that resemble lesions identified in human patients with fatal HME, including interstitial pneumonitis, myeloid hyperplasia of bone marrow, and hepatic apoptosis and erythrophagocytosis. In this context, the IOE-mouse model represents a promising system for investigating the immunity and pathogenesis of HME (203, 252).
CLINICAL FEATURES
Characteristics of Disease
The early disease manifestations of HME are relatively constant and, with few exceptions, are shared by a vast array of infectious and noninfectious processes. As the disease progresses, involvement of multiple organ systems may complicate the clinical course and result in various life-threatening scenarios.
General clinical features.
Within 1 to 2 weeks (median, 9 days) following exposure to an infecting tick, patients experience a prodrome characterized by malaise, low-back pain, or gastrointestinal symptoms or may develop sudden onset of fever (often >39°C). Patients with HME are most likely to seek medical attention within 3 to 4 days after the onset of symptoms, and the presenting clinical features frequently include fever (>95%), headache (60 to 75%), myalgias (40 to 60%), nausea (40 to 50%), arthralgias (30 to 35%), and malaise (30 to 80%) (99, 105). During the course of the illness, other manifestations of multisystem disease develop in approximately 10 to 40% of patients, including cough, pharyngitis, lymphadenopathy, diarrhea, vomiting, abdominal pain, and changes in mental status (99, 105, 204, 254). Less frequently reported manifestations include conjunctivitis (32, 250), dysuria (109, 179), and peripheral edema (98).
Large case series of HME in the general population report rashes in approximately 30 to 40% of patients, although a rash is reported more frequently among adult persons infected with HIV (206) and may occur in as many as two-thirds of pediatric patients (95, 139). In comparison, rash is a component of approximately 90% of cases of Rocky Mountain spotted fever (248). Rash patterns associated with HME are variable in character, distribution, and temporal occurrence. This pleomorphism includes petechiae (50, 103, 177, 206, 215, 250), macules (103, 126), maculopapules (32, 200, 241, 243), and diffuse erythema (28, 103, 173, 206). Rash generally occurs later in the course of disease (median of 5 days after onset) (105), may be fleeting or transient (27, 103), and may involve the extremities, trunk, face or, rarely, the palms and soles (96, 124).
Hematologic and biochemical abnormalities.
Multilineage cytopenias are a hallmark laboratory feature of HME early in the course of the illness and may provide early presumptive clues to the diagnosis (105, 246). Mild to moderate leukopenia is observed in approximately 60 to 70% of patients during the first week of illness, with the largest decreases occurring in the total lymphocyte count (99, 105, 139, 255). A relative and absolute lymphocytosis (approximately 45 to 85% of the total leukocyte count) is seen in most patients during recovery and is characterized predominantly by the expansion of activated T cells expressing the γ/δ T-cell receptor (45, 99). Thrombocytopenia is the most frequently identified cytopenia, being seen in 70 to 90% of patients during their illness (105, 206). Although some patients may develop very low platelet levels (e.g., <20,000/μl), platelet nadirs are generally between 60,000 and 120,000/μl (105). The majority of patients present with a normal hematocrit; however, anemia eventually develops in approximately half of HME patients, occurring within 2 weeks following the onset of illness (99, 105, 139, 254, 255).
Mildly or moderately elevated hepatic transaminase levels are noted in approximately 80 to 90% of patients at some point during their illness (99, 105, 200, 254). Alkaline phosphatase and bilirubin levels are less likely to be elevated; however, these markers can be elevated in 25 to 60% of patients (99, 200, 255). Mild to moderate hyponatremia has been reported in as many as 50% of adult patients and 70% of pediatric patients (99, 139). Serum sodium levels of <130 mEq/liter are frequently observed in persons with severe disease (16, 32, 177, 181, 206).
Various other biochemical abnormalities may occur, reflecting progression of the illness to multisystem involvement. These include prolonged activated partial thromboplastin and prothrombin times, increased levels of fibrin degradation products, elevations in the levels of serum creatinine, lactate dehydrogenase, creatine phosphokinase, and amylase, and electrolyte abnormalities including hypocalcemia, hypomagnesemia, and hypophosphatemia (94, 99, 138, 177, 206). The pathophysiologic processes responsible for electrolyte abnormalities are not well understood. In some patients, diminished concentrations of albumin and protein in serum are also noted (1, 126, 138), which may affect the measurement of some divalent cations.
Severe or unusual manifestations.
HME generally manifests as a moderate to severe disease, and approximately 60 to 70% of patients in contemporary case series have been hospitalized (48, 105, 254, 255). In some patients, untreated disease may progress to death as early as the second week of illness (79, 109, 179, 209) or may cause a febrile illness lasting 2 to 3 weeks (106). Multisystem involvement often develops in patients with severe disease and may include acute renal failure, metabolic acidosis, respiratory failure, profound hypotension, disseminated intravascular coagulopathy, hepatic failure, adrenal insufficiency, and myocardial dysfunction (103, 138, 174, 177, 179, 194, 206, 246, 254, 261, 279). The factors responsible for disease severity and involvement of specific organ systems are incompletely understood.
Approximately 20% of persons infected with E. chaffeensis develop signs and symptoms of central nervous system disease (99, 105). Neurologic findings may suggest a meningitis syndrome (meningismus, photophobia, severe headache, lethargy, confusion, or cranial nerve palsies), or an encephalitis or encephalopathy syndrome (delirium, obtundation, coma, seizures, hyperreflexia, clonus, broad-based gait, or ataxia) (72, 222). Cognitive impairment is the most predictive indicator of abnormalities in the cerebrospinal fluid (CSF), which are generally characterized by a mild to moderate lymphocytic pleocytosis and a moderately elevated protein level (222). CSF white blood cell counts in adult patients with meningitis are generally lower than 250 cells/mm3, although counts in children may be higher, occasionally exceeding 500 cells/mm3 (32, 103, 246, 253). Morulae are rarely visualized in CSF mononuclear cells and, if found, are typically in severely ill patients (32, 94, 222). Long-term sequelae of central nervous system infections are not well documented; however, persistence of various symptoms, including headache and photophobia (32), facial or ocular palsies (50, 99), tremors (16), diminished memory (138) and confusion (222), for one to several weeks has been reported. Impairment of cognitive performance has been described for some pediatric patients following HME (139).
Cough or other respiratory symptoms are described in 20 to 25% of all patients with HME (105, 204); however, pulmonary manifestations, including interstitial pneumonitis (16, 59, 138), pleural effusions (109, 173, 243), pulmonary edema (109, 277), and acute respiratory distress syndrome (155, 211, 213, 215, 246, 271), are frequent components of severe disease.
Patients may develop profound thrombocytopenia and coagulopathies and occasionally develop hemorrhagic manifestations including epistaxsis (208), pulmonary hemorrhage (89, 109, 180, 208), gastrointestinal bleeding (1, 89, 174, 263, 275), subdural hematomas (180, 206), hematuria (263), and conjunctival hemorrhage (27, 103, 173, 261).
The estimated case-fatality ratio for HME is approximately 3% (188). Fatal disease has been described most frequently in males (approximately 70%), older patients (median age, 51 years; range, 6 to 80 years), and patients debilitated by underlying disease or immunodeficiencies including HIV infection, malignancy, asplenia, chronic ethanol abuse, and corticosteroid therapy. Half of all deaths occur during the second week of illness (range, 7 to 68 days), and death is generally attributed to multisystem organ failure, catastrophic hemorrhage, or secondary bacterial or fungal infections (23, 79, 89, 92, 109, 179, 180, 206, 208, 209, 222).
Secondary infections, including those caused by cytomegalovirus, Candida, and Aspergillus spp., have occurred in some severely ill patients (92, 104) suggesting that infection with E. chaffeensis may induce suppression of the host immune system (275). The occurrence of pathogen-mediated immune dysfunction has also been proposed for animals and patients infected with A. phagocytophila (87, 227).
Dual infections.
Lone star ticks harbor or vector several other pathogenic or potentially pathogenic bacteria including the spirochete “Borrelia lonestari” (22, 44, 140), Francisella tularensis (132), various spotted fever group rickettsiae (118), and E. ewingii (282). However, there are relatively few well-documented laboratory confirmed cases of concurrent infection with E. chaffeensis and another tick-borne agent. PCR-confirmed HME occurring synchronously with a spotted fever rickettsiosis has been described (247), and several prospective epidemiologic studies have demonstrated simultaneous seroconversions to E. chaffeensis and spotted fever group rickettsiae among military personnel exposed to A. americanum-infested habitats (185, 284). Descriptions of patients with simultaneous HME and Lyme disease (3, 27, 220) require cautious interpretation (27), particularly because the lone star tick is not a competent vector of Borrelia burgdorferi (217). However, in states where populations of A. americanum may be sympatric with I. scapularis, antibodies reactive with E. chaffeensis have been detected in patients with well-documented erythema chronicum migrans (176), suggesting that patients with Lyme disease may be exposed simultaneously or sequentially to other tick species carrying E. chaffeensis or other antigenically related ehrlichiae. A fatal case of HME and babesiosis in an 85-year-old man has been reported from New Jersey (141).
“Asymptomatic” Infection
Military training exercises involving troops exposed to tick-infested habitats where E. chaffeensis is highly endemic have permitted prospective investigations of seroconversions among individuals to E. chaffeensis or related antigens following known tick exposure (185, 216, 284). In one study, more than two-thirds of persons demonstrating seroconversion reported no clinical illness (284) while a subsequent investigation conducted at the same location found that 80% of individuals developing antibodies reactive with E. chaffeensis reported an illness compatible with ehrlichiosis (185). Although these studies suggest that asymptomatic infections with E. chaffeensis occur, a definitive interpretation of the data is precluded by the potential for antigenically related ehrlichiae to elicit antibodies that cross-react in serologic tests.
The possibility for asymptomatic or subclinical HME is also suggested by the relative paucity of described cases of disease in children, as well as relatively high seroprevalences of antibodies reactive with E. chaffeensis among children residing in several regions of the southeastern and south-central United States where this agent is endemic. In one study of children 1 to 17 years of age evaluated at major medical centers in Arkansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee, age-adjusted prevalence rates of antibody reactive with E. chaffeensis (or antigenically related ehrlichiae) at titers of ≥1:80 ranged from 2 to 22%. At most of these locations, the age-adjusted seroprevalence exceeded 10% (178). Among the first 250 cases of ehrlichiosis described, fewer than 10% were in individuals aged 2 to 13 years (95). Children are known to be exposed to tick-borne pathogens at levels similar to or greater than those for adults, as documented by the very high incidences of Rocky Mountain spotted fever and Lyme disease among children aged 5 to 9 years (55, 67). There is no reason to believe that children are less commonly exposed than adults to A. americanum, suggesting that infection with E. chaffeensis in the pediatric population in general results in less severe illness relative to HME in adults.
Differential Diagnoses
E. chaffeensis ehrlichiosis is a multisystem disease with protean manifestations, but because it lacks a pathognomonic clinical feature, the differential diagnosis of HME is often broad. Initial symptoms may be generalized and relatively vague, and diagnoses frequently include “viral syndrome” in the context of gastroenteritis, upper respiratory infection, pneumonia, or meningoencephalitis. Localized findings may lead to suspicion of pharyngitis, urinary tract infection, epididymitis, or prostatitis (99, 206). Abdominal pain may mimic cholecystitis, and cholecystectomies have been performed in some patients with HME before the correct diagnosis was made (50, 209). Marked hypotension or laboratory abnormalities associated with HME may be interpreted as indicators of sepsis, thrombotic thrombocytopenic purpura, or hematologic neoplasia (103, 138, 180).
A history of recent tick bite or exposure can be elicited from most patients; however, this feature is absent in approximately 10 to 30% of cases (99, 106, 204, 254). The clinical presentation of HME may be similar to that of other tick-associated illnesses, especially other ehrlichioses, caused by A. phagocytophila or E. ewingii, and Rocky Mountain spotted fever. Because most patients with HME who are treated with doxycycline show obvious clinical improvement within 42 to 72 h, failure to improve within this interval generally supports an alternative diagnosis (273).
Comparison with Other Ehrlichioses
The usual symptom complex of fever, headache, myalgia, and malaise, coupled with thrombocytopenia, leukopenia, and elevated hepatic transaminase levels, are features shared by HME, HGE, and E. ewingii ehrlichiosis. However, some differences in the frequencies of disease manifestations exist between HME and the other forms of human ehrlichiosis. Rash, central nervous system involvement, and gastrointestinal disturbances are reported more often for patients with HME than for patients with HGE. Comparisons of general measures of disease severity, including hospitalization rates (2, 20, 30) and case-fatality ratios (188), suggest that severe or life-threatening disease occurs more frequently among patients with HME than among persons with HGE (Table 1). Descriptions of E. ewingii ehrlichiosis exist for only eight patients, precluding broad comparisons of severity. However, no known deaths have been attributed to E. ewingii, and analysis of a small group of HIV-infected patients coinfected with E. chaffeensis or E. ewingii suggests that these patients develop fewer disease manifestations and complications than do HIV-infected patients with HME (206).
TABLE 1.
Selected clinical characteristics and outcomes of ehrlichioses for patients infected with E. chaffeensis, A. phagocytophila, or E. ewingii, extracted from case series and state surveillance activities
| Clinical characteristic or outcome | No. of patients with positive results/no. for whom data were available (%) for:
|
||
|---|---|---|---|
| HMEa | HGEb | E. ewingii ehrlichiosisc | |
| Fever | 256/262 (98) | 151/154 (98) | 8/8 (100) |
| Headache | 189/244 (77) | 110/143 (77) | 5/8 (63) |
| Myalgias | 158/242 (65) | 99/122 (81) | 3/8 (37) |
| Vomiting | 83/231 (36) | 16/65 (25) | 1/4 (25) |
| Rash | 95/270 (35) | 11/111 (10) | 0/4 (0) |
| Cough | 54/215 (25) | 13/65 (20) | 0/4 (0) |
| Mental-status changes | 39/197 (20) | 9/65 (14) | NAd |
| Hospitalization | 196/306 (64) | 61/128 (48) | 6/8 (75) |
| Death | 9/287 (3.1) | 3/266 (1.1) | 0/8 (0) |
Treatment and Prevention
In vitro susceptibility testing has shown that E. chaffeensis is resistant to representatives of most classes of antibiotics including aminoglycosides (gentamicin), fluoroquinolones (ciprofloxacin), penicillins (penicillin), macrolides and ketolides (erythromycin and telithromycin), and sulfa-containing drugs (co-trimoxazole) (18, 41, 234). Clinical experience supports the results of these tests and indicates that other classes of antibiotics, including cephalosporins, are equally ineffective. Rifampin exerts rapidly bactericidal effects in vitro (41); however, there are no clinical data that evaluate the use of this antibiotic in patients with HME. Relatively little is known of the biochemical mechanisms responsible for the resistance of E. chaffeensis to various antimicrobials; however, a molecular basis for resistance of E. chaffeensis and closely related Ehrlichia spp. to fluoroquinolones appears to be associated with natural mutations in the quinolone resistance-determining region of gyrA, the gene encoding the A subunit of DNA gyrase (182). Specifically, the presence of alanine residues at two positions of the dimer interface in the DNA binding area of the A subunit of this enzyme confers resistence to the activity of fluoroquinolone antibiotics (182).
E. chaffeensis is susceptible to tetracyclines and their derivatives, broad-spectrum antimicrobials which act by inhibiting protein synthesis of various bacterial species by reversibly binding to the 30S ribosomal subunit to prevent the addition of new amino acids during the formation of peptide chains. Many other human pathogens, including rickettsiae, chlamydiae, borreliae, mycoplasmas, Actinomyces spp., Vibrio spp., Bartonella spp., and some Mycobacterium spp. and protozoa, are also susceptible to tetracyclines. These drugs, particularly doxycyline, represent the treatment of choice for all persons with HME. Most patients become afebrile within 1 to 3 days following treatment with a tetracycline (99, 105, 106); however, fever may persist in some severely ill persons even after several days of therapy (105, 107, 221, 274, 277). The optimal duration of therapy has not been established definitively; however, a treatment course of 7 to 10 days, or at least 3 days after the abatement of fever, is widely accepted (18). For most patients, leukocyte and platelet counts and serum sodium levels correct to normal values within 3 to 7 days and hepatic transaminase levels normalize within 1 to 4 weeks (99, 105, 263). Although susceptibility data are limited to evaluations of a single isolate (41), there is no clinical evidence to suggest that tetracycline-resistant strains of E. chaffeensis exist.
In vitro data have shown that E. chaffeensis is resistant to chloramphenicol (41), and several anecdotal reports describe treatment failures with this antibiotic (103, 174, 250). Paradoxically, there are also reports of apparent treatment successes with chloramphenicol, particularly in children (28, 96, 124, 221). However, because the efficacy of chloramphenicol remains incompletely defined, this drug should not be considered primary therapy for HME, even in young children (8).
Reducing contact with infected ticks lowers the risk of acquiring HME. Because it is unreasonable to assume that a person can eliminate all activities that may result in these contacts, prevention techniques primarily involve personal protection. Wearing light-colored clothing that facilitates the detection of crawling or attached ticks and the use of repellents containing DEET (n,n-diethyl-m-toluamide) can minimize the risk of tick bites. However, the best protective measure consists of a thorough body examination for ticks after returning from potentially tick-infested areas. It is not known how long A. americanum must remain attached before it can transmit E. chaffeensis to a host; however, because other tick species generally require several hours of attachment before bacteria are transmitted (131, 143), frequent inspections for and prompt removal of attached ticks by using forceps or tweezers is an important method to minimize the risk of HME.
LABORATORY DIAGNOSIS
A diagnosis of HME can be confirmed by several laboratory methods. In order of their routine application, these are serologic tests to measure specific antibody titers, detection of morulae in peripheral blood or in CSF leukocytes, detection of ehrlichial DNA by PCR of blood or CSF, direct detection of ehrlichiae in tissue samples by immunohistochemistry (discussed above under Pathology), and isolation of bacteria.
Serologic Testing
The most widely available laboratory diagnostic tests detect and measure antibody reactive with E. chaffeensis (273). Although these assays remain the most frequently utilized confirmatory methods, there are several caveats to their use. Currently available serologic tests may return negative results for the majority of patients during the first week of illness. Additionally, the discovery of other pathogenic species of related bacteria that share cross-reacting antigens (e.g., A. phagocytophila and E. ewingii) requires careful interpretation and correlation of diagnostic test results with clinical and epidemiologic findings to avoid incorrect designation of the specific agent.
Indirect immunofluorescence assay.
Most patients with HME have been diagnosed by the indirect immunofluorescence assay (IFA). The original IFA format for detecting antibodies reactive with E. chaffeensis used a surrogate antigen, E. canis, as substrate (78). Currently, the standard IFA for HME uses the Arkansas strain of E. chaffeensis (9) cultivated in DH82 cells or Vero cells (75) as substrate. Paired sera collected during a 3- to 6-week interval represent the preferred specimens for serologic evaluation of HME. Both immunoglobulin M (IgM) and IgG antibodies can be measured using the IFA (61); however, the IgG IFA test is negative in as many as 80% of patients during the first week of illness and the IgM titers may also be uninformative at this time (61). It is important to obtain a convalescent-phase serum specimen since most (>80%) patients have developed diagnostic IFA titers by 6 weeks postinfection (61, 255). Unfortunately for the purposes of diagnosis, individuals with HME initially present for care a median of 4 days after disease onset (105), and often this initial visit is the only time at which a serum sample is obtained. The impact of these observations on surveillance and underreporting of HME has been discussed (60, 61). Few data are available to describe the kinetics of IFA-detectable antibody for E. chaffeensis infections (78), and none have been published using E. chaffeensis antigen as a testing substrate.
The diagnosis of ehrlichiosis in a person with a clinically compatible illness can be confirmed by seroconversion or a fourfold or greater change in antibody titer (sometimes limited to a rise in antibody titer [273]) between acute- and convalescent-phase samples (51). Recommendations for diagnosing HME in a patient with compatible illness promulgated by the Task Force on Consensus Approach for Ehrlichiosis include a single reciprocal titer of ≥256 as sufficient to confirm disease and a titer of 64 as indicating probable HME (273); however, national surveillance efforts have considered cases with a single IFA titer of ≥64 as only probable HME, regardless of the magnitude of the end-point titer (188).
Antibodies cross-reactive with a number of ehrlichial antigens (57) and different Ehrlichia species are well documented in humans (48, 64). Western immunoblotting analyses using purified E. chaffeensis and A. phagocytophila proteins suggest that patient antibodies dually reactive with these agents are recognizing homologous heat shock proteins, not major OMPs (268). Because cross-reactivity among ehrlichial species is seen in 10 to 30% of patient sera, sera should be tested against both E. chaffeensis and A. phagocytophila antigens when ascribing specific etiology (64). In general, a fourfold or higher end-point IFA titer is useful in discriminating between etiologic agents when PCR has been used to confirm the diagnosis. Many end-point IFA titers to E. chaffeensis and A. phagocytophila antigens are within a twofold range, precluding the use of IFA serologic testing from ascribing specific etiology (64). Cross reactivity among antibodies to a number of ehrlichial species has been an important feature in defining new human pathogens. Just as E. canis provided a useful surrogate antigen for diagnosing HME until E. chaffeensis was cultured, E. chaffeensis antigens have been used to diagnose some cases of ehrlichiosis caused by E. ewingii (42).
Negative serologic results for the acute-phase sample do not necessarily exclude the diagnosis. Similarly, the lack of seroconversion does not rule out HME. Some small fraction of patients do not develop measurable antibody following infection with E. chaffeensis. In some instances, this failure to seroconvert can be attributed to immune impairment (208, 209) or to early death due to rapidly progressive disease, as seen with some cases of Rocky Mountain spotted fever (207), but in other instances the reasons are unclear (255). Early treatment with a tetracycline-class antibiotic occassionally reduces or abrogates the antibody response to R. rickettsii (245), and it appears that a similar phenomenon occurs with E. chaffeensis (254).
Western blotting.
The use of Western blotting has permitted the identification of antigenic variability among isolates of E. chaffeensis and identified variability in the reactivity of patient sera to a number of E. chaffeensis antigens (40, 56). The majority of HME patients with detectable IFA reactivity to whole E. chaffeensis preparations have antibody reactive with the 120-kDa protein (56), and a recombinant 120-kDa protein has potential application as a serodiagnostic antigen (288). A recombinant major OMP of E. chaffeensis (rP30) has been used as antigen in immunoblot analysis to determine specific reactivity to E. chaffeensis among serum samples dually reactive with this agent and with A. phagocytophila (268). Western blotting of E. chaffeensis antigen has also been useful in diagnosing human infections with E. ewingii, which was first recognized as a human pathogen in 1999. Because E. ewingii has not yet been cultured, homologous IFA antigens are unavailable. However, human serum samples from patients infected with E. ewingii fail to react with the 28-kDa antigen of E. chaffeensis (42).
Other assays.
Enzyme linked-immunosorbent assays using whole cell antigen or recombinant protein antigens hold promise for the future diagnosis of HGE (134) but are still in the developmental stage for diagnosis of HME. An E. chaffeensis homolog of the major antigenic protein 2 of E. ruminantium has been cloned and sequenced (35). This 21-kDa protein from E. chaffeensis has been expressed in Escherichia coli and successfully adapted to an enzyme-linked immunosorbent assay format to detect antibodies from patients with HME (5). Preliminary findings with 20 human serum samples indicated a diagnostic sensitivity of 95%, using the IFA as the “gold standard,” and a diagnostic specificity of 100%.
Visualization of Morulae and Staining Methods
Morulae have been identified in smears of peripheral blood, buffy coat preparations, and bone marrow aspirates by using various eosin-azure (Romanovsky)-type stains, including Wright's, Diff-Quik, Giemsa's, and Leishman's. Although this technique offers the most rapid method of diagnosis, it is considered relatively insensitive and is seldom confirmatory in clinical practice. In this context, morula-positive smears are characteristically seen in a minority of patients, even in patients from whom the organism has been isolated (61, 73, 209, 255, 262). Even when visualized, morulae are generally detected in fewer than 5% of circulating leukocytes. Case series describing patients with culture or PCR-confirmed HME report sensitivities of approximately 20 to 30% for morula visualization as an independent diagnostic indicator (61, 99, 255). However, this figure may be biased to the high side due to (i) intensified retrospective review of smears in patients initially deemed negative but in whom the diagnosis was later confirmed by other methods (208) or (ii) small numbers of patients and inclusion of a disproportionate number of individuals who are immunocompromised and are more likely to develop highly concentrated bacteremias. Sensitivity issues are further confounded by inconsistencies in the number of cells or smears examined and the relative experience of the microscopist.
Other inclusions in leukocytes, including Döhle bodies, toxic granulations, Auer rods, phagocytosed bacteria or fungi, and superimposed platelets or debris, may be confused with morulae (104, 273). Similarly, familial conditions, including May-Hegglin anomaly, Alder-Reilly anomaly, and Chédiak-Higashi syndrome, are associated with intraleukocytic inclusions potentially mistaken for morulae by inexperienced observers. In some severely ill patients, morulae of E. chaffeensis have been identified in mononuclear cells in the CSF (32, 94, 253).
PCR Amplification
PCR assays to identify DNA from Ehrlichia spp. in whole blood, CSF, and serum are becoming standard complements to serologic assays. Frequently, positive results can be obtained by PCR using an acute-phase whole-blood sample from E. chaffeensis patients at a time when serologic testing is still negative (61). Several of the genes described above have been used to various extents to diagnose or characterize E. chaffeensis infections; however, their relative analytic and diagnostic sensitivities have not been systematically investigated. The Task Force on Consensus Approach for Ehrlichiosis recognizes the necessity for each laboratory to establish and validate its own molecular assays for the diagnosis of HME (273). However, systematic sample collections from well-characterized patients, such as those from whom isolates of E. chaffeensis are obtained, are needed for assay validation and any future attempts at standardization of PCR. Historically, the 16S rRNA gene has been the primary molecular target for diagnosing E. chaffeensis infections in humans (11, 99). This gene has also been the most widely used to identify E. chaffeensis DNA in ticks (10, 135, 235) and vertebrate reservoirs (150, 170).
The groESL heat shock operon may be a useful target for a species-specific diagnostic PCR because of variation in the length and content of the spacer region between E. chaffeensis and A. phagocytophla (260) and because it appears to be completely conserved among isolates (209). The 120-kDa antigen gene has been used less extensively than the 16S rRNA gene as a diagnostic target, but characterization of amplicons resulting from its use have potential application to molecular epidemiologic studies (59, 258, 259, 287).
Like the 120-kDa antigen gene, the VLPT gene has a variable number of tandem repeats and other variations at the nucleotide level. The frequency of the number of repeat units found among different E. chaffeensis isolates obtained from humans suggests that VLPT profiles represented by four and five repeats are the most frequently encountered, while a six-repeat strain has been detected only once in a human (255, 259). There may be some geographic variation among the prevalence of strains with different repeat units based on sampling of tick pools, since three- and six-repeat variants of the VLPT were absent from sites in Maryland (258).
The 28-kDa outer membrane proteins (p28) of E. chaffeensis are encoded by a multigene family homologous to major antigenic protein 1 in E. ruminantium (202, 226, 291). This family of genes has not been investigated as a diagnostic PCR target for E. chaffeensis infection but has multiple potential diagnostic applications (226).
Isolation
The isolation of Ehrlichia species from blood, CSF, and other tissues requires a laboratory capable of processing clinical specimens using cell culture techniques. In this context, a clinical laboratory equipped for virus isolations could potentially culture E. chaffeensis. In some cases, primary isolation has required several weeks (73, 90, 209), although morulae have been identified in some primary cultures as early as 2 days following inoculation (206, 255). Most isolates of E. chaffeensis have been obtained from EDTA-anticoagulated whole-blood specimens collected from patients during the acute phase of their illnesses. In addition to blood, E. chaffeensis has been isolated from CSF (255) and bone marrow aspirate material (Centers for Disease Control and Prevention [CDC], unpublished data). Because culturing of these bacteria is seldom undertaken, the sensitivity of isolation compared with other laboratory methods has been investigated in only a few circumstances (61).
EPIDEMIOLOGY AND ECOLOGY
The epidemiology and ecology of E. chaffeensis are incompletely understood. Although this was the first ehrlichia identified as a human pathogen in the United States, research on the epidemiology and natural history of this pathogen has lagged behind similar efforts afforded to A. phagocytophila. Because A. phagocytophila is transmitted by ticks belonging to the genus Ixodes which also transmit the spirochete B. burgdorferi, it has been possible to build on the epidemiologic knowledge and public health infrastructure accumulated over nearly two decades of study of Lyme disease in the northeastern and north-central United States.
Geographic Distribution
Although there are an increasing number of reports of potential human infections caused by E. chaffeensis in countries other than the United States, these studies have relied on serologic tests that lack the specificity to ascribe etiology to the level of ehrlichial species. Other reports have identified DNA of E. chaffeensis or closely related bacteria in ticks from countries where human disease has not been described. Because the data from countries other than the United States indicating the presence of E. chaffeensis and human disease caused by this agent are currently equivocal, these topics are dealt with separately.
United States.
The majority of cases of HME are reported from states in the south-central and southeastern United States, where this pathogen's primary tick vector, Amblyomma americanum, reaches its highest population densities and human exposure is greatest (98, 105, 188). The geographic distribution of >500 reported cases of HME (Fig. 4) reflects both the region where the pathogen is endemic and locations from which serum samples submitted for testing to the CDC have yielded positive results. In some instances, these cases have resulted from exposure to ticks following recent travel to states where HME is endemic rather than autochthonous acquisition in the reporting state (16, 174, 181, 194, 224). However, it is not always possible to sort out these factors from information provided with the submitted samples (60, 188). Estimates of the incidence of HME indicate a region of highest risk from central Texas through Oklahoma and Missouri east to Virginia and all states to the south. Sporadic cases of HME, which occasionally represent serologic cross-reactivity, are reported up the East Coast, most notably along the Atlantic coastal plain.
FIG. 4.
Presumptive cases of HME diagnosed by IFA at CDC, 1986 to 1997. The numbers within the states indicate the origin of the sample tested but not necessarily the state of exposure.
In addition to variation in surveillance and reporting, antibodies resulting from infections with A. phagocytophila and E. ewingii are variably cross-reactive with E. chaffeensis antigens (42, 64, 268). Because reliance on serologic test results alone may preclude the ascription of etiologic agent, surveillance reports and distribution maps of the different human ehrlichioses that have relied primarily on serologic tests conducted with a limited number of antigens should be interpreted as general indicators of regions of endemic disease. Although 46 states in the continental United States have reported HME (Fig. 4), confirmed cases based on the isolation of E. chaffeensis or identification of ehrlichial DNA in human samples through PCR amplification and sequencing of amplicons are generally restricted to states in the southeastern, south-central, and central Atlantic United States (59, 209, 209, 255, 262). However, PCR-based surveys from other regions in the United States have documented E. chaffeensis DNA in A. americanum ticks from most locations where sufficient samples have been collected, including the northeastern states (135). Therefore, regions where E. chaffeensis is endemic should be considered dynamic, and HME may continue to be recognized from additional states as surveillance and access to laboratory testing improve.
Other locations.
Although E. chaffeensis has been isolated only from sources in the United States, there are increasing data to suggest that ehrlichiae which are closely related or identical to E. chaffeensis occur throughout the world. Patients with antibodies reactive with E. chaffeensis or antigenically related ehrlichiae have been reported from Argentina (232), Israel (147), Italy (199, 244), Mali (267), Mexico (120), Portugal (192), Korea (240), and Thailand (129). Because the only proven tick vector of E. chaffeensis is restricted to North America, caution is required in interpreting data based solely on serologic testing (39). Ehrlichia spp. that are closely related or identical to E. chaffeensis have been identified from other species of ticks collected in Japan, Russia, and China (4, 47, 223, 249), although the significance of these findings for human disease is unclear.
Surveillance for HME
Human ehrlichioses were made nationally reportable to CDC in 1999 (54), although not all state health departments in states where the ehrlichioses are notifiable conditions are currently reporting cases to the weekly national database through the National Electronic Telecommunications Surveillance System. Through December 2000, ehrlichioses were notifiable diseases in 36 states. The current case definition for ehrlichiosis includes a broad clinical description and specific laboratory criteria for confirmation of disease. Three categories of confirmed or probable ehrlichiosis are reportable to CDC: (i) human ehrlichiosis caused by E. chaffeensis, (ii) human ehrlichiosis caused by A. phagocytophila, and (iii) human ehrlichiosis (other or unspecified agent), which includes cases that cannot be easily classified by available laboratory techniques and cases caused by newly recognized ehrlichial pathogens of humans, such as E. ewingii (49).
Laboratory criteria for a diagnosis of confirmed HME as defined by the Council of State and Territorial Epidemiologists include demonstration of a fourfold or greater change in antibody titer to E. chaffeensis antigen by IFA in paired serum samples, or a positive PCR assay and confirmation of E. chaffeensis DNA, or identification of morulae in leukocytes and a positive IFA titer to E. chaffeensis antigen, or immunostaining of E. chaffeensis antigen in a biopsy or autopsy sample, or culture of E. chaffeensis from a clinical specimen (51). A confirmed case of HME requires a patient to have a clinically compatible illness that is laboratory confirmed. A probable case of HME requires a patient to have a clinically compatible illness with either a single IFA titer at or above the cutoff dilution or the visualization of morulae in leukocytes.
Passive surveillance.
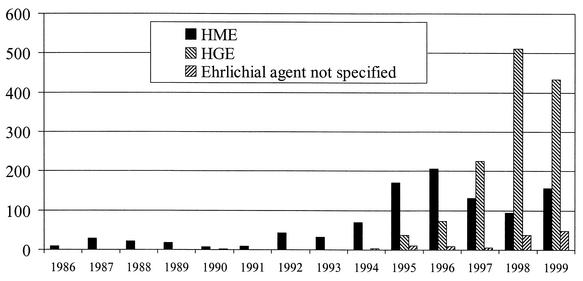
A total of 200 cases of confirmed or probable HME were reported to CDC from state health departments during 2000. In comparison, 156 cases were reported to CDC during 1999 (49; unpublished data) (Fig. 5). Even when a high level of national reporting is achieved, passive surveillance for HME underestimates true incidence of this disease for various reasons. Many states do not have adequate surveillance systems for the reporting and laboratory diagnosis of HME (188), and reliance on serologic testing misses many individuals during the acute phase of disease before antibodies have developed (60, 61). Despite these limitations, passive surveillance for ehrlichiosis has revealed annual reported rates from states and counties that are similar to those reported for Rocky Mountain spotted fever (125, 265). In a review of >700 cases of HME compiled by CDC from 1986 to 1997, the five states with the highest average annual incidence were Arkansas (5.53 per million population), North Carolina (4.72), Missouri (3.05), Oklahoma (2.90), and New Jersey (1.47) (188).
FIG. 5.
Cases of human ehrlichioses in the United States during 1986 through 1999, compiled through reports from individual states and CDC records.
Active surveillance.
Reliable incidence data for HME based on active surveillance are sparse and have been restricted to a few geographic regions. In general, incidence based on active surveillance is approximately 10-fold higher than the highest rates reported by individual states for passive surveillance. The estimated incidence for hospitalized cases of HME was 5.5 per 100,000 persons in southeastern Georgia, higher than that for Rocky Mountain spotted fever in the same population during the study period (106). Provisional estimates of 8 and 14 cases of HME per 100,000 persons during 1997 and 1998 were obtained by active surveillance in southeast Missouri (204). The incidence of ehrlichiosis was slightly greater than that of Rocky Mountain spotted fever in a cohort of patients presenting with fever and a history of tick bite over a 2-year period in central North Carolina (48). In comparison, the incidence for HGE was estimated by prospective population-based surveillance to range between 24 cases per 100,000 and 51 cases per 100,000 persons per year from 1997 to 1999 in 12 towns within the Lyme disease-endemic area of Connecticut (133).
Mechanisms of Transmission and Seasonality of Infection
Ehrlichiae affecting humans in the United States are transmitted almost invariably through the bites of infected ticks. Cases of HGE have been reported in persons for whom the only known risk factor was exposure to deer blood and tissues during the processing of fresh animal carcasses, suggesting that direct contact with potentially infectious blood from a vertebrate reservoir may initiate an infection (21). However, routes of transmission for E. chaffeensis other than tick bite are potential threats but are epidemiologically insignificant or unproven at present. Because E. chaffeensis can survive in refrigerated, anticoagulated blood for at least 11 days (187), there is a theoretical risk of acquiring the pathogen from transfused blood.
HME has been reported during March thorough November, although approximately 70% of cases occur during May through July (105, 106, 254). This seasonality corresponds approximately to the peak feeding activity periods of nymphal and adult Amblyomma americanum ticks throughout much of their range. Reports of HME occurring in late fall and winter are unusual but may be more frequent in the southern range of A. americanum, such as in Texas (224).
Patient Demographics and Risk Factors for HME
HME is most commonly diagnosed in adults, and the majority of patients are >40 years of age. Men are diagnosed more frequently than women in all age groups, with an overall male-to-female ratio of >2:1 (105, 204). Infections described in children comprise a small fraction of the total cases (95). Most cases of HME occur as sporadic infections. Recreational or occupational activities that place individuals in rural habitats infested by ticks are well-documented risk factors. A history of a tick bite has been reported by 68% of patients in national surveys (105), but that number can approach ≥80% in smaller investigations or case series (99, 204, 254).
Outbreaks of HME have been documented in several locations associated with recreational or occupational activities. In Tennessee, golfers with poorer scores were more likely to have antibodies reactive with E. chaffeensis, presumably because their lack of skill placed them more frequently in the wooded or grassy locations adjacent to the fairway, where ticks abound (254). Outbreaks of HME or infections caused by antigenically related ehrlichiae are well- documented among military personnel participating in field exercises in New Jersey (216) and Arkansas (15, 284). A focus of tick-borne disease at Fort Chaffee, Ark., provided the first isolate of E. chaffeensis (73) and continues to be a location of exceptionally high risk for acquiring infections from tick bite (185).
Tick Vectors
A. americanum.
As a geographic portrait of HME emerged in the late 1980s, investigators identified a predominance of patients from the south-central, southeastern, and mid-Atlantic states. Because this region closely approximates the recognized distribution of Amblyomma americanum (commonly known as the lone star tick for the silvery white spot on its dorsal surface), this tick was soon implicated as a potential vector for E. chaffeensis (98). This hypothesis was supported further by amplification of E. chaffeensis DNA from pools of A. americanum adults collected from various locations in the eastern United States (10), experimental transmission of E. chaffeensis among white-tailed deer by adult and nymphal lone star ticks (100), and retrospective studies demonstrating temporal and spatial associations between lone star tick infestations and the presence of antibodies reactive with E. chaffeensis in white-tailed deer (166, 167).
A. americanum is a three-host, hard tick distributed from west-central Texas north to Iowa and eastward in a broad belt spanning the southeastern United States. Along the Atlantic coast, the range of this species extends north through coastal areas of New England. It is found predominantly in woodland habitats, particularly young second-growth forests with dense understory vegetation (123). Lone star ticks are notorious for their aggressive and relatively nonspecific feeding behavior. Adult A. americanum ticks feed on medium-sized and large mammals, and larvae and nymphs infest various ground-feeding birds, medium-sized and large mammals, and, rarely, small mammals (62, 151). All three stages bite humans. Adult and nymphal lone star ticks are most active during April through June and decline markedly in abundance and activity as summer progresses (71, 123, 137). The seasonal activity of adult and nymphal ticks, which precedes that of larvae, increases the probability of that larvae will acquire the pathogen from an infected vertebrate during the first blood meal.
E. chaffeensis has been detected in female and male lone star ticks collected in Alabama, Arkansas, Connecticut, Georgia, Florida, Indiana, Kansas, Kentucky, Maryland, Missouri, North Carolina, New Jersey, Rhode Island, and Virginia (10, 43, 135, 170, 235, 256, 257, 278). Infection prevalences presumably vary intrinsically across location, sampling period, and life stage of Amblyomma ticks tested and extrinsically with the assaying method (e.g., numbers of ticks sampled, sampling methods, and sensitivity of the assay), making generalizations difficult. E. chaffeensis DNA has been found by PCR in approximately 5 to 15% of adult ticks collected from areas where the agent is endemic and tested individually (135, 170, 235, 278). Several studies have obtained a crude minimum infection ratio (MIR) from pools of adult ticks, but this method characteristically underestimates the level of infection (170, 257). Accordingly, infection prevalences determined by pooling are lower than those observed with individual ticks and generally range from 1 to 5% (Table 2). As molecular assays become increasingly sensitive, more accurate estimates of infection prevalence in ticks may be possible (101).
TABLE 2.
Reported prevalences of infection with E. chaffeensis in lone star ticksa as determined by PCR
| Location | Yr of collection | Individuals or pooled | Direct or nested PCR | % Infected or MIR (total no. tested) | Reference(s) |
|---|---|---|---|---|---|
| Multiple counties, Mo. | NSb | Pooled | Direct | 1.2b (85) | 10, 287 |
| Multiple counties, N.C. | NS | Pooled | Direct | 1.2b (436) | 10, 287 |
| Bullitt Co., Ky. | NS | Pooled | Direct | 3.6b (28) | 10, 287 |
| Clarke Co., Ga. | 1993-1995 | Individual | Nested | 12.0 (50) | 170 |
| Clarke Co., Ga. | 1993-1995 | Pooled | Nested | 3.5b (402) | 170 |
| Multiple counties, Mo. | 1995 | Individual | Nested | 23.0c (48) | 235 |
| Multiple counties, Ind. | 1995 | Pooled | Direct | 4.9b (430) | 43 |
| Multiple counties, Ind. | 1997 | Pooled | Direct | 1.6b,c (920) | 256 |
| Multiple counties, Ind. | 1998 | Pooled | Direct | 3.8b (262) | 136 |
| Harford Co., Md. | 1997 | Pooled | Nested | 3.5b (138) | 258 |
| Multiple counties, Ga. | NS | Individual | Nested | 5.2 (250) | 278 |
| Bristol Co., R.I. | 1992 | Individual | Direct | 11.5 (52) | 135 |
| Multiple counties, Conn. | 1996-1998 | Individual | Direct | 7.6c (106) | 135 |
| Multiple counties, Fla. | 1998 | Individual | Nested | 13.6 (323) | CDC, unpublished data |
Adult ticks, unless otherwise specified.
NS, not specified
Stage(s) not specified.
Infections in nymphal A. americanum ticks have been described from Connecticut, Florida, Kansas, Maryland, Missouri, New Jersey, and North Carolina (235, 257, 258). In the largest sample evaluated to date, 81 pools representing 2,723 nymphal ticks collected from Harford County, Md., showed an overall MIR of 0.8%. By contrast, the MIR of adult ticks collected at the same location was 3.5% (258). In general, infection prevalences appear to be lower among nymphal ticks than among adults, and failure to detect E. chaffeensis in nymphs collected at sites with confirmed infections in adult A. americanum has been described by several investigators (10, 43).
As with many other tick-transmitted pathogens, infections of tick populations may appear spatially or temporally discontinuous, and wide variations in infection prevalences can be expected among ticks collected from closely spaced geographic locales (43, 278) or among ticks collected at the same site during different years (256). In this context, infection may not always be evident among ticks at a specific location at a particular time of sampling (43, 278). These differences have been ascribed to natural variations of infection of ticks by ehrlichiae, clustering of infected ticks, or the sampling techniques used (256).
Little is known about the vector-pathogen relationship between A. americanum and E. chaffeensis. Detection of ehrlichiae in questing nymphal and adult ticks and successful transmission of the pathogen between deer by nymphs and adult ticks infected during the previous life stage confirm that E. chaffeensis is passaged transstadially (100). Detection of E. chaffeensis in larval A. americanum has been described in a single report (257); however, there are no other data to suggest that transovarial transmission occurs, and absence of transovarial transmission has been demonstrated in studies of the closely related species E. canis and its vector, Rhipicephalus sanguineus (122). Nothing is known of the development of E. chaffeensis in the vector or the exact mechanism by which ehrlichiae are transmitted to the vertebrate host during feeding. The risk that the bite of an infected lone star tick will successfully transmit E. chaffeensis, even if detectable by molecular methods, remains unknown. Lone star ticks may harbor other recently identified species of ehrlichiae. In this context, A. americanum is the putative vector of E. ewingii (13, 282) and an as yet unamed Ehrlichia sp. of white-tailed deer (36, 169).
Other tick species.
PCR has been used to detect DNA of E. chaffeensis in other tick species, including the dog tick, Dermacentor variabilis (10, 152, 235), the Western blacklegged tick, Ixodes pacificus (152), Ixodes ricinus in Russia (4), and the ticks Amblyomma testudinarium and Haemaphysalis yeni collected from domesticated and wild animals in southern China (47). Detection of DNA of ehrlichiae within a particular tick species does not conclusively incriminate that tick as an efficient vector (83), and the role of these or other tick species as natural vectors of HME has not been established definitively. Similarly, the Gulf Coast tick (Amblyomma maculatum) has been implicated as a potential vector because of feeding proclivities and a range distribution similar to those of the lone star tick, although insufficient data exist to support or refute the role of this tick in the transmission of E. chaffeensis (149).
Vertebrate Reservoirs
E. chaffeensis is maintained in nature as a complex zoonosis, potentially involving many vertebrate species that serve as competent reservoirs for the bacterium, as sources of blood for tick vectors, or as both. The catholic feeding proclivity of Amblyomma americanum for the blood of a wide range of mammalian and avian species is well described (33, 123). Considerably less is known about which vertebrates can serve as competent reservoirs for E. chaffeensis (Table 3). It is unknown if all strains of E. chaffeensis isolated from or detected in various animal species are pathogenic to humans.
TABLE 3.
Mammalian species implicated as reservoir hosts of E. chaffeensisa
| Species | Evidence | Authority |
|---|---|---|
| Odocoileus virginianus (white-tailed deer) | Antibody, PCR, isolation, experimental transmission | 70, 100, 167, 169 |
| Ovis species (domestic goat) | Antibody, PCR, isolation | 85 |
| Canis familiarus or C. lupus (domestic dog) | Antibody, PCR, experimental transmission | 74, 198 |
| Canis latrans (coyote) | PCR | 150 |
| Vulpes vulpes (red fox) | Antibody, experimental transmission | 69 |
| Procyon lotor (raccoon) | Antibody | 65, 170 |
| Peromyscus leucopus (white-footed mouse) | Antibody | 175 |
| Didelphis virginianus (Virginia possum) | Antibody | 170 |
Negative results have been obtained for serologic tests conducted on P. leucopus, Sigmodon hispidus, Sciurus carolinensis, Sciurus niger, Orizomys pulustris, Mus musculus, Rattus norvegicus, Reithrodontomys humulis, Ochrotomys nuttali, and Tamias striatus from the southeastern United States (168, 170) and P. leucopus, S. hispidus, and M. musculus from North Carolina (251).
White-tailed deer.
The white-tailed deer (Odocoileus virginianus) currently stands as the sole vertebrate species recognized as a complete and sufficient host for maintaining the transmission cycle of E. chaffeensis (Fig. 6). White-tailed deer are an important source of blood for adult and immature stages of A. americanum (33, 123). These deer are also naturally infected with E. chaffeensis in the southeastern United States based on PCR results (170) and isolation of the bacterium (169). Deer experimentally infected with various isolates of E. chaffeensis have maintained viable bacteremias for at least several weeks to months postinfection.
FIG. 6.
A life cycle of E. chaffeensis. Noninfected larvae obtaining blood from a bacteremic vertebrate reservoir host (e.g., white-tailed deer [shaded]) become infected and maintain ehrlichiae to the nymphal stage. Infected nymphs may transmit E. chaffeensis to susceptible reservoir hosts (unshaded) or to humans during acquisition of blood. Infected adult ticks, having acquired ehrlichiae either by transstadial transmission from infected nymphal stage or during blood meal acquisition as noninfected nymphs on infected deer, may also pass E. chaffeensis to humans or other susceptible reservoirs. Transovarial transmission has not been demonstrated, and eggs and unfed larvae are presumably not infected.
Recently infected white-tailed deer may circulate the highest levels of ehrlichiae in their blood during the 3 weeks after initial infection (80). In general, bacteremias in white-tailed deer appear to be relatively low, as evidenced by difficulty in detecting infection by PCR when isolation is successful (70), and morulae have been visualized in the peripheral blood of only one, severely debilitated animal (163). Experimentally infected deer can infect laboratory-reared larval and nymphal A. americanum ticks, which transstadially maintain infections (100), but this has not been consistently demonstrated (70). Infection with E. chaffeensis has been established in white-tailed deer tissue collected in 1985 by using PCR (163), a date prior to the description of the first case of HME.
The prevalence of E. chaffeensis infections among populations of white-tailed deer in nature is difficult to determine. The duration of patent infection in this reservoir can be months, and recrudescence or persistent infection occurs. In natural settings, yearling deer may be particularly susceptible to persistent bacteremia and therefore may represent important components in the epizootiology of E. chaffeensis (170). Antibody surveys have demonstrated high seroprevalences (frequently >50% at sites where any antibody-positive animals were present) of antibody reactive with E. chaffeensis antigens among white-tailed deer populations (76, 136, 170, 197), and field data have confirmed a site-specific correlation between antibody prevalence and the presence of A. americanum (166). However, deer can be infected and coinfected with several different Ehrlichia and Anaplasma species, which are antigenically related to different degrees (81, 164). Studies using only serologic testing cannot routinely distinguish between antibodies resulting from infection with E. chaffeensis and those resulting from infection with antigenically related species, including the white-tailed deer agent and E. ewingii.
Goats.
Domestic goats (Ovis species) may serve as hosts for all stages of the life cycle of A. americanum (33, 123, 162). Although knowledge of goats as potential reservoir species for E. chaffeensis in the United States is limited to a single report, this detailed study found reactive antibody in 28 (74%) of 38 animals and ehrlichial DNA in whole blood of 6 (16%) of 38 goats (85). An isolate of E. chaffeensis was obtained from a single goat sampled at two time points 40 days apart, suggesting that a long-lived or persistent infection may occur in this species. Apart from E. chaffeensis isolates from deer, this is the only other naturally occurring isolate from a mammal other than humans.
Domestic dogs.
Dogs (Canis familiaris or C. lupus) are potentially the most important reservoir for any zoonotic pathogen affecting humans because of their numbers (>61,000,000 in the United States as of March 1998 [www.apapets.com/petstats2.htm]), their presence throughout the United States, their usual free-roaming life-styles which give them access to tick-infested habitats, and their proximity to humans. Domestic dogs can serve as hosts for all stages of the life cycle of A. americanum (33, 123) and provide a convenient vehicle for transport of ticks from various habitats into the peridomestic environment. In the first description of canine infections with E. chaffeensis, 28 (38%) of 74 dogs from southeastern Virginia were shown to have reactive antibody and 8 (42%) of 19 had ehrlichial DNA in whole-blood samples detected by PCR (74). A survey conducted in Oklahoma using similar methods found 7 (10.8%) of 65 dogs with antibody reactive to E. chaffeensis antigens by IFA and 4 animals with E. chaffeensis DNA in whole-blood samples detected by PCR (198).
Dogs are susceptible to disease caused by E. chaffeensis and by several other closely related pathogens including E. canis, E. ewingii, Anaplasma platys (37) and possibly other closely related Ehrlichia species (7). The potential for coinfection or sequential infections with different agents may complicate clinical descriptions of naturally acquired canine disease caused by E. chaffeensis (110). Studies suggest that dogs infected by E. chaffeensis remain infected or can be reinfected with this organism after doxycycline treatment (37, 110).
Coyotes.
Coyotes (Canis latrans) have expanded their range in North America since the 1800s, when they were restricted to the Great Plains and the western United States, and are currently found throughout the United States and most of Canada (29, 191). These carnivores exist in most habitats and thrive in suburban areas (17). Although most abundant in the southwestern and midwestern United States, where population densities can reach >2 animals per km2 (148), populations are increasing in New England and West Coast locations. Since 1980, populations of coyotes in the southeastern United States have increased dramatically, as evidenced by an increase in the harvest of these animals in Mississippi from 500 in 1975 to 40,000 in 1988 (191). Coyotes serve as hosts for adult and nymphal stages of A. americanum (34, 66). Coyotes naturally infected with E. chaffeensis have been identified in Oklahoma (150). Infection among wild coyotes occurred at a very high prevalence (15 of 21 [71%]), suggesting that these animals could be a significant reservoir for E. chaffeensis over their extensive geographic range.
Other species.
Red foxes (Vulpes vulpes) can serve as hosts for all stages of A. americanum (266). In a single experiment, red foxes, but not gray foxes (Urocyon cinereoargenteus), were susceptible to infection with a white-tailed deer isolate of E. chaffeensis, and ehrlichiae could be reisolated for 14 days postinfection. Antibody has been detected in field surveys among both red and gray foxes (69), but until additional evidence becomes available, the role of this carnivore in the maintenance of E. chaffeensis is uncertain.
Raccoons (Procyon lotor) are frequently parasitized by all life stages of A. americanum (33, 266). Raccoons occur throughout much of North America and reach some of their highest population densities in areas coinhabited by humans (231), making them a potentially important reservoir for E. chaffeensis. Antibody reactive with E. chaffeensis was identified in 82 (20%) of 411 raccoons sampled from eight states (65). A high prevalence of antibodies (20%) was also found among raccoons sampled from an E. chaffeensis-enzootic site in Georgia (170), but until additional evidence becomes available, the role of this carnivore in the maintenance of E. chaffeensis is uncertain.
The Virginia opossum (Didelphis virginianus) can serve as a host for nymphal A. americanum (33) and is a common animal throughout its range in North America (113). Antibodies reactive with E. chaffeensis were identified among 3 (8%) of 38 opossums sampled at an E. chaffeensis-enzootic site (168). As with several other wildlife species, additional evidence will have to be established before the role, if any, for this marsupial in the natural history of E. chaffeensis can be determined.
The contribution of rodents to the ecology of E. chaffeensis is inconclusive, although larval and nymphal A. americanum ticks parasitize several species of rodents (123). Antibodies reactive with E. chaffeensis at titers of ≥80 were identified in 31 (10.6%) of 294 white-footed mice sampled from Connecticut (175); however, there were no molecular or isolation data to identify the causative agent. No antibodies reactive with E. chaffeensis were identified among 281 rodents of eight species sampled from the southern United States, including animals obtained from E. chaffeensis-enzootic sites (168).
The role of birds as a natural reservoir for E. chaffeensis has yet to be investigated, although many ground-feeding species can serve as important sources of blood for immature stages of A. americanum (123). The lone star tick is commonly found on wild turkeys (Meleagris gallopavo), and this species has been called the “turkey tick” because of this close association (82). The remarkable recovery of the wild turkey in the northeastern United States from its near extirpation in the 1800s may be contributing to increases in the range and abundance of A. americanum (189). Other ehrlichiae, including an organism identified as E. chaffeensis, have been identified by PCR from I. ricinus ticks recovered from several species of migratory passerine birds in Russia. Ehrlichiae were identified from cofeeding larvae and nymphs on the same bird, suggesting that transmission of bacteria among ticks could occur without systemic infection of the host (4).
Factors Influencing the Emergence of HME
As with most emerging infections, the boundary between contemporary recognition and true biological emergence is difficult to discern for HME. As of 2002, many questions relating to the epidemiology and natural history, and pathogenesis of disease caused by E. chaffeensis, remain to be answered. However, as detailed below, it appears likely that fundamental changes in the host-vector ecology are largely responsible for the emergence of this disease in human populations (Table 4).
TABLE 4.
Examples of factors in the emergence of E. chaffeensis ehrlichiosis
| Factor in emergence | Authority or example |
|---|---|
| Increase in A. americanum population density | 115 |
| Increase in A. americanum geographic distribution | 146, 189 |
| Increase in vertebrate host populations (wild turkeys, white-tailed deer) for A. americanum | 184, 189 |
| Increase in reservoir host (i.e., white-tailed deer) populations for E. chaffeensis | 184 |
| Increased human contact with natural foci of infection through recreational and occupational activities | 209, 254 |
| Increased frequency or severity of disease in aging or immunocompromised populations | 105, 208 |
| Increasing size and longevity of population >60 years of age and immunocompromised populations in region of enzootic infection | 53, 210, 238, 267 |
| Availability of diagnostic reagents and improved surveillance and reporting | 73, 188 |
Recent clinical recognition or new disease?
In 1986, routine perusal of a peripheral blood smear by a relatively inexperienced observer precipitated the chain of events that led to the first identification of HME (104). In retrospect, it is likely that the same hematologic abnormalities had repeatedly gone unnoticed or unappreciated prior to the identification of E. chaffeensis as the cause of HME. Given the ubiquity of peripheral blood smear evaluation prior to widespread use of automated cytometry, it can be reasonably assumed that morulae had been noted but the connection to an ehrlichiae was missed or left uninvestigated. Even 15 years after the first documentation of HME, experienced hematologists may have trouble differentiating the morulae caused by E. chaffeensis from other intracytoplasmic inclusions associated with conditions other than ehrlichiosis. Without an index case to focus diagnostic suspicion, it is likely that these inclusions would have been attributed to other causes or noted but not considered relevant to the disease process.
There are tantalizing hints that investigators had suspected or linked ehrlichiae to individual cases of disease and even to an outbreaks of disease decades earlier than its formal recognition. An Ehrlichia sp. may have been identified in the bone marrow of an immunodeficient persons as early as 1972 (C. A. Kallick, S. Levin, and K. T. Reddi, Prog. Abstr. 13th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1, 1973). A mysterious infectious disease affecting approximately 1,000 troops training at Camp Bullis, Tex. during 1942 to 1943 was linked to exposure to lone star ticks (283). The unexplained disease shared sufficient epidemiologic and clinical features with HME to suggest to contemporary scientists that an Ehrlichia sp. may have been responsible (116, 117).
Evidence for recent emergence.
Regardless of speculations concerning the earliest appearance of HME, it is most likely that recognition of this disease is attributable to identifiable, and in some cases quantified, changes in biological, demographic, and environmental factors over the past several decades. The interaction of these factors has not produced a new pathogen or disease; rather, they have acted to increase the incidence of HME to a level at which recognition became probable. Another example of a tick-borne disease with dramatically changed incidence in the United States within the past two decades is Lyme disease (153), which is an environmentally driven zoonosis that shares many ecologic features with HME and especially HGE (6). In these instances, changes in wildlife and tick vector populations have been identified as major factors in disease emergence (68).
Growth and geographic expansion of reservoir and vector populations.
It appears likely that the greatest influence on the emergence of HME has been the explosive growth of white-tailed deer populations in the United States. The white-tailed deer is a major, and perhaps the keystone, vertebrate reservoir for E. chaffeensis and serves as a primary source of blood for A. americanum ticks of all life stages. Estimated numbers of white-tailed deer document an approximately 50-fold increase during the 20th century from an estimated 350,000 animals in 1900 to at least 17 million animals by the mid-1990s (184). In many south central and southeastern states during 1973 through 1993, populations swelled three- to sevenfold (Fig. 7). This remarkable increase in numbers has been matched by an equally impressive range expansion throughout most suitable habitat types in the eastern, central, and southern United States. In recent decades, lone star ticks have become more abundant in some areas of the southeast and northeast compared to historic collections (102, 115). Higher population densities of A. americanum observed within the expanding range of this tick appear to be influenced largely by population growth and geographical extension of host animals, particularly white-tailed deer (196).
FIG. 7.
The white-tailed deer harvest in selected states in which E. chaffeensis is endemic mirrors a dramatic increase in population numbers. Deer were virtually eliminated from many of these states in the early 1900s. Data from reference 234 and from Brian Murphy, Quality Deer Management Association (personal communication).
Other important vertebrate hosts for A. americanum have undergone similarly impressive increases in abundance and geographic distribution. Wild-turkey populations have increased throughout their historic geographic range, and their success has been shared by the lone star ticks. Concurrent range expansion and increases in turkey and tick populations have been reported at the extremes of known distribution of lone star ticks, as in New York to the north (189) and Kansas to the west (190). Coyotes, which serve as hosts to lone star ticks and as potential reservoirs for E. chaffeensis (34, 150), have become established throughout the eastern and southern United States since the 1960s (130, 191). During the last several decades, coyote populations have surged dramatically in some regions of the southeastern United States (191).
Improved diagnostics and surveillance.
The availability of diagnostic reagents, changes in surveillance activities, and requirements for national notification will obviously have a major impact on our understanding of the epidemiology of HME. However, reporting remains inconsistent or nonexistent in several southern states, where HME is a special concern. It is anticipated that further recognition of HME in the southeastern United States will lead to increases in case reporting over the next few years. Enhanced surveillance and education programs are required to raise the level of diagnostic suspicion for HME as the full spectrum of disease is incompletely known. As an example, tick-borne pathogens have been identified as important causes of nonspecific febrile illness in Wisconsin (31), and similar efforts in southern locales will undoubtedly lead to a better appreciation of the public health impact of HME.
Growth of susceptible human populations.
One of the fundamental factors contributing to the emergence of new pathogens and diseases has been changes in host susceptibility, operating through the mechanism of immunosupression, affecting large segments of the population through aging, malignancy, or infectious causes (153, 193). The demographics of the United States indicate an aging population, and HME is a disease that occurs predominantly adults. The U.S. Census Bureau estimates that the percentage of the population ≥45 years of age will increase from 34.9% in 2000 to 41.3% in 2025 (254, 269).
Although the absolute prevalence of HIV among persons in nonmetropolitan areas remains significantly lower than in urban centers, the number of HIV-infected persons residing in nonmetropolitan areas has increased rapidly during the last 15 years (114). The diffusion of HIV into rural populations is particularly evident in the southeastern United States (63, 238, 270) where the risk for acquiring HME is greatest (188). Combination antiretroviral regimens have significantly slowed the progression of HIV disease in many persons, with concomitant declines in hospitalization rates, morbidity, and mortality in patients for whom these drugs are available (210). In this context, new therapies for HIV offer a level of health that facilitates occupational and recreational pursuits that perhaps were not previously possible. Some of these activities, including hunting, hiking, camping, and working outdoors, have been associated with acquisition of tick-borne diseases, including HME (206). The emergence of a healthier HIV-infected patient population exposed to increasingly diverse environments may paradoxically accentuate rises in the incidences of some vector-borne or zoonotic diseases.
CONCLUSION
Seventeen years have elapsed since the first described patient with HME presented to medical attention. During this interval, much has been learned about the pathogen, the disease, and the multiple ecological elements involved in the maintenance of this zoonosis. However, as is true of all emerging pathogens, many questions remain. Among the numerous areas for future research include studies that provide a better understanding of the interactions between the pathogen and the vector, that define pathogenic mechanisms involved in the maintenance of E. chaffeensis in vertebrate reservoirs and factors influencing disease and immunity in human hosts, and that estimate the incidence of disease in areas where E. chaffeensis is endemic. Certainly, there are few, if any, areas relating to this fascinating pathogen that have been completely elucidated.
REFERENCES
- 1.Abbott, K. C., S. J. Vukelja, C. E. Smith, C. K. McAllister, K. A. Konkol, T. J. O'Rourke, C. J. Holland, and M. Ristic. 1991. Hemophagocytic syndrome: a cause of pancytopenia in human ehrlichiosis. Am. J. Hematol. 38:230-234. [DOI] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld, M. E., H. W. Horowitz, G. P. Wormser, D. F. McKenna, J. Nowakowski, J. Munoz, and J. S. Dumler. 1996. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann. Intern. Med. 125:904-908. [DOI] [PubMed] [Google Scholar]
- 3.Ahkee, S., and J. Ramirez. 1997. A case of concurrent Lyme meningitis with ehrlichiosis. Scand. J. Infect. Dis. 28:527-528. [DOI] [PubMed] [Google Scholar]
- 4.Alekseev, A. N., H. V. Dubinina, A. V. Semenov, and C. V. Bolshakov. 2001. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J. Med. Entomol. 38:471-474. [DOI] [PubMed] [Google Scholar]
- 5.Alleman, A. R., A. F. Barbet, M. V. Bowie, H. L. Sorenson, S. J. Wong, and M. Belanger. 2000. Expression of a gene encoding the major antigenic protein 2 homolog of Ehrlichia chaffeensis and potential application for serodiagnosis. J. Clin. Microbiol. 38:3705-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen, L. J., and P. J. Cormier. 1996. Environmentally driven epizootics. Math. Biosci. 131:51-80. [DOI] [PubMed] [Google Scholar]
- 7.Allsopp, M. T., and B. A. Allsopp. 2001. Novel Ehrlichia genotype detected in dogs in South Africa. J. Clin. Microbiol. 39:4204-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. 2000. Rocky Mountain spotted fever, p. 491-493. In L. K. Pickering (ed.), 2000 Red Book: report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, Ill.
- 9.Anderson, B. E., J. E. Dawson, D. C. Jones, and K. H. Wilson. 1991. Ehrlichia chaffeensis, a new species associated with human ehrlichoisis. J. Clin. Microbiol. 29:2838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson, B. E., K. G. Sims, J. G. Olson, J. E. Childs, J. F. Piesman, C. M. Happ, G. O. Maupin, and B. J. Johnson. 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 49:239-244. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, B. E., J. W. Sumner, J. E. Dawson, T. Tzianabos, C. R. Greene, J. G. Olson, D. B. Fishbein, M. Olsen-Rasmussen, B. P. Holloway, and E. H. George. 1992. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 30:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antony, S. J., J. S. Dummer, and E. Hunter. 1995. Human ehrlichiosis in a liver transplant recipient. Transplantation 60:879-881. [PubMed] [Google Scholar]
- 13.Anziani, O. S., S. A. Ewing, and R. W. Barker. 1990. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 51:929-931. [PubMed] [Google Scholar]
- 14.Arav-Boger, R., J. H. Knepp, J. J. Walls, and J. S. Dumler. 2000. Human monocytic ehrlichiosis in a child with leukemia. Pediatr. Infect. Dis. J. 19:173-175. [DOI] [PubMed] [Google Scholar]
- 15.Arguin, P. M., J. Singleton, Jr., L. D. Rotz, E. Marston, T. A. Treadwell, K. Slater, M. Chamberland, A. Schwartz, J. G. Olson, J. E. Childs, and the Transfusion-associated Tick-borne illness Task Force. 1999. An investigation of possible transmission of tick-borne pathogens via blood transfusion. Transfusion 39:828-833. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong, R. W. 1992. Ehrlichiosis in a visitor to Virginia. West. J. Med. 157:182-184. [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson, K. T., and D. M. Shakleton. 1991. Coyote, Canis latrans, ecology in a rural-urban environment. Can. Field Nat. 105:49-54. [Google Scholar]
- 18.Bakken, J. S., and J. S. Dumler. 1999. Ehrlichia species, p. 546-554. In V. L. Yu, T. C. Merigan, Jr., and S. L. Barriere (ed.), Antimicrobial therapy and vaccines. The Williams & Wilkins Co., Baltimore, Md.
- 19.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 20.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 21.Bakken, J. S., J. K. Krueth, T. Lund, D. Malkovitch, K. Asanovich, and J. S. Dumler. 1996. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin. Infect. Dis. 23:198. [DOI] [PubMed] [Google Scholar]
- 22.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 23.Barenfanger, J., P. G. Patel, J. S. Dumler, and D. H. Walker. 1996. Identifying human ehrlichiosis. Lab. Med. 27:372-374. [Google Scholar]
- 24.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 67:2258-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 62:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnewall, R. E., Y. Rikihisa, and E. H. Lee. 1997. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect. Immun. 65:1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton, L. L., J. E. Dawson, G. W. Letson, A. Luisiri, and A. J. Scalzo. 1990. Simultaneous ehrlichiosis and Lyme disease. Pediatr. Infect. Dis. J. 9:127-129. [DOI] [PubMed] [Google Scholar]
- 28.Barton, L. L., and T. M. Foy. 1989. Ehrlichia canis infection in a child. Pediatrics 4:580-584. [PubMed] [Google Scholar]
- 29.Bekoff, M. 1982. Coyote, p. 447-459. In J. A. Chapman and G. A. Feldhamer (ed.), Wild mammals of North America: biology, management, economics. The Johns Hopkins University Press, Baltimore, Md.
- 30.Belongia, E. A., K. D. Reed, P. D. Mitchell, P. H. Chyou, N. Mueller-Rizner, M. F. Finkel, and M. E. Schriefer. 1999. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin. Infect. Dis. 29:1472-1477. [DOI] [PubMed] [Google Scholar]
- 31.Belongia, E. A., K. D. Reed, P. D. Mitchell, N. Mueller-Rizner, M. Vandermause, M. F. Finkel, and J. J. Kazmierczak. 2001. Tickborne infections as a cause of nonspecific febrile illness in Wisconsin. Clin. Infect. Dis. 32:1434-1439. [DOI] [PubMed] [Google Scholar]
- 32.Berry, D. S., R. S. Miller, J. A. Hooke, R. F. Massung, J. Bennett, and M. G. Ottolini. 1999. Ehrlichial meningitis with cerebrospinal fluid morulae. Pediatr. Infect. Dis. J. 18:552-555. [DOI] [PubMed] [Google Scholar]
- 33.Bishopp, F. C., and H. L. Trembley. 1945. Distribution and hosts of certain North American ticks. J. Parasitol. 31:1-55. [Google Scholar]
- 34.Bloemer, S. R., and R. H. Zimmerman. 1988. Ixodid ticks on the coyote and gray fox at Land-between-the-Lakes, Kentucky-Tennessee, and implications for tick dispersal. J. Med. Entomol. 25:5-8. [DOI] [PubMed] [Google Scholar]
- 35.Bowie, M. V., G. R. Reddy, S. M. Semu, S. M. Mahan, and A. F. Barbet. 1999. Potential value of major antigenic protein 2 for serological diagnosis of heartwater and related ehrlichial infections. Clin. Diagn. Lab Immunol. 6:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandsma, A. R., S. E. Little, J. M. Lockhart, W. R. Davidson, D. E. Stallknecht, and J. E. Dawson. 1999. Novel Ehrlichia organism (Rickettsiales: Ehrlichieae) in white-tailed deer associated with lone star tick (Acari: Ixodidae) parasitism. J. Med. Entomol. 36:190-194. [DOI] [PubMed] [Google Scholar]
- 37.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brouqui, P., M. L. Birg, and D. Raoult. 1994. Cytopathic effect, plaque formation, and lysis of Ehrlichia chaffeensis grown on continuous cell lines. Infect. Immun. 62:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouqui, P., and J. S. Dumler. 2000. Serologic evidence of human monocytic and granulocytic ehrlichiosis in Israel. Emerg. Infect. Dis. 6:314-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouqui, P., C. Lecam, J. Olson, and D. Raoult. 1994. Serologic diagnosis of human monocytic ehrlichiosis by immunoblot analysis. Clin. Diagn. Lab. Immunol. 1:645-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brouqui, P., and D. Raoult. 1992. In-vitro antibiotic susceptibility of the newly recognized agent of ehrlichiosis in humans Ehrlichia chaffeensis. Antimicrob. Agents Chemother. 36:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buller, R. S., M. Arens, S. P. Hmiel, C. D. Paddock, J. W. Sumner, Y. Rikhisa, Unver, M. Gaudreault-Keener, F. A. Manian, A. M. Liddell, N. Schmulewitz, and G. A. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148-155. [DOI] [PubMed] [Google Scholar]
- 43.Burket, C. T., C. N. Vann, R. R. Pinger, C. L. Chatot, and F. E. Steiner. 1998. Minimum infection rate of Ambylomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in southern Indiana. J. Med. Entomol. 35:653-659. [DOI] [PubMed] [Google Scholar]
- 44.Burkot, T. R., G. R. Mullen, R. Anderson, B. S. Schneider, C. M. Happ, and N. S. Zeidner. 2001. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg. Infect. Dis. 7:471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldwell, C. W., E. D. Everett, G. McDonald, Y. W. Yesus, and W. E. Roland. 1995. Lymphocytosis of gamma/delta T cells in human ehrlichiosis. Am. J. Clin. Pathol. 103:761-766. [DOI] [PubMed] [Google Scholar]
- 46.Caldwell, C. W., E. D. Everett, G. McDonald, Y. W. Yesus, W. E. Roland, and H. M. Huang. 1996. Apoptosis of gamma/delta T cells in human ehrlichiosis. Am. J. Clin. Pathol. 105:640-646. [DOI] [PubMed] [Google Scholar]
- 47.Cao, W. C., Y. M. Gao, P. H. Zhang, X. T. Zhang, Q. H. Dai, J. S. Dumler, L. Q. Fang, and H. Yang. 2000. Identification of Ehrlichia chaffeensis by nested PCR in ticks from southern China. J. Clin. Microbiol. 38:2778-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter, C. F., T. K. Gandhi, L. K. Kong, G. R. Corey, S. M. Chen, D. H. Walker, Dumler, JS, E. Breitschwerdt, B. Hegarty, and D. J. Sexton. 1999. The incidence of ehrlichial and rickettsial infection in patients with unexplained fever and recent history of tick bite in central North Carolina. J. Infect. Dis. 180:900-903. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. 2002. Summary of notifiable diseases, United States 2000. Morb. Mortal. Wkly. Rep. 49:1-100. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. 1996. Human ehrlichiosis—Maryland, 1994. Morb. Mortal. Wkly. Rep. 45:798-802. [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. Morb. Mortal. Wkly. Rep. RR-10:46. [PubMed]
- 52.Centers for Disease Control and Prevention. 1998. Statewide surveillance for ehrlichiosis—Connecticut and New York, 1994-1997. Morb. Mortal. Wkly. Rep. 47:476-480. [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. 1999. HIV AIDS Surveillance Report. 11(1):1-43. [Google Scholar]
- 54.Centers for Disease Control and Prevention. 1999. Summary of notifiable diseases, United States, 1999. Morb. Mortal. Wkly. Rep. 48(53):34-35. [Google Scholar]
- 55.Centers for Disease Control and Prevention. 2000. Surveillance for Lyme disease—United States, 1992-1998 CDC Surveillance Summaries, 28 April 2000. Morb. Mortal. Wkly. Rep. 49(SS-3):1-11. [PubMed] [Google Scholar]
- 56.Chen, S. M., L. C. Cullman, and D. H. Walker. 1997. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin. Diagn. Lab. Immunol. 4:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen, S. M., J. S. Dumler, H. M. Feng, and D. H. Walker. 1994. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am. J. Trop. Med. Hyg. 50:52-58. [PubMed] [Google Scholar]
- 58.Chen, S. M., V. L. Popov, H. M. Feng, J. Wen, and D. H. Walker. 1995. Cultivation of Ehrlichia chaffeensis in mouse embryo, Vero, BGM, and L929 cells and study of Ehrlichia-induced cytopathic effect and plaque formation. Infect. Immun. 63:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen, S. M., X. J. Yu, V. L. Popov, E. L. Westerman, F. G. Hamilton, and D. H. Walker. 1997. Genetic and antigenic diversity of Ehrlichia chaffeensis: comparative analysis of a novel human strain from Oklahoma and previously isolated strains. J. Infect. Dis. 175:856-863. [DOI] [PubMed] [Google Scholar]
- 60.Childs, J. E., J. H. McQuiston, J. W. Sumner, W. L. Nicholson, J. A. Comer, R. F. Massung, S. M. Standaert, and C. D. Paddock. 1999. Human monocytic ehrlichiosis due to Ehrlichia chaffeensis: how do we count the cases? p. 287-293. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 61.Childs, J. E., J. W. Sumner, W. L. Nicholson, R. F. Massung, S. M. Standaert, and C. D. Paddock. 1999. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J. Clin. Microbiol. 37:2997-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clymer, B. C., D. E. Howell, and J. A. Hair. 1970. Animal hosts of economically important ticks (Acarina) in east-central Oklahoma. Ann. Entomol. Soc. Am. 63:612-614. [Google Scholar]
- 63.Cohn, S. E., J. D. Klein, J. E. Mohr, C. M. van der Horst, and D. J. Weber. 1994. The geography of AIDS: patterns of urban and rural migration. South. Med. J. 87:599-606. [DOI] [PubMed] [Google Scholar]
- 64.Comer, J. A., W. L. Nicholson, J. G. Olson, and J. E. Childs. 1999. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J. Clin. Microbiol. 37:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Comer, J. A., W. L. Nicholson, C. D. Paddock, J. W. Sumner, and J. E. Childs. 2000. Detection of antibodies reactive with Ehrlichia chaffeensis in the raccoon. J. Wildl. Dis. 36:705-712. [DOI] [PubMed] [Google Scholar]
- 66.Cooley, R. A., and G. M. Kohls. 1944. The genus Amblyomma (Ixodidae) in the U. S. J. Parasitol. 30:77-111. [Google Scholar]
- 67.Dalton, M. J., M. J. Clarke, R. C. Holman, J. W. Krebs, D. B. Fishbein, J. G. Olson, and J. E. Childs. 1995. National surveillance for Rocky Mountain spotted fever, 1981-1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am. J. Trop. Med. Hyg. 52:405-413. [DOI] [PubMed] [Google Scholar]
- 68.Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2001. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 78:103-116. [DOI] [PubMed] [Google Scholar]
- 69.Davidson, W. R., J. M. Lockhart, D. E. Stallknecht, and E. W. Howerth. 1999. Susceptibility of red and gray foxes to infection by Ehrlichia chaffeensis. J. Wildl. Dis. 35:696-702. [DOI] [PubMed] [Google Scholar]
- 70.Davidson, W. R., J. M. Lockhart, D. E. Stallknecht, E. W. Howerth, J. E. Dawson, and Y. Rechav. 2001. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 37:538-546. [DOI] [PubMed] [Google Scholar]
- 71.Davidson, W. R., D. A. Siefken, and L. H. Creekmore. 1994. Seasonal and annual abundance of Amblyomma americanum (Acari: Ixodidae) in central Georgia. J. Med. Entomol. 31:67-71. [DOI] [PubMed] [Google Scholar]
- 72.Davis, L. E., C. D. Paddock, and J. E. Childs. 2000. Ehrlichiosis and the nervous system, p. 499-520. In L. E. Davis and P. G. E. Kennedy (ed.), Infectious diseases of the nervous system. Butterworth-Heinemann, Oxford, United Kingdom.
- 73.Dawson, J. E., B. E. Anderson, D. B. Fishbein, J. L. Sanchez, C. S. Goldsmith, K. H. Wilson, and C. W. Duntley. 1991. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 29:2741-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawson, J. E., K. L. Biggie, C. K. Warner, K. Cookson, S. Jenkins, J. Levine, and J. G. Olson. 1996. Polymerase chain reaction evidence of Ehrlichia chaffeensis, etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am. J. Vet. Res. 57:1175-1179. [PubMed] [Google Scholar]
- 75.Dawson, J. E., F. J. Candal, V. G. George, and E. W. Ades. 1993. Human endothelial cells as an alternative to DH82 cells for isolation of Ehrlichia chaffeensis, E. canis, and Rickettsia rickettsii. Pathobiology 61:293-296. [DOI] [PubMed] [Google Scholar]
- 76.Dawson, J. E., J. E. Childs, K. L. Biggie, C. Moore, D. Stallknecht, J. Shaddock, J. Bouseman, E. Hofmeister, and J. G. Olson. 1994. White-tailed deer as a potential reservoir of Ehrlichia spp. J. Wildl. Dis. 30:162-168. [DOI] [PubMed] [Google Scholar]
- 77.Dawson, J. E., and S. A. Ewing. 1992. Susceptibility of dogs to infection with Ehrlichia chaffeensis causative agent of human ehrlichiosis. Am. J. Vet. Res. 53:1322-1327. [PubMed] [Google Scholar]
- 78.Dawson, J. E., D. B. Fishbein, T. R. Eng, M. A. Redus, and N. R. Green. 1990. Diagnosis of human ehrlichiosis with the indirect fluorescent antibody test: kinetics and specificity. J. Infect. Dis. 162:91-95. [DOI] [PubMed] [Google Scholar]
- 79.Dawson, J. E., C. D. Paddock, C. K. Warner, P. W. Greer, J. H. Bartlett, S. A. Ewing, U. G. Munderloh, and S. R. Zaki. 2001. Tissue diagnosis of Ehrlichia chaffeensis, in patients with fatal ehrlichiosis, by using immunohistochemistry, in situ hybridization, and polymerase chain reaction. Am. J. Trop. Med. Hyg. 65:603-609. [DOI] [PubMed] [Google Scholar]
- 80.Dawson, J. E., D. E. Stallknecht, E. W. Howerth, C. Warner, K. Biggie, W. R. Davidson, J. M. Lockhart, V. F. Nettles, J. G. Olson, and J. E. Childs. 1994. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J. Clin. Microbiol. 32:2725-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dawson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olson. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 82.Demaree, H. A., Jr. 1986. Ticks of Indiana. Pittman-Robertson Bull. 16:1-178. [Google Scholar]
- 83.Des Vignes, F., M. L. Levin, and D. Fish. 1999. Comparative vector competence of Dermacentor variabilis and Ixodes scapularis (Acari: Ixodidae) for the agent of human granulocytic ehrlichiosis. J. Med. Entomol. 36:182-185. [DOI] [PubMed] [Google Scholar]
- 84.Doran, T. I., R. T. Parmley, P. C. Logas, and S. Chamblin. 1989. Infection with Ehrlichia canis in a child. J. Pediatr. 114:809-812. [DOI] [PubMed] [Google Scholar]
- 85.Dugan, V. G., S. E. Little, D. E. Stallknecht, and A. D. Beall. 2000. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 38:448-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dumler, J. S., and J. S. Bakken. 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis. 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 87.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 88.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 89.Dumler, J. S., P. Brouqui, J. Aronson, J. P. Taylor, and D. H. Walker. 1991. Identification of Ehrlichia in human tissue. N. Engl. J. Med. 325:1109-1110. [DOI] [PubMed] [Google Scholar]
- 90.Dumler, J. S., S. M. Chen, K. Asanovich, E. Trigiani, V. L. Popov, and D. H. Walker. 1995. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J. Clin. Microbiol. 33:1704-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dumler, J. S., J. E. Dawson, and D. H. Walker. 1993. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum. Pathol. 24:391-396. [DOI] [PubMed] [Google Scholar]
- 92.Dumler, J. S., W. L. Sutker, and D. H. Walker. 1993. Persistent infection with Ehrlichia chaffeensis. Clin. Infect. Dis. 17:903-905. [DOI] [PubMed] [Google Scholar]
- 93.Dumler, J. S., and D. H. Walker. 2001. Tick-borne ehrlichioses. Lancet Infect. Dis. (April) 2001:21-28. [Google Scholar]
- 94.Dunn, B. E., T. P. Monson, J. S. Dumler, C. C. Morris, A. B. Westbrook, J. L. Duncan, J. E. Dawson, K. G. Sims, and B. E. Anderson. 1992. Identification of Ehrlichia chaffeensis morulae in cerebrospinal fluid mononuclear cells. J. Clin. Microbiol. 30:2207-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Edwards, M. S. 1994. Ehrlichiosis in children. Semin. Pediatr. Infect. Dis. 5:143-147. [Google Scholar]
- 96.Edwards, M. S., J. E. Jones, D. L. Leass, J. W. Whitmore, J. E. Dawson, and D. B. Fishbein. 1988. Childhood infection caused by Ehrlichia canis or a closely related organism. Pediatr. Infect. Dis. J. 7:651-654. [DOI] [PubMed] [Google Scholar]
- 97.Eng, T. R., D. B. Fishbein, J. E. Dawson, C. R. Greene, and M. Redus. 1990. Surveillance of human ehrlichiosis in the United States: 1988. Ann. N. Y. Acad. Sci. 590:306-307. [DOI] [PubMed] [Google Scholar]
- 98.Eng, T. R., J. R. Harkess, D. B. Fishbein, J. E. Dawson, C. N. Greene, M. A. Redus, and F. T. Satalowich. 1990. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA 264:2251-2258. [PubMed] [Google Scholar]
- 99.Everett, E. D., K. A. Evans, R. B. Henry, and G. McDonald. 1994. Human ehrlichiosis in adults after tick exposure: diagnosis using polymerase chain reaction. Ann. Intern. Med. 120:730-735. [DOI] [PubMed] [Google Scholar]
- 100.Ewing, S. A., J. E. Dawson, A. A. Kocan, R. W. Barker, C. K. Warner, R. J. Panciera, J. C. Fox, K. M. Kocan, and E. F. Blouin. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32:368-374. [DOI] [PubMed] [Google Scholar]
- 101.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2001. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felz, M. W., L. A. Durden, and J. H. Oliver. 1996. Ticks parasitizing humans in Georgia and South Carolina. J. Parasitol. 82:505-508. [PubMed] [Google Scholar]
- 103.Fichtenbaum, C. J., L. R. Peterson, and G. J. Weil. 1993. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am. J. Med. 95:351-357. [DOI] [PubMed] [Google Scholar]
- 104.Fishbein, D. B. 1990. Human ehrlichiosis in the United States, p. 100-111. In J. C. Williams and I. Kakoma (ed.), Ehrlichiosis. Kluwer Academic Publishers, Amsterdam, The Netherlands.
- 105.Fishbein, D. B., J. E. Dawson, and L. E. Robinson. 1994. Human ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 120:736-743. [DOI] [PubMed] [Google Scholar]
- 106.Fishbein, D. B., A. Kemp, J. E. Dawson, N. R. Greene, M. A. Redus, and D. H. Fields. 1989. Human ehrlichiosis: prospective active surveillance in febrile hospitalized patients. J. Infect. Dis. 160:803-809. [DOI] [PubMed] [Google Scholar]
- 107.Fishbein, D. B., L. A. Sawyer, C. J. Holland, E. B. Hayes, W. Okoroanyanwu, D. Williams, R. K. Sikes, M. Ristic, and J. E. McDade. 1987. Unexplained febrile illnesses after exposure to ticks. Infection with an ehrlichia? JAMA 257:3100-3104. [PubMed] [Google Scholar]
- 108.Foley, J. E., L. Crawford-Miksza, J. S. Dumler, C. Glaser, J. S. Chae, E. Yeh, D. Schnurr, R. Hood, W. Hunter, and J. E. Madigan. 1999. Human granulocytic ehrlichiosis in Northern California: two case descriptions with genetic analysis of the Ehrlichiae. Clin. Infect. Dis. 29:388-392. [DOI] [PubMed] [Google Scholar]
- 109.Fordham, L. A., C. J. Chung, B. B. Specter, D. F. Merten, and D. L. Ingram. 1998. Ehrlichiosis: findings on chest radiographs in three pediatric patients. Am. J. Roentgenol. 171:1421-1424. [DOI] [PubMed] [Google Scholar]
- 110.Frank, J. R., and E. B. Breitschwerdt. 1999. A retrospective study of ehrlichiosis in 62 dogs from North Carolina and Virginia. J. Vet. Intern. Med. 13:194-201. [DOI] [PubMed] [Google Scholar]
- 111.Fritz, C. L., and C. A. Glaser. 1998. Ehrlichiosis. Infect. Dis. Clin. North Am. 12:123-136. [DOI] [PubMed] [Google Scholar]
- 112.Ganta, R. R., M. J. Wilkerson, C. Cheng, A. M. Rokey, and S. K. Chapes. 2002. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 70:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gardner, A. L. 1982. Virginia opossum, p. 3-36. In J. A. Chapman and G. A. Feldhamer (eds.), Wild mammals of North America: biology, management, economics. The Johns Hopkins University Press, Baltimore, Md.
- 114.Gardner, L. I., J. F. Brundage, D. S. Burke, J. G. McNeil, R. Visintine, and R. N. Miller. 1989. Evidence for spread of the human immunodeficiency virus epidemic into low prevalence areas of the United States. J. Acquir. Immune Defic. Syndr. 2:521-532. [PubMed] [Google Scholar]
- 115.Ginsberg, H. S., C. P. Ewing, A. F. O'Connell, E. M. Bosler, J. G. Daley, and M. W. Sayre. 1991. Increased population densities of Amblyomma americanum (Acari: Ixodidae) on Long Island, New York. J. Parasitol. 77:493-495. [PubMed] [Google Scholar]
- 116.Goddard, J. 1988. Was Bullis fever actually ehrlichiosis? JAMA 260:3006-3007. [PubMed] [Google Scholar]
- 117.Goddard, J. 2000. The mystery of Bullis fever: what can we learn from an outbreak 57 years ago? Infect. Med. 17:24-26. [Google Scholar]
- 118.Goddard, J., and B. R. Norment. 1986. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 23:465-472. [DOI] [PubMed] [Google Scholar]
- 119.Golden, S. E. 1989. Aseptic meningitis associated with Ehrlichia canis infection. Pediatr. Infect. Dis. J. 8:335-337. [PubMed] [Google Scholar]
- 120.Gongora-Biachi, R. A., J. Zavala-Velazquez, C. J. Castro-Sansores, and P. Gonzalez-Martinez. 1999. First case of human ehrlichiosis in Mexico. Emerg. Infect. Dis. 5:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grant, A. C., S. Hunter, and W. C. Partin. 1997. A case of acute monocytic ehrlichiosis with prominent neurologic signs. Neurology 48:1619-1623. [DOI] [PubMed] [Google Scholar]
- 122.Groves, M. G., G. L. Dennis, H. L. Amyx, and D. L. Huxsoll. 1975. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 36:937-940. [PubMed] [Google Scholar]
- 123.Hair, J. A., and D. E. Howell. 1970. Lone star ticks. Their biology and control in Ozark recreation areas, p. 1-47. Oklahoma State University Agricultural Experiment Station, Bulletin B-679.
- 124.Harkess, J. R. 1991. Ehrlichiosis. Infect. Dis. Clin. North Am. 5:37-51. [PubMed] [Google Scholar]
- 125.Harkess, J. R., S. A. Ewing, J. M. Crutcher, J. Kudlac, G. McKee, and G. R. Istre. 1989. Human ehrlichiosis in Oklahoma. J. Infect. Dis. 159:576-579. [DOI] [PubMed] [Google Scholar]
- 126.Harkess, J. R., D. Stucky, and S. A. Ewing. 1990. Neurologic abnormalities in a patient with human ehrlichiosis. South. Med. J. 83:1341-1343. [DOI] [PubMed] [Google Scholar]
- 127.Hawkins, M. M. 1995. Human ehrlichiosis: a case report from the South Carolina lowcountry. J. S. C. Med. Assoc. 91:228-229. [PubMed] [Google Scholar]
- 128.Heimer, R., D. Tisdale, and J. E. Dawson. 1998. A single tissue culture system for the propagation of the agents of the human ehrlichioses. Am. J. Trop. Med. Hyg. 58:812-815. [DOI] [PubMed] [Google Scholar]
- 129.Heppner, D. G., C. Wongsrichanalai, D. S. Walsh, P. McDaniel, C. Eamsila, B. Hanson, and H. Paxton. 1997. Human ehrlichiosis in Thailand. Lancet 350:785-786. [DOI] [PubMed] [Google Scholar]
- 130.Hill, E. P., P. W. Sumner, and J. B. Wooding. 1987. Human influences on range expansion of coyotes in the southeast. Wildl. Soc. Bull. 15:521-524. [Google Scholar]
- 131.Hodzic, E., D. Fish, C. M. Maretzki, A. M. de Silva, S. L. Feng, and S. W. Barthold. 1998. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36:3574-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hopla, C. E. 1953. Experimental studies on tick transmission of tularemia organisms. Am. J. Hyg. 58:101-108. [DOI] [PubMed] [Google Scholar]
- 133.IJdo, J. W., J. I. Meek, M. L. Cartter, L. A. Magnarelli, C. Y. Wu, S. W. Tenuta, E. Fikrig, and R. W. Ryder. 2000. The emergence of another tickborne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J. Infect. Dis. 181:1388-1393. [DOI] [PubMed] [Google Scholar]
- 134.IJdo, J. W., C. Wu, L. A. Magnarelli, and E. Fikrig. 1999. Serodiagnosis of human granulocytic ehrlichiosis by a recombinant HGE-44-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3540-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.IJdo, J. W., C. Wu, L. A. Magnarelli, K. C. Stafford, J. F. Anderson, and E. Fikrig. 2000. Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum ticks in Connecticut and Rhode Island. J. Clin. Microbiol. 38:4655-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Irving, R. P., R. R. Pinger, C. N. Vann, J. B. Olesen, and F. E. Steiner. 2000. Distribution of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum in southern Indiana and prevalence of E. chaffeensis—reactive antibodies in white-tailed deer in Indiana and Ohio in 1998. J. Med. Entomol. 37:595-600. [DOI] [PubMed] [Google Scholar]
- 137.Jackson, L. K., D. M. Gaydon, and J. Goddard. 1996. Seasonal activity and relative abundance of Amblyomma americanum in Mississippi. J. Med. Entomol. 33:128-131. [DOI] [PubMed] [Google Scholar]
- 138.Jackson, R. T., and J. W. Jackson. 1997. Ehrlichiosis with systemic sepsis syndrome. Tenn. Med. 90:185-186. [PubMed] [Google Scholar]
- 139.Jacobs, R. F., and G. E. Schutze. 1997. Ehrlichiosis in children. J. Pediatr. 131:184-192. [DOI] [PubMed] [Google Scholar]
- 140.James, A. M., D. Liveris, G. P. Wormser, I. Schwartz, M. A. Montecalvo, and B. J. Johnson. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183:1810-1814. [DOI] [PubMed] [Google Scholar]
- 141.Javed, M. Z., M. Srivastava, S. Zhang, and M. Kandathil. 2001. Concurrent babesiosis and ehrlichiosis in an elderly host. Mayo Clin. Proc. 76:563-565. [DOI] [PubMed] [Google Scholar]
- 142.Reference deleted.
- 143.Katavolos, P., P. M. Armstrong, J. E. Dawson, and S. R. Telford. 1998. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J. Infect. Dis. 177:1422-1425. [DOI] [PubMed] [Google Scholar]
- 144.Kawahara, M., C. Suto, Y. Rikihisa, S. Yamamoto, and Y. Tsuboi. 1993. Characterization of ehrlichial organisms isolated from a wild mouse. J. Clin. Microbiol. 31:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawahara, M., C. Suto, S. Shibata, M. Futohashi, and Y. Rikihisa. 1997. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol. Immunol. 40:575-581. [DOI] [PubMed] [Google Scholar]
- 146.Keirans, J. E., and E. H. Lacombe. 1998. First records of Amblyomma americanum, Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J. Parasitol. 84:629-631. [PubMed] [Google Scholar]
- 147.Keysary, A., L. Amram, G. Keren, Z. Sthoeger, I. Potasman, A. Jacob, C. Strenger, J. E. Dawson, and T. Waner. 1999. Serologic evidence of human monocytic and granulocytic ehrlichiosis in Israel. Emerg. Infect. Dis. 5:775-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Knowlton, F. F. 1972. Preliminary interpretations of coyote population mechanics with some management implications. J. Wildl. Manage. 36:369-382. [Google Scholar]
- 149.Kocan, A. A., S. A. Ewing, D. Stallknecht, G. L. Murphy, S. Little, L. C. Whitworth, and R. W. Barker. 2000. Attempted transmission of Ehrlichia chaffeensis among white-tailed deer by Amblyomma maculatum. J. Wildl. Dis. 36:592-594. [DOI] [PubMed] [Google Scholar]
- 150.Kocan, A. A., G. C. Levesque, L. C. Whitworth, G. L. Murphy, S. A. Ewing, and R. W. Barker. 2000. Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg. Infect. Dis. 6:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kollars, T. M. 1993. Ticks (Acari: Ixodidae) infesting medium-sized wild mammals in southwestern Tennessee. J. Med. Entomol. 30:896-900. [DOI] [PubMed] [Google Scholar]
- 152.Kramer, V. L., M. P. Randolph, L. T. Hui, W. E. Irwin, A. G. Gutierrez, and D. J. Vugia. 1999. Detection of the agents of human ehrlichioses in ixodid ticks from California. Am. J. Trop. Med. Hyg. 60:62-65. [DOI] [PubMed] [Google Scholar]
- 153.Krause, R. M. 1994. Dynamics of emergence. J. Infect. Dis. 170:265-271. [DOI] [PubMed] [Google Scholar]
- 154.Lam, N. S., and K. B. Liu. 1994. Spread of AIDS in rural America, 1982-1990. J. Acquir. Immune Defic. Syndr. 7:485-490. [PubMed] [Google Scholar]
- 155.Laudicina, R. J., and A. E. Hilger. 1997. Human ehrlichiosis. A case review. Clin. Lab. Sci. 10:149-166. [Google Scholar]
- 156.Lederberg, J., R. E. Shope, and S. C. Oaks, Jr. 1992. Emerging infections, p. 294. National Academy Press, Washington, D.C. [PubMed]
- 157.Lee, E. H., and Y. Rikihisa. 1996. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect. Immun. 64:4211-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee, E. H., and Y. Rikihisa. 1997. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κβ. Infect. Immun. 65:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee, E. H., and Y. Rikihisa. 1998. Protein kinase A-mediated inhibition of gamma interferon-induced tyrosine phosphorylation of Janus kinases and latent cytoplasmic transcription factors in human monocytes by Ehrlichia chaffeensis. Infect. Immun. 66:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 161.Liddell, A. M., J. W. Sumner, C. D. Paddock, Y. Rikihisa, A. Unver, R. S. Buller, and G. A. Storch. 2002. Reinfection with Ehrlichia chaffeensis in a liver transplant patient. Clin. Infect. Dis. 34:1644-1647. [DOI] [PubMed] [Google Scholar]
- 162.Liebisch, A. 1997. General review of the tick species which parasitize sheep and goats world-wide. Parassitologia 39:123-129. [PubMed] [Google Scholar]
- 163.Little, S. E., and E. W. Howerth. 1999. Ehrlichia chaffeensis in archived tissues of a white-tailed deer. J. Wildl. Dis. 35:596-599. [DOI] [PubMed] [Google Scholar]
- 164.Little, S. E., D. E. Stallknecht, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 1998. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. J. Parasitol. 84:897-901. [PubMed] [Google Scholar]
- 165.Lockhart, J. M., and W. R. Davidson. 1999. Evaluation of C3H/HeJ mice for xenodiagnosis of infection with Ehrlichia chaffeensis. J. Vet. Diagn. Investig. 11:55-59. [DOI] [PubMed] [Google Scholar]
- 166.Lockhart, J. M., W. R. Davidson, J. E. Dawson, and D. E. Stallknecht. 1995. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J. Wildl. Dis. 31:119-124. [DOI] [PubMed] [Google Scholar]
- 167.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, and J. E. Dawson. 1996. Site-specific geographic association between Amblyomma americanum (Acari: Ixodidae) infestations and Ehrlichia chaffeensis-reactive (Rickettsiales: Ehrlichieae) antibodies in white-tailed deer. J. Med. Entomol. 33:153-158. [DOI] [PubMed] [Google Scholar]
- 168.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, and J. E. Dawson. 1998. Lack of seroreactivity to Ehrlichia chaffeensis among rodent populations. J. Wildl. Dis. 34:392-396. [DOI] [PubMed] [Google Scholar]
- 169.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and E. W. Howerth. 1997. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 35:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and S. E. Little. 1997. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the piedmont physiographic province of Georgia. J. Parasitol. 83:887-894. [PubMed] [Google Scholar]
- 171.Long, S. W., X. F. Zhang, H. Qi, S. Standaert, D. H. Walker, and X. J. Yu. 2002. Antigenic variation of Ehrlichia chaffeensis resulting from differential expression of the 28-kilodalton protein gene family. Infect. Immun. 70:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lotric-Furlan, S., M. Petrovec, T. A. Zupanc, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 1998. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin. Infect. Dis. 27:424-428. [DOI] [PubMed] [Google Scholar]
- 173.Ludmerer, K. M., and J. M. Kissane. 1998. Fever, nausea, and rash in a 37-year-old man. Am. J. Med. 104:596-601. [PubMed] [Google Scholar]
- 174.Maeda, K., N. Markowitz, R. C. Hawley, M. Ristic, D. Cox, and J. E. McDade. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316:853-856. [DOI] [PubMed] [Google Scholar]
- 175.Magnarelli, L. A., J. F. Anderson, K. C. Stafford, and J. S. Dumler. 1997. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. J. Wildl. Dis. 33:466-473. [DOI] [PubMed] [Google Scholar]
- 176.Magnarelli, L. A., J. S. Dumler, J. F. Anderson, R. C. Johnson, and E. Fikrig. 1995. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J. Clin. Microbiol. 33:3054-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manian, F. A., J. Weidner, J. Costello, D. B. Fishbein, and J. E. Dawson. 1989. Human ehrlichiosis. Missouri Med. 86:691-695. [PubMed] [Google Scholar]
- 178.Marshall, G. S., R. F. Jacobs, G. E. Schutze, H. Paxton, S. C. Buckingham, J. P. DeVincenzo, M. A. Jackson, V. H. San Joaquin, S. M. Standaert, C. R. Woods, and The Tick-Borne Infections in Children Study Group. 2002. Ehrlichia chaffeensis seroprevalence among children in the southeast and south-central regions of the United States. Arch. Pediatr. Adolesc. Med. 156:166-170. [DOI] [PubMed] [Google Scholar]
- 179.Martin, G. S., B. W. Christman, and S. M. Standaert. 1999. Rapidly fatal infection with Ehrlichia chaffeensis. N. Engl. J. Med. 341:763-764. [DOI] [PubMed] [Google Scholar]
- 180.Marty, A. M., J. S. Dumler, G. Imes, H. P. Brusman, L. L. Smrkovski, and D. M. Frisman. 1995. Ehrlichiosis mimicking thrombotic thrombocytopenic purpura. Case report and pathological correlation. Hum. Pathol. 26:920-925. [DOI] [PubMed] [Google Scholar]
- 181.Mathisen, G. E., P. J. Weiss, and C. A. Kennedy. 1993. Pneumonia, aseptic meningitis, and leukopenia in a 28-year-old man. Clin. Infect. Dis. 16:809-815. [DOI] [PubMed] [Google Scholar]
- 182.Maurin, M., C. Abergel, and D. Raoult. 2001. DNA gyrase-mediated natural resistance to fluoroquinolones in Ehrlichia spp. Antimicrob. Agents Chemother. 45:2098-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McBride, J. W., X. J. Yu, and D. H. Walker. 2000. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and Ehrlichia canis. Infect. Immun. 68:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McCabe, T. R., and R. E. McCabe. 1997. Recounting whitetails past, p. 11-26. In W. J. McShea, H. B. Underwood, and J. H. Rappole (ed.), The science of over abundance: deer ecology and population management. Smithsonian Institution Press, Washington, D.C.
- 185.McCall, C. L., A. T. Curns, J. S. Singleton, J. A. Comer, J. G. Olson, L. D. Rotz, T. A. Treadwell, P. Arguin, and J. E. Childs. 2001. Fort Chaffee revisited: the epidemiology of tickborne diseases at a persistent focus. Vector Borne Zoonotic Dis. 2:119-127. [DOI] [PubMed] [Google Scholar]
- 186.McDade, J. E. 1990. Ehrlichiosis—a disease of animals and humans. J. Infect. Dis. 161:609-617. [DOI] [PubMed] [Google Scholar]
- 187.McKechnie, D. B., K. S. Slater, J. E. Childs, R. F. Massung, and C. D. Paddock. 2000. Survival of Ehrlichia chaffeensis in refrigerated, ADSOL-treated RBCs. Transfusion 40:1041-1047. [DOI] [PubMed] [Google Scholar]
- 188.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Means, R. G., and D. J. White. 1997. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. J. Vector Ecol. 22:133-145. [PubMed] [Google Scholar]
- 190.Mock, D. E., R. D. Applegate, and L. B. Fox. 2001. Preliminary survey of ticks (Acari: Ixodidae) parasitizing wild turkeys (Aves: Phasianidae) in eastern Kansas. J. Med. Entomol. 38:118-121. [DOI] [PubMed] [Google Scholar]
- 191.Moore, G. C., and G. R. Parker. 1992. Colonization by the eastern coyote (Canis latrans), p. 21-37. In A. H. Boer (ed.), Ecology and management of the eastern coyote. Wildlife Research Unit, University of New Brunswick, Fredericton, N.B., Canada.
- 192.Morais, J. D., J. E. Dawson, C. Greene, A. R. Filipe, L. C. Galhardas, and F. Bacellar. 1991. First European case of ehrlichiosis. Lancet 338:633-634. [DOI] [PubMed] [Google Scholar]
- 193.Morris, J. G., Jr., and M. Potter. 1997. Emergence of new pathogens as a function of changes in host susceptibility. Emerg. Infect. Dis. 3:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Moskovitz, M., R. Fadden, and T. Min. 1991. Human ehrlichiosis: a rickettsial disease associated with severe cholestasis and multisystemic disease. J Clin. Gastroenterol. 13:86-90. [PubMed] [Google Scholar]
- 195.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mount, G. A., D. G. Haile, D. R. Barnard, and E. Daniels. 1993. New version of LSTSIM for computer simulation of Amblyomma americanum (Acari: Ixodidae) population dynamics. J. Med. Entomol. 30:843-857. [DOI] [PubMed] [Google Scholar]
- 197.Mueller-Anneling, L., M. J. Gilchrist, and P. S. Thorne. 2000. Ehrlichia chaffeensis antibodies in white-tailed deer, Iowa, 1994 and 1996. Emerg. Infect. Dis. 6:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murphy, G. L., S. A. Ewing, L. C. Whitworth, J. C. Fox, and A. A. Kocan. 1998. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 79:325-339. [DOI] [PubMed] [Google Scholar]
- 199.Nuti, M., D. A. Serafini, D. Bassetti, A. Ghionni, F. Russino, P. Rombola, G. Macri, and E. Lillini. 1998. Ehrlichia infection in Italy. Emerg. Infect. Dis. 4:663-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nutt, A. K., and J. Raufman. 1999. Gastrointestinal and hepatic manifestations of human ehrlichiosis: 8 cases and a review of the literature. Dig. Dis. 17:37-43. [DOI] [PubMed] [Google Scholar]
- 201.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Okada, H., T. Tajima, M. Kawahara, and Y. Rikihisa. 2001. Ehrlichial proliferation and acute hepatocellular necrosis in immunocompetent mice experimentally infected with the HF strain of Ehrlichia, closely related to Ehrlichia chaffeensis. J. Comp. Pathol. 124:165-171. [DOI] [PubMed] [Google Scholar]
- 204.Olano, J. P., E. Masters, L. Cullman, W. Hogrefe, X. J. Yu, and D. H. Walker. 1999. Human monocytotropic ehrlichiosis (HME): epidemiological, clinical and laboratory diagnosis of a newly emergent infection in the United States, p. 262-268. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Elsevier, Paris, France.
- 205.Olano, J. P., and D. H. Walker. 2002. Human ehrlichioses. Med. Clin. North Am. 86:375-392. [DOI] [PubMed] [Google Scholar]
- 206.Paddock, C. D., S. M. Folk, G. M. Shore, L. J. Machado, M. M. Huycke, L. N. Slater, A. M. Liddell, R. S. Buller, G. A. Storch, T. P. Monson, D. Rimland, J. W. Sumner, J. Singleton, K. C. Bloch, Y. W. Tang, S. M. Standaert, and J. E. Childs. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586-1594. [DOI] [PubMed] [Google Scholar]
- 207.Paddock, C. D., P. W. Greer, T. L. Ferebee, J. Singleton, D. B. McKechnie, T. A. Treadwell, J. W. Krebs, M. J. Clarke, R. C. Holman, J. G. Olson, J. E. Childs, and S. R. Zaki. 1999. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically uncnfirmed disease. J. Infect. Dis. 179:1469-1476. [DOI] [PubMed] [Google Scholar]
- 208.Paddock, C. D., D. P. Suchard, K. L. Grumbach, W. K. Hadley, R. L. Kerschmann, N. W. Abbey, J. E. Dawson, B. E. Anderson, K. G. Sims, and J. S. Dumler. 1993. Brief report: fatal seronegative ehrlichiosis in a patient with HIV infection. N. Engl. J. Med. 329:1164-1167. [DOI] [PubMed] [Google Scholar]
- 209.Paddock, C. D., J. W. Sumner, G. M. Shore, D. C. Bartley, R. C. Elie, J. G. McQuade, C. R. Martin, C. S. Goldsmith, and J. E. Childs. 1997. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J. Clin. Microbiol. 35:2496-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 211.Paparone, P. W., P. Ljubich, G. A. Rosman, and N. T. Nazha. 1995. Ehrlichiosis with pancytopenia and ARDS. N. J. Med. 92:381-385. [PubMed] [Google Scholar]
- 212.Parola, P., and D. Raoult. 2001. Ticks and tickborne diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32:897-928. [DOI] [PubMed] [Google Scholar]
- 213.Patel, R. G., and M. A. Byrd. 1999. Near fatal acute respiratory distress syndrome in a patient with human ehrlichiosis. South. Med. J. 92:333-335. [DOI] [PubMed] [Google Scholar]
- 214.Perez, M., Y. Rikihisa, and B. Wen. 1996. Ehrlichia canis -like agent isolated from a man in Venezuela: antigenic and genetic characterization. J. Clin. Microbiol. 34:2133-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Peters, T. R., K. M. Edwards, and S. M. Standaert. 2000. Severe ehrlichiosis in an adolescent taking trimethoprim-sulfamethoxazole. Pediatr. Infect. Dis. J. 19:170-172. [DOI] [PubMed] [Google Scholar]
- 216.Petersen, L. R., L. A. Sawyer, D. B. Fishbein, P. W. Kelley, R. J. Thomas, L. A. Magnarelli, M. Redus, and J. E. Dawson. 1989. An outbreak of ehrlichiosis in members of an army reserve unit exposed to ticks. J. Infect. Dis. 159:562-658. [DOI] [PubMed] [Google Scholar]
- 217.Piesman, J., and C. M. Happ. 1997. Ability of the Lyme disease spirochete Borrelia burgdorferi to infect rodents and three species of human-biting ticks (blacklegged tick, American dog tick, lone star tick) (Acari: Ixodidae). J. Med. Entomol. 34:451-456. [DOI] [PubMed] [Google Scholar]
- 218.Popov, V. L., S. M. Chen, H. M. Feng, and D. H. Walker. 1995. Ultrastructural variation of cultured Ehrlichia chaffeensis. J. Med. Microbiol. 43:411-421. [DOI] [PubMed] [Google Scholar]
- 219.Popov, V. L., X. Yu, and D. H. Walker. 2000. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 28:71-80. [DOI] [PubMed] [Google Scholar]
- 220.Raad, I., V. Singh, and T. J. Quan. 1989. Concurrent positive serology for ehrlichiosis and Lyme disease. J. Infect. Dis. 160:727-728. [DOI] [PubMed] [Google Scholar]
- 221.Rathore, M. H., and K. Meyer. 1993. Human ehrlichiosis in Florida. J. Fla. Med. Assoc. 80:327-329. [PubMed] [Google Scholar]
- 222.Ratnasamy, N., E. D. Everett, W. E. Roland, G. McDonald, and C. W. Caldwell. 1996. Central nervous system manifestations of human ehrlichiosis. Clin. Infect. Dis. 23:314-319. [DOI] [PubMed] [Google Scholar]
- 223.Ravyn, M. D., E. I. Korenberg, J. A. Oeding, Y. V. Kovalevskii, and R. C. Johnson. 1999. Monocytic Ehrlichia in Ixodes persulcatus ticks from Perm, Russia. Lancet 353:722-723. [DOI] [PubMed] [Google Scholar]
- 224.Rawlings, J. 1996. Human ehrlichiosis in Texas. J. Spir. Tick-borne Dis. 3:94-97. [Google Scholar]
- 225.Reddy, G. R., and C. P. Streck. 1999. Variability in the 28-kDa surface antigen protein multigene locus of isolates of the emerging disease agent Ehrlichia chaffeensis suggests that it plays a role in immune evasion. Mol. Cell Biol. Res. Commun. 1:167-175. [DOI] [PubMed] [Google Scholar]
- 226.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247:636-643. [DOI] [PubMed] [Google Scholar]
- 227.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rikihisa, Y. 1999. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1:367-376. [DOI] [PubMed] [Google Scholar]
- 229.Rikihisa, Y. 2000. Ehrlichial strategy for survival and proliferation in leukocytes. Subcell. Biochem. 33:517-538. [DOI] [PubMed] [Google Scholar]
- 230.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Riley, S. P. D., J. Hadidian, and D. A. Manski. 1998. Population density, survival, and rabies in raccoons in an urban national park. Can. J. Zool. 76:1153-1164. [Google Scholar]
- 232.Ripoll, C. M., C. E. Remondegui, G. Ordonez, R. Arazamendi, H. Fusaro, M. J. Hyman, C. D. Paddock, S. R. Zaki, J. G. Olson, and C. A. Santos-Buch. 1999. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am. J. Trop. Med. Hyg. 61:350-354. [DOI] [PubMed] [Google Scholar]
- 233.Rohrbach, B. W., J. R. Harkess, S. A. Ewing, J. Kudlac, G. L. McKee, and G. R. Istre. 1990. Epidemiologic and clinical characteristics of persons with serologic evidence of E. canis infection. Am. J. Publ. Health 80:442-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rolain, J. M., M. Maurin, A. Bryskier, and D. Raoult. 2000. In vitro activities of telithromycin (HMR 3647) against Rickettsia rickettsii, Rickettsia conorii, Rickettsia africae, Rickettsia typhi, Rickettsia prowazekii, Coxiella burnetii, Bartonella henselae, Bartonella quintana, Bartonella bacilliformis, and Ehrlichia chaffeensis. Antimicrob. Agents Chemother. 44:1391-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roland, W. E., E. D. Everett, T. L. Cyr, S. Z. Hasan, C. B. Dommaraju, and G. A. McDonald. 1998. Ehrlichia chaffeensis in Missouri ticks. Am. J. Trop. Med. Hyg. 59:641-643. [DOI] [PubMed] [Google Scholar]
- 236.Rooney, T. B., T. E. McGue, and K. C. Delahanty. 2001. A Naval Academy midshipman with ehrlichiosis after summer field exercises in Quantico, Virginia. Mil. Med. 166:191-193. [PubMed] [Google Scholar]
- 237.Roundtree, S. E., and G. A. Nixon. 1999. Human monocytic ehrlichiosis in a thirteen year old: a case report. J. S. C. Med. Assoc. 95:300-302. [PubMed] [Google Scholar]
- 238.Rumley, R. L., N. C. Shappley, L. E. Waivers, and J. D. Esinhart. 1991. AIDS in rural eastern North Carolina—patient migration: a rural AIDS burden. AIDS 5:1373-1378. [DOI] [PubMed] [Google Scholar]
- 239.Rydkina, E., V. Roux, and D. Raoult. 1999. Determination of the genome size of Ehrlichia spp., using pulsed field gel electrophoresis. FEMS Microbiol. Lett. 176:73-78. [DOI] [PubMed] [Google Scholar]
- 240.Sachar, D. S. 2000. Ehrlichia chaffeensis infection in an active duty soldier stationed in Korea. Medical Surveillance Monthly Report 6:9-11. [Google Scholar]
- 241.Sadikot, R., M. J. Shaver, and W. B. Reeves. 1999. Ehrlichia chaffeensis in a renal transplant recipient. Am. J. Nephrol. 19:674-676. [DOI] [PubMed] [Google Scholar]
- 242.Safdar, N., R. B. Love, and D. G. Maki. 2002. Severe Ehrlichia chaffeensis infection in a lung transplant recipient: a review of ehrlichiosis in the immunocompromised patient. Emerg. Infect. Dis. 8:320-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Salgado, J. H., M. E. Evans, A. D. Hoven, and R. C. Noble. 1995. Ehrlichiosis in Kentucky. J. Ky. Med. Assoc. 93:132-135. [PubMed] [Google Scholar]
- 244.Santino, I., A. Iori, R. Sessa, C. Sulli, G. Favia, M. Del Piano, H. W. Horowitz, E. Kilchevsky, S. Haber, M. Aguero-Rosenfeld, R. Kranwinkel, E. K. James, S. J. Wong, F. Chu, D. Liveris, and I. Schwartz. 1998. Borrelia burgdorferi s.l. and Ehrlichia chaffeensis in the National Park of Abruzzo. FEMSMicrobiol. Lett. 164:1-6. [DOI] [PubMed] [Google Scholar]
- 245.Schubert, J. H. 1952. Serologic titers in rickettsial infection as affected by antibiotic treatment. Publ. Health Lab. 10:38-41. [Google Scholar]
- 246.Schutze, G. E., and R. F. Jacobs. 1997. Human monocytic ehrlichiosis in children. Pediatrics 100:E10. [DOI] [PubMed] [Google Scholar]
- 247.Sexton, D. J., G. R. Corey, C. Carpenter, L. Q. Kong, T. Gandhi, E. Breitschwerdt, B. Hegarty, S. M. Chen, H. M. Feng, X. J. Yu, J. Olano, D. H. Walker, and S. J. Dumler. 1998. Dual infection with Ehrlichia chaffeensis and a spotted fever group rickettsia: a case report. Emerg. Infect. Dis. 4:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sexton, D. J., and G. R. Corey. 1992. Rocky Mountain “spotless” and “almost spotless” fever: a wolf in sheep's clothing. Clin. Infect. Dis. 15:439-448. [DOI] [PubMed] [Google Scholar]
- 249.Shibata, S., M. Kawahara, Y. Rikihisa, H. Fujita, Y. Watanabe, C. Suto, and T. Ito. 2000. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 38:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Simmons, B. P., and J. R. Hughey. 1989. Ehrlichia in Tennessee. South. Med. J. 82:669. [DOI] [PubMed] [Google Scholar]
- 251.Solberg, V. B., J. G. Olson, L. R. Boobar, J. R. Burge, and P. G. Lawyer. 1996. Prevalence of Ehrlichia chaffeensis, spotted fever group rickettsia, and Borrela spp. infections in ticks and rodents at Fort Bragg, North Carolina. J. Vector Ecol. 21:81-84. [Google Scholar]
- 252.Sotomayor, E. A., V. L. Popov, H. M. Feng, D. H. Walker, and J. P. Olano. 2001. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. Pathol. 158:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Standaert, S. M., L. A. Clough, W. Schaffner, J. S. Adams, and K. M. Neuzil. 1998. Neurologic manifestations of human monocytic ehrlichiosis. Infect. Dis. Clin. Pract. 7:358-362. [Google Scholar]
- 254.Standaert, S. M., J. E. Dawson, W. Schaffner, J. E. Childs, K. L. Biggie, J. Singleton, Jr., R. R. Gerhardt, M. L. Knight, and R. H. Hutcheson. 1995. Ehrlichiosis in a golf-oriented retirement community. N. Engl. J. Med. 333:420-425. [DOI] [PubMed] [Google Scholar]
- 255.Standaert, S. M., T. Yu, M. A. Scott, J. E. Childs, C. D. Paddock, W. L. Nicholson, J. Singleton, and M. J. Blaser. 2000. Primary isolation of Ehrlichia chaffeensis from patients with febrile illnesses: clinical and molecular characteristics. J. Infect. Dis. 181:1082-1088. [DOI] [PubMed] [Google Scholar]
- 256.Steiner, F. E., R. R. Pinger, and C. N. Vann. 1999. Infection rates of Amblyomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) and prevalence of E. chaffeensis-reactive antibodies in white-tailed deer in southern Indiana, 1997. J. Med. Entomol. 36:715-719. [DOI] [PubMed] [Google Scholar]
- 257.Stromdahl, E. Y., S. R. Evans, J. J. O'Brien, and A. G. Gutierrez. 2001. Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J. Med. Entomol. 38:67-74. [DOI] [PubMed] [Google Scholar]
- 258.Stromdahl, E. Y., M. P. Randolph, J. J. O'Brien, and A. G. Gutierrez. 2000. Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) infection in Amblyomma americanum (Acari: Ixodidae) at Aberdeen Proving Ground, Maryland. J. Med. Entomol. 37:349-356. [DOI] [PubMed] [Google Scholar]
- 259.Sumner, J. W., J. E. Childs, and C. D. Paddock. 1999. Molecular cloning and characterization of the Ehrlichia chaffeensis variable-length PCR target: an antigen-expressing gene that exhibits interstrain variation. J. Clin. Microbiol. 37:1447-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tal, A., and D. Shannahan. 1995. Ehrlichiosis presenting as a life-threatening illness. Am. J. Med. 98:318-319. [DOI] [PubMed] [Google Scholar]
- 262.Tan, H. P., J. S. Dumler, W. R. Maley, A. S. Klein, J. F. Burdick, P. F. Fred, P. J. Thuluvath, and J. S. Markowitz. 2001. Human monocytic ehrlichiosis: an emerging pathogen in transplantation. Transplantation 71:1678-1680. [DOI] [PubMed] [Google Scholar]
- 263.Taylor, J. P., T. G. Betz, D. B. Fishbein, M. A. Roberts, J. Dawson, and M. Ristic. 1988. Serological evidence of possible human infection with ehrlichia in Texas. J. Infect. Dis. 158:217-220. [DOI] [PubMed] [Google Scholar]
- 264.Telford, S. R., III, and J. E. Dawson. 1996. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet. Microbiol. 52:103-112. [DOI] [PubMed] [Google Scholar]
- 265.Treadwell, T. A., R. C. Holman, M. J. Clarke, J. W. Krebs, C. D. Paddock, and J. E. Childs. 2000. Rocky Mountain spotted fever in the United States during 1993 through 1996. Am. J. Trop. Med. Hyg. 63:21-26. [DOI] [PubMed] [Google Scholar]
- 266.Tugwell, P., and J. L. Lancaster, Jr. 1962. Results of a tick-host study in northwest Arkansas. J. Kans. Entomol. Soc. 35:202-211. [Google Scholar]
- 267.Uhaa, I. J., J. D. MacLean, C. R. Green, and D. B. Fishbein. 1992. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am. J. Trop. Med. Hyg. 46:161-164. [DOI] [PubMed] [Google Scholar]
- 268.Unver, A., S. Felek, C. D. Paddock, N. Zhi, H. W. Horowitz, G. P. Wormser, L. C. Cullman, and Y. Rikihisa. 2001. Western blot analysis of sera reactive to human monocytic ehrlichiosis and human granulocytic ehrlichiosis agents. J. Clin. Microbiol. 39:3982-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.U.S. Census Bureau. 1999. Statistical abstract of the United States, p. 1-1005. U.S. Census Bureau, Washington, D.C.
- 270.Verghese, A., S. L. Berk, and F. Sarubbi. 1989. Urbs in rure: human immunodeficiency virus infection in rural Tennessee. J Infect Dis. 160:1051-1055. [DOI] [PubMed] [Google Scholar]
- 271.Vugia, D. J., E. Holmberg, E. M. Steffe, M. S. Ascher, and D. Gallo. 1996. A human case of monocytic ehrlichiosis with adult respiratory distress syndrome in northern California. West. J. Med. 164:525-528. [PMC free article] [PubMed] [Google Scholar]
- 272.Walker, D. H. 1998. Tick-transmitted infectious diseases in the United States. Annu. Rev. Public Health 19:237-269. [DOI] [PubMed] [Google Scholar]
- 273.Walker, D. H. 2000. Diagnosing human ehrlichioses: current status and recommendations. ASM News 66:287-291. [Google Scholar]
- 274.Walker, D. H., A. G. Barbour, J. H. Oliver, R. S. Lane, J. S. Dumler, D. T. Dennis, D. H. Persing, A. F. Azad, and E. McSweegan. 1996. Emerging bacterial zoonotic and vector-borne diseases. Ecological and epidemiological factors. JAMA 275:463-469. [PubMed] [Google Scholar]
- 275.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 121:785-791. [PubMed] [Google Scholar]
- 276.Wallace, B. J., G. Brady, D. M. Ackman, S. J. Wong, G. Jacquette, E. E. Lloyd, and G. S. Birkhead. 1998. Human granulocytic ehrlichiosis in New York. Arch. Intern. Med. 158:769-773. [DOI] [PubMed] [Google Scholar]
- 277.Weaver, R. A. R., G. Virella, and A. Weaver. 1999. Ehrlichiosis with severe pulmonary manifestations despite early treatment. South. Med. J. 92:336-339. [DOI] [PubMed] [Google Scholar]
- 278.Whitlock, J. E., Q. Q. Fang, L. A. Durden, and J. H. Oliver, Jr. 2000. Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia coast and Barrier Islands. J. Med. Entomol. 37:276-280. [DOI] [PubMed] [Google Scholar]
- 279.Williams, J. D., R. M. Snow, and J. G. Arciniegas. 1995. Myocardial involvement in a patient with human ehrlichiosis. Am. J. Med. 98:414-415. [DOI] [PubMed] [Google Scholar]
- 280.Winslow, G. M., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66:3892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Winslow, G. M., E. Yager, K. Shilo, E. Volk, A. Reilly, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wolf, L., T. McPherson, B. Harrison, B. Engber, A. Anderson, and P. Whitt. 2000. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J. Clin. Microbiol. 38:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Woodland, J. C., M. M. McDowell, and J. T. Richards. 1943. Bullis fever (Lone Star tick fever-tick fever). JAMA 122:1156-1160. [Google Scholar]
- 284.Yevich, S. J., J. L. Sanchez, R. F. DeFraites, C. C. Rives, J. E. Dawson, I. J. Uhaa, B. J. Johnson, and D. B. Fishbein. 1995. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J. Infect. Dis. 171:1266-1273. [DOI] [PubMed] [Google Scholar]
- 285.Yu, X., P. Brouqui, J. S. Dumler, and D. Raoult. 1993. Detection of Ehrlichia chaffeensis in human tissue by using a species-specific monoclonal antibody. J. Clin. Microbiol. 31:3284-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yu, X., J. W. McBride, X. Zhang, and D. H. Walker. 2000. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene 248:59-68. [DOI] [PubMed] [Google Scholar]
- 287.Yu, X., J. F. Piesman, J. G. Olson, and D. H. Walker. 1997. Short report: geographic distribution of different genetic types of Ehrlichia chaffeensis. Am. J. Trop. Med. Hyg. 56:679-680. [DOI] [PubMed] [Google Scholar]
- 288.Yu, X. J., P. Crocquet-Valdes, L. C. Cullman, and D. H. Walker. 1996. The recombinant 120-kilodalton protein of Ehrlichia chaffeensis, a potential diagnostic tool. J. Clin. Microbiol. 34:2853-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yu, X. J., P. Crocquet-Valdes, and D. H. Walker. 1997. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene 184:149-154. [DOI] [PubMed] [Google Scholar]
- 290.Yu, X. J., P. A. Crocquet-Valdes, L. C. Cullman, V. L. Popov, and D. H. Walker. 1999. Comparison of Ehrlichia chaffeensis recombinant proteins for serologic diagnosis of human monocytotropic ehrlichiosis. J. Clin. Microbiol. 37:2568-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yu, X. J., J. W. McBride, and D. H. Walker. 1999. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yu, X. J., and D. H. Walker. 1997. Sequence and characterization of an Ehrlichia chaffeensis gene encoding 314 amino acids highly homologous to the NAD A enzyme. FEMS Microbiol. Lett. 154:53-58. [DOI] [PubMed] [Google Scholar]