Abstract
Of the many types of human papillomavirus (HPV), more than 30 infect the genital tract. The association between certain oncogenic (high-risk) strains of HPV and cervical cancer is well established. Although HPV is essential to the transformation of cervical epithelial cells, it is not sufficient, and a variety of cofactors and molecular events influence whether cervical cancer will develop. Early detection and treatment of precancerous lesions can prevent progression to cervical cancer. Identification of precancerous lesions has been primarily by cytologic screening of cervical cells. Cellular abnormalities, however, may be missed or may not be sufficiently distinct, and a portion of patients with borderline or mildly dyskaryotic cytomorphology will have higher-grade disease identified by subsequent colposcopy and biopsy. Sensitive and specific molecular techniques that detect HPV DNA and distinguish high-risk HPV types from low-risk HPV types have been introduced as an adjunct to cytology. Earlier detection of high-risk HPV types may improve triage, treatment, and follow-up in infected patients. Currently, the clearest role for HPV DNA testing is to improve diagnostic accuracy and limit unnecessary colposcopy in patients with borderline or mildly abnormal cytologic test results.
INTRODUCTION
Human papillomavirus (HPV) is one of the most common causes of sexually transmitted disease in both men and women worldwide and is thought to be the most common sexually transmitted viral disease in the United States. Genital HPV infection is not a reportable disease, so actual incidence and prevalence figures are not known; however, it is estimated that the incidence of new infections in the United States ranges from 1 million to 5.5 million per year, and the prevalence is estimated to be as high as 20 million (20). HPV continues to be an important topic, as rates of infection appear to continue to be rapidly increasing.
Papillomaviruses are ubiquitous and have been detected in a wide variety of animals as well as in humans and are specific for their respective hosts. More than 200 types of HPV have been recognized on the basis of DNA sequence data showing genomic differences. Eighty-five HPV genotypes are well characterized. An additional 120 isolates are partially characterized potential new genotypes (133). HPVs can infect basal epithelial cells of the skin or inner lining of tissues and are categorized as cutaneous types or mucosal types. Cutaneous types of HPV are epidermitrophic and target the skin of the hands and feet. Mucosal types infect the lining of the mouth, throat, respiratory tract, or anogenital epithelium. Based on their association with cervical cancer and precursor lesions, HPVs can also be grouped to high-risk and low-risk HPV types. Low-risk HPV types include types 6, 11, 42, 43, and 44. High-risk HPV types include types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70. Included in the high-risk group are some HPV types that are less frequently found in cancers but are often found in squamous intraepithelial lesions (SILs) (Table 1). Some authors refer to these HPV types as intermediate-risk. Low-risk subtypes are also occasionally found in cervical carcinomas.
TABLE 1.
HPV type and disease associationa
| Disease | HPV typeb |
|---|---|
| Plantar warts | 1, 2, 4, 63 |
| Common warts | 2, 1, 7, 4, 26, 27, 29, 41, 57, 65, 77, 1, 3, 4, 10, 28 |
| Flat warts | 3, 10, 26, 27, 28, 38, 41, 49, 75, 76 |
| Other cutaneous lesions (e.g., epidermoid cysts, laryngeal carcinoma) | 6, 11, 16, 30, 33, 36, 37, 38, 41, 48, 60, 72, 73 |
| Epidermodysplasia verruciformis | 2, 3, 10, 5, 8, 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, 36, 37, 38, 47, 50 |
| Recurrent respiratory papillomatosis | 6, 11 |
| Focal epithelial hyperplasia of Heck | 13, 32 |
| Conjunctival papillomas/carcinomas | 6, 11, 16 |
| Condyloma acuminata (genital warts) | 6, 11, 30, 42, 43, 45, 51, 54, 55, 70 |
| Cervical intraepithelial neoplasia | |
| Unspecified | 30, 34, 39, 40, 53, 57, 59, 61, 62, 64, 66, 67, 68, 69 |
| Low risk | 6, 11, 16, 18, 31, 33, 35, 42, 43, 44, 45, 51, 52, 74 |
| High risk | 16, 18, 6, 11, 31, 34, 33, 35, 39, 42, 44, 45, 51, 52, 56, 58, 66 |
| Cervical carcinoma | 16, 18, 31, 45, 33, 35, 39, 51, 52, 56, 58, 66, 68, 70 |
HPV is associated with a variety of clinical conditions that range from innocuous lesions to cancer (Table 1). Most HPV infections are benign. HPV was first recognized as the cause of cutaneous warts (plantar warts, common warts, flat warts) on the hands and feet. Warts are areas of hypertrophied skin filled with keratin and are considered to be mainly a cosmetic nuisance; generally, they resolve spontaneously within 1 to 5 years. Strains that target the face make skin cancer more likely. Other strains that grow primarily in the lining of the mouth produce small elevated nodules that can develop into fatal squamous cell cancers. Focal epithelial hyperplasia of the oral cavity (Heck's disease) is caused predominantly by HPV-13 and tends to regress spontaneously. Epidermodysplasia veruciformis, a rare genetic disease with HPV-associated warts on the trunk and upper extremities, can develop into invasive squamous cell carcinomas. Conjunctival papillomas and carcinomas associated with HPV have been described. Recurrent respiratory papillomatosis is primarily a disease of the larynx in young children at a median age of 3 years but can also occur in adults. Because a high percentage of mothers have a history of HPV and because similar HPV types cause anogenital warts and recurrent respiratory papillomatosis, the infection in young children is thought to be acquired by passage through an infected birth canal. There is also some suggestion that the disease may be acquired in utero since cases have been documented at birth after cesarean section. Respiratory tract lesions may undergo malignant transformation.
Cervical cancer is the third most common cancer in women in the United States, preceded by skin cancer and breast cancer as the first and second most common causes, respectively (93). In developing countries, cervical cancer is often the most common cancer in women and may constitute up to 25% of all female cancers (45). Cervical cancer is preceded only by breast cancer as the most common cause of death from cancer in women worldwide (54). The link between genital HPV infections and cervical cancer was first demonstrated in the early 1980s by Harold zur Hausen, a German virologist. Since then, the link between HPV and cervical squamous cell carcinoma has become well established. The magnitude of the association between HPV and cervical squamous cell carcinoma is higher than that for the association between smoking and lung cancer (37). In 1996, the World Health Association, along with the European Research Organization on Genital Infection and Neoplasia and the National Institutes of Health Consensus Conference on Cervical Cancer, recognized HPV as an important cause of cervical cancer. Scientists have identified about 30 HPV types that are spread through sexual contact and infect primarily the cervix, vagina, vulva, penis, and anus. Of these, four are most often found within the malignant cells of cervical cancers, with type 16 accounting for about half of the cases in the United States and Europe and types 18, 31, and 45 accounting for an additional 25 to 30% of cases (45). HPV has been implicated in 99.7% of cervical squamous cell cancer cases worldwide (124). Adenocarcinomas of the cervix are also related to HPV, but the correlation is less pronounced and is age dependent (3). In women younger than 40 years, HPV was present in 89% of adenocarcinomas, whereas in women aged 60 years and older, HPV was observed in only 43%.
BASIC VIROLOGY
Papillomaviruses are members of the Papovaviridae family, which also includes polyomavirus and simian vacuolating virus. HPV is a relatively small, nonenveloped virus, 55 nm in diameter. It has an icosahedral capsid composed of 72 capsomers, which contain at least two capsid proteins, L1 and L2. Each capsomer is a pentamer of the major capsid protein, L1 (8). Each virion capsid contains several copies (about 12 per virion) of the minor capsid protein, L2 (98). The virus is said to somewhat resemble a golf ball when viewed by electron microscopy. The HPV genome consists of a single molecule of double-stranded, circular DNA containing approximately 7,900 bp associated with histones (34). All open reading frame (ORF) protein-coding sequences are restricted to one strand. The genome is functionally divided into three regions (Fig. 1): (i) The first is a noncoding upstream regulatory region of 400 to 1,000 bp, which has been referred to as the noncoding region, the long control region (LCR), or the upper regulatory region. This region contains the p97 core promoter along with enhancer and silencer sequences that regulate DNA replication by controlling the transcription of the ORFs. This region also contains the highest degree of variation in the viral genome (6). (ii) The second is an early region, consisting of ORFs E1, E2, E4, E5, E6, and E7, which are involved in viral replication and oncogenesis. (iii) The third is a late region, which encodes the L1 and L2 structural proteins for the viral capsid.
FIG. 1.
Schematic representation of the circular HPV DNA genome.
By definition, the nucleotide sequences of the E6, E7, and L1 ORFs of a new HPV type should be no more than 90% homologous to the corresponding sequences of known HPV types (117). HPVs have further been classified into subtypes, when they have 90 to 98% sequence similarity to the corresponding type and variants when they show no more than 98% sequence homology to the prototype. Some naturally occurring variants have different biological and biochemical properties important in cancer risk.
EPIDEMIOLOGY
Transmission of HPV occurs primarily by skin-to-skin contact. Epidemiologic studies clearly indicate that the risk of contracting genital HPV infection and cervical cancer is influenced by sexual activity. HPV is very resistant to heat and desiccation, and nonsexual transmission via fomites can also occur, such as by prolonged exposure to shared contaminated clothing (94). An individual is at greater risk of becoming infected with HPV if he or she has had multiple sexual partners at any time or is the partner of someone who has had multiple sexual partners. Sexual activity at an early age also places an individual at increased risk, as does a history of other sexually transmitted diseases, genital warts, abnormal Pap smears, or cervical or penile cancer in an individual or sexual partner. Condom usage may not adequately protect individuals from exposure to HPV since HPV can be transmitted by contact with infected labial, scrotal, or anal tissues that are not protected by a condom.
In addition to sexual activity, age is an important determinant of risk of HPV infection (1, 18). Most cervical cancers arise at the squamocolumnar junction between the columnar epithelium of the endocervix and the squamous epithelium of the ectocervix. At this site, there are continuous metaplastic changes. The greatest risk of HPV infection coincides with greatest metaplastic activity. Greatest metaplastic activity occurs at puberty and first pregnancy and declines after menopause. HPV infection is most common in sexually active young women, 18 to 30 years of age. There is a sharp decrease in prevalence after 30 years of age. However, cervical cancer is more common in women older than 35 years, suggesting infection at a younger age and slow progression to cancer. Persistence of infection is more common with the high-risk oncogenic HPV types and is an important determinant in the development of cervical cancer.
Detection of high-risk HPV is necessary but may not be sufficient for the development of cervical cancer. Studies suggest that whether a woman will develop cervical cancer depends on a variety of additional factors that act in concert with cancer-associated HPV types in the process that leads to cervical cancer. The primary immune response to HPV infection is cell mediated; therefore, conditions that impair cell-mediated immunity such as renal transplantation or human immunodeficiency virus disease increase the risk of acquisition and progression of HPV (19, 28, 117). The upstream regulatory region of HPV contains sequences similar to the glucocorticoid responsive elements that are inducible by steroid hormones such as progesterone (the active component of oral contraceptives) and dexamethasone. Long-term use of oral contraceptives is a significant risk factor for high-grade cervical disease according to some studies but not in others (1, 16). Cervical cancer risk also seems to be independently influenced by other variables including current smoking and parity (1). Local immune suppression induced by smoking and the mutagenic activity of cigarette components have been demonstrated in cervical cells and may contribute to persistence of HPV or to malignant transformation similar to that seen in the lung (87, 121, 128). It appears that smoking is the most important risk factor independent of HPV infection for higher grades of cervical disease (1). Smoking shows little or no relationship to low grades of cervical disease. Multiple pregnancies were a significant independent risk factor among women with histopathologic evidence of HPV infection in biopsy specimens and among women with moderate- to high-grade cervical disease. In women with mild cervical disease, only the presence of high-risk HPV infection was a significant risk factor. Other factors such as alcohol consumption and diet have not been well established.
There has been some suggestion that sexually transmitted viruses may serve as cofactors in the development of cervical cancer. It has been postulated that coinfection with herpes simplex virus type 2 may play a role in the initiation of cervical cancer (131). Cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), and HHV-7 have also been detected in the cervix. Coinfection offers the opportunity for these viruses to interact with HPV. Putative oncogenes and transforming factors have been proposed for CMV and HHV-6, but epidemiologic and in vitro data are not conclusive of a causal association with cervical cancer (23,89,95,102). Recent studies using PCR to detect CMV, HHV-6, and HHV-7 in women with abnormal cervical cytologic test results indicate that these viruses are only bystanders rather than cofactors in the development of cervical cancer (21).
It has been proposed that the viral load correlates directly with the severity of disease. Studies using quantitative type-specific PCR for high-risk HPV-16, -18, -31, -33, and -45 and low-risk HPV-6 and -11 have shown that HPV-16 can reach much higher viral loads than the other types and that only for HPV-16 does increased viral load correlate with increased severity of cervical disease (111, 130). High-risk HPVs of all types are able to induce malignant tumors even when they are present at low levels (132).
An important emerging factor in the development of cervical neoplasia is the role of HPV variants (39). HPV variants differ in biological and chemical properties and pathogenicity (27, 120). Based on sequence variation of the L1, L2, and LCR regions of HPV-16, five naturally occurring phylogenetic clusters have been defined for HPV-16: European (E), Asian (As), Asian-American (AA), African-1 (Af1), and African-2 (Af2). Intratypic sequence variation has also been found in the E2, E4, E5, E6, and E7 genes of HPV-16. Since the LCR contains several E2 binding sites in addition to binding sites for several transcription factors, nucleotide sequence variation in the LCR, E2, E6, and E7 genes may be of functional significance. The oncogenicity of specific HPV variants appears to vary geographically and also with the ethnic origin of the population studied. One study suggested that because of increased transcriptional activity and changes in the progesterone response elements, Asian-American variants might have enhanced oncogenic activity compared to European isolates (120). European HPV variants with point mutations in the LCR binding sites have enhanced transcriptional activity compared to the European prototype (32). In a large clinical study of 10,000 women in Costa Rica, the European HPV-16 prototype and three variants were seen (46). The most common variant, EP[a], contained a single point mutation and was not associated with disease. A second variant, EL, contained single point mutations at locations other than that of EP[a] and was associated with normal cytology and some high-grade squamous intraepithelial lesions (HSILs). Another variant, NE, contained multiple substitutions within the LCR and was associated with HSIL and cancer at rates much higher than expected. Mechansims for this association are not known, and since transformation is a complex process, mutations could directly affect transcription by increasing the activity of promoters, could affect other regions of the viral genome, or could affect the relationship between HPV variants and nonviral factors such as HLA and p53. The study also reported a statistically significant association of the NE variant with the presence of HLA class II alleles. Other studies have also reported the association of HLA class II alleles with cervical HPV disease; however, these associations appear to be relatively weak (2, 5, 13). Intratypic sequence variation has also been analyzed for other high- and intermediate-risk HPV types and for low-risk types 6 and 11. Sequence variation in low-risk types was not associated with increased activity of the promoters responsible for E6 and E7 protein expression (39).
A genetic predisposition to colorectal cancer, lung cancer, and melanoma has long been recognized and is widely accepted. Genetic predisposition was found to be even a greater component in cervical cancer when the same method of analysis was used (73). Genetic heritability was found to account for 27% of the effect of underlying factors for tumor development. Heritability could affect many factors contributing to the development of cervical cancer, including susceptibility to HPV infection, ability to clear HPV infection, and time to development of disease. The effect of shared familial environment was shown to be small at 2% and was found only between sisters and not between mother and daughter.
Studies have shown that infections with multiple types of HPV can occur (52, 59, 88). Multiple-infection rates up to 39% have been seen. The presence of multiple HPV genotypes tended to increase with the severity of cervical disease. Multiple genotypes, usually with at least one type classified as high risk, were found in 11.8% of patients with normal cytology or ASCUS (see below) and in 34.5% of patients with mild or moderate dyskaryosis (59). However, the prevalence of multiple genotypes was much lower in cervical carcinoma tissue samples (4.4%) (59). The majority of multiple infections contain two HPV genotypes, but samples with three, four, or five genotypes were also seen (52, 88).
Coinfection with adeno-associated virus is associated with a significantly reduced risk of cervical neoplasia (26). Adeno-associated virus replication gene product Rep78 disrupts the transcription of the HPV-16 E6 and E7 oncogenes by interfering with the binding of the TATA binding protein to the p97 core promoter in the LCR region of the HPV genome (110). This interference does not require HPV gene products.
CLINICAL MANIFESTATIONS OF SEXUALLY TRANSMITTED INFECTIONS
Sexually transmitted HPV infection leads to one of three possible results, depending largely on which HPV type is involved. (i) The first is anogenital warts (condyloma acuminatum) on or around the genitals and anus in both men and women. Anogenital warts are generally associated with HPV-6 and HPV-11 and do not lead to cancer. Most are asymptomatic and may spontaneously resolve in 3 to 4 months, remain the same, or increase in size and number. Treatment options include ablation, excision, or topical agents such as 0.5% podophyllin (Podocon) or 5.0% imiquimod (Aldara). When anogenital warts are red-brown pigmented, they should be subjected to biopsy since they may actually be Bowenoid papulosis which is caused by HPV-16 or HPV-18 and histologically shows condylomatous architecture with intraepithelial neoplasia (83). These lesions may rarely evolve into carcinoma in situ (30, 83).
(ii) The second result is latent or inactive infection, in which few people know they are infected since noticeable symptoms are seldom produced and the infected area remains cytologically normal. HPV DNA is present in approximately 10% of women with cytologically normal cervical epithelium. The HPV DNA detected was primarily of low-risk HPV-6, -11, and others (Table 1).
(iii) The third result is active infection, which is associated with high-risk HPV types in which the virus causes changes in infected cells which may result in penile, urethral, bladder, vaginal, vulvar, or cervical intraepithelial neoplasia. High-risk HPV types (Table 1) include types associated with high-grade lesions and cervical cancers and types identified as intermediate risk that are less commonly represented in cancers but are frequently seen in SIL (9, 10, 15, 38, 42, 65, 66, 67, 68, 69, 70, 71, 72, 76, 79, 80, 82, 90, 91, 105, 122). These infections can lead to cervical cancer. Prospective studies have shown that 15 to 28% of women in whom HPV DNA was detected developed SIL within 2 years, compared to only 1 to 3% of women in whom HPV DNA was not detected. In particular, the risk of progression for HPV-16 and -18 was greater (approximately 40%) than for other HPV types.
PATHOGENESIS
Basal cells of stratified squamous epithelium may be infected by HPV. Other cell types appear to be relatively resistant. Attempts to reproduce HPV replication in standard cell culture have been unsuccessful, largely because replication is linked to the differentiation process of keratinocytes and because of the difficulty in recreating the stratified structure of the epithelium in vitro. Some HPVs have been successfully propagated using either xenograft biological models or organotypic raft culture systems. These systems have been important tools for the study of HPV replication and host cell interactions. HPV-6, -11, -16, and -40 have been successfully propagated in human skin and cervical tissues that have been grafted into athymic (nude) or severe combined immunodeficiency (SCID) mice (36, 114). Several epithelial cell lines derived from HPV-infected patients, W12E cells harboring HPV-16 and CIN612-9E cells harboring HPV-31b, have been successfully cultured on rafts (11, 50, 53, 77). HPV raft cultures are reproducible systems that re-create in vitro the morphologic and physiologic differentiation process of epithelial cells and allow HPV replication. In HPV raft cultures, epithelial cell lines containing latent HPV are grown with a collagen matrix maintained on a solid support. The epithelial cells form stratified layers and develop characteristics of normal terminal differentiation. Raft culture systems are prepared by creating a base composed of bovine tendon type 1 collagen. Human foreskin fibroblasts mixed with collagen are placed on the collagen base and allowed to contract. Epithelial cells are precultured on Swiss 3T3 fibroblast feeders and plated on the collagen plug. After 4 days of incubation, the raft is placed on a cotton pad and fed with cornification medium containing 12-O-tetradecanoyl phorbol-13-acetate (TPA) to induce differentiation. Differentiating suprabasal cells permit the replication of HPV.
To study the effects of mutations in the HPV genome on the replication cycle, naturally infected or experimentally transfected human foreskin keratinocytes have been used. This method is limited because of the limited life span of human foreskin keratinocytes in cell culture. A spontaneously immortalized human foreskin keratinocyte cell line, BC-1-Ep/SL, has been transfected with molecularly cloned HPV-16 and placed in a raft culture system for induction of terminal differentiation (36). This system supports the full HPV life cycle.
It is assumed that the HPV replication cycle begins with entry of the virus into the cells of the stratum germinativum (basal layer) of the epithelium. It is likely that HPV infection of the basal layer requires mild abrasion or microtrauma of the epidermis. α6-Integrin has been proposed as the epithelial cell receptor for HPV-6 but is not obligatory for attachment of HPV-11 or HPV-33 (33, 41, 55). HPV-16 and HPV-33, like many other viruses, attach to host cells via cell surface heparan sulfate (41). A secondary receptor or stabilizing proteoglycans may also be involved in HPV attachment (41). The cellular factors necessary for virion uptake are not known. Once inside the host cell, HPV DNA replicates as the basal cells differentiate and progress to the surface of the epithelium. In the basal layers, viral replication is considered to be nonproductive and the virus establishes itself as a low-copy-number episome by using the host DNA replication machinery to synthesize its DNA on average once per cell cycle (35, 40). In the differentiated keratinocytes of the suprabasal layers of the epithelium, the virus switches to a rolling-circle mode of DNA replication, amplifies its DNA to high copy number, synthesizes capsid proteins, and causes viral assembly to occur (36).
Since HPVs encode only 8 to 10 proteins, they must employ host cell factors to regulate viral transcription and replication. HPV replication begins with host cell factors which interact with the LCR region of the HPV genome and begin transcription of the viral E6 and E7 genes. The E6 and E7 gene products deregulate the host cell growth cycle by binding and inactivating tumor suppressor proteins, cell cyclins, and cyclin-dependent kinases (Fig. 2) (113). The function of the E6 and E7 gene products during a productive HPV infection is to subvert the cell growth-regulatory pathways and modify the cellular environment in order to facilitate viral replication in a cell that is terminally differentiated and has exited the cell cycle (113). Cell growth is regulated largely by two cellular proteins, the tumor suppressor protein, p53, and the retinoblastoma gene product, pRB. Unlike in many other cancers, the p53 in cervical cancer is usually wild type and is not mutated (116). The HPV E6 gene product binds to p53 and targets it for rapid degradation via a cellular ubiquitin ligase (116). This degradation has the same effect as an inactivating mutation (116). As a consequence, the normal activities of p53 which govern G1 arrest, apoptosis, and DNA repair are abrogated. Low-risk HPV E6 proteins do not bind p53 at detectable levels and have no effect on p53 stability in vitro. The HPV E6 proteins also can form complexes with at least six other cellular proteins which are not well characterized. The HPV E7 gene product binds to the hypophosphorylated form of the RB family of proteins. This binding disrupts the complex between pRB and the cellular transcription factor E2F-1, resulting in the liberation of E2F-1, which allows the transcription of genes whose products are required for the cell to enter the S phase of the cell cycle. The E7 gene product can also associate with other mitotically interactive cellular proteins such as cyclin E (113). The outcome is stimulation of cellular DNA synthesis and cell proliferation. The E7 protein from low-risk HPV types binds pRB with decreased affinity. Next, the E5 gene product induces an increase in mitogen-activated protein kinase activity, thereby enhancing cellular responses to growth and differentiation factors. This results in continuous proliferation and delayed differentiation of the host cell. The E1 and E2 gene products are synthesized next. The E2 gene product is a DNA binding protein which blocks transcription of the E6 and E7 genes and permits the E1 gene product to bind to the viral origin of replication located within the LCR. This binding initiates replication of the viral genome as extrachromosomal elements in the S phase of the cell cycle. Genome copy number is maintained at a constant level in these cells, and a low level of transcripts is expressed. The E2-mediated down-regulation of E6 and E7 transcription results in the release of the p53 and pRB proteins, and the normal differentiation process of the host cell is allowed to continue. Then, a putative late promoter activates the capsid genes, L1 and L2. Viral particles are assembled in the nucleus, and complete virions are released as the cornified layers of the epithelium are shed. The E4 gene product plays a role in the maturation and release of papillomavirus particles. The process does not appear to be cytolytic. In the replication process, viral DNA becomes established throughout the entire thickness of the epithelium but intact virions are found only in the upper layers of the tissue. In warts or condylomata, viral replication is associated with proliferation of all epidermal layers except the basal layer. This leads to acanthosis, parakeratosis, hyperkeratosis, and deepening of rete ridges, creating the typical papillomatous cytoarchitecture seen histologically. Some infected cells transform into koilocytes, which are large, usually polygonal, squamous cells with a shrunken nucleus inside a cytoplasmic vacuole (Fig. 3). Excessive proliferation of cells in the basal layer accompanied by large number of mitoses, some abnormal, is a feature of malignant and premalignant disease.
FIG. 2.
Pathogenesis of oncogenic HPV. HPV E6 and E7 genes encode multifunctional proteins that bind primarily to cellular p53 and pRB proteins, disrupt their functions, and alter cell cycle regulatory pathways, leading to cellular transformation.
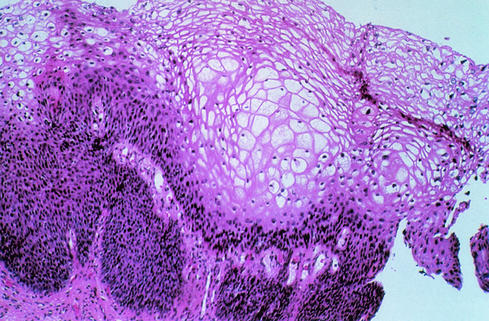
FIG. 3.
Cervical condyloma showing proliferation of the suprabasal epithelial layers and koilocytic atypia, consisting of nuclear pyknosis and a well-defined perinuclear cavity associated with peripherally thickened cytoplasm. Reprinted with permission of S. Kini.
Cervical cancer is one of the best understood examples of how viral infection can lead to malignancy. Infection with high-risk HPV types interferes with the function of cell proteins and also with the expression of cellular gene products. Microarray (gene chip) analysis of cells infected with HPV-31 has shown that 178 cellular genes are up-regulated and 150 cellular genes are down-regulated by HPV (22). The genes that are down-regulated are primarily those involved in regulation of cell growth, some keratinocyte-specific genes, and interferon (IFN)-responsive genes. High-risk HPV types can be distinguished from other HPV types largely by the structure and function of the E6 and E7 gene products. In benign lesions caused by HPV, viral DNA is located extrachromosomally in the nucleus. In high-grade intraepithelial neoplasias and cancers, HPV DNA is generally integrated into the host genome. In some cases, episomal and integrated HPV DNAs are carried simultaneously in the host cell (129). Integration of HPV DNA specifically disrupts or deletes the E2 ORF, which results in loss of its expression (129). This interferes with the function of E2, which normally down-regulates the transcription of the E6 and E7 genes and leads to an increased expression of E6 and E7. In high-risk HPV types, the E6 and E7 proteins have a high affinity for p53 and pRB. Binding disrupts the normal function of these cellular proteins and can give rise to an increased proliferation rate and genomic instability. As a consequence, the host cell accumulates more and more damaged DNA that cannot be repaired. Efficient immortalization of keratinocytes requires the cooperation of the E6 and E7 gene proteins; however, the E7 gene product alone at high levels can immortalize host cells (44). Eventually, mutations accumulate that lead to fully transformed cancerous cells. In addition to the effects of activated oncogenes and chromosome instability, potential mechanisms contributing to transformation include methylation of viral and cellular DNA, telomerase activation, and hormonal and immunogenetic factors. Progression to cancer generally takes place over a period of 10 to 20 years. Some lesions become cancerous more rapidly, sometimes within a year or two (48).
DIAGNOSIS
Decades of studies have confirmed that cervical infection by high-risk HPV types is a precursor event to cervical cancer. The natural history of cervical cancer as a continuous single disease process progressing gradually from mild cervical cervical intraepithelial neoplasia (CIN1) to more severe degrees of neoplasia and microinvasive lesions (CIN2 or CIN3) and finally to invasive disease has been the basis for diagnosis, therapeutic measures, and secondary preventive strategies (48). It is plausible that high-risk HPV infection occurs early in life, may persist, and, in association with other factors promoting cell transformation, may lead to a gradual progression to more severe disease. Some investigators have correlated HPV type with different degrees of CIN and have suggested that CIN1 and CIN2-CIN3 are distinct processes, with CIN1 indicating a self-limited sexually transmitted HPV infection and CIN2 or CIN3 being the only true cervical cancer precursor (58,125). Most mild and moderate dysplasias are more likely to regress than progress (48). The risk of progression of mild dysplasia to severe dysplasia was only 1% per year, while the risk of progression of moderate dysplasia to severe dysplasia was 16% within 2 years and 25% within 5 years. Nonetheless, it is agreed that early detection and subsequent early treatment of HPV in precancerous lesions can prevent progression to cancer (109). As mentioned above, HPV cannot be cultured in the laboratory from clinical specimens and immunologic assays are not adequate for detection of HPV infections. The primary diagnostic tools have been cytology and histology. Recently, molecular methods to detect HPV DNA sequences in clinical specimens have been introduced.
Conventional Cytology
The primary method for detection of high-risk HPV is still the Papanicolaou-stained (Pap) smear. This method was named for pathologist George Papanicolaou, who introduced the method in 1949 before the cause of cervical cancer was known (86). Since its introduction, the Pap smear has helped reduce cervical cancer incidence and mortality rates by roughly half to two-thirds (61). The Pap smear is a screening tool that looks for changes in cells of the transformation zone of the cervix. Often these changes are caused by HPV.
The Pap smear reporting classification has evolved and been refined over time. The current reporting system is the Bethesda System (Table 2), which was introduced in 1988, amended in 1991 to replace the CIN System, and updated again in 1999 (17, 108). The CIN System is based on tissue architecture and was introduced in 1973 to promote the concept of a disease continuum from precursor lesions to invasive cancer (92). The Bethesda System was developed to reflect an advanced understanding of cervical neoplasia and to introduce uniform descriptive diagnostic histologic terminology. Inclusion of a statement regarding the adequacy of the specimen as an integral part of the report was also a significant innovation of the Bethesda System. The Bethesda System was modified in 1991 to reflect actual laboratory and clinical experience after its implementation. It was modified again in 2001, taking into account increased utilization of new cervical screening technologies, adjunctive molecular tests, lessons learned from litigation, and a better understanding of the biology of cervical neoplasia (108). The Bethesda System 2001 classifies squamous cell abnormalities into four categories: (i) ASC (atypical squamous cells), (ii) LSIL (low-grade squamous intraepithelial lesions), (iii) HSIL (high-grade squamous intraepithelial lesions), and (iv) squamous cell carcinoma (Table 2). The ASC category was termed “atypical cells of undetermined significance (ASCUS)” in the previous version of the Bethesda System and is considered to be a category for reporting borderline or equivocal results. In the previous version of the Bethesda System, pathologists were encouraged to qualify ASCUS with respect to whether a reactive process or SIL was favored. Many laboratories appended comments such as “favor SIL,” “favor repair,” “favor a high-grade lesion,” “favor a low-grade lesion,” and “ASCUS, not otherwise specified.” This reporting was confusing to clinicians. In attempt to provide clearer terminology, the “ASCUS favor repair” comment has been eliminated in the Bethesda System 2001, and these cases are now called “negative.” The new ASC category contains two subcategories: the “atypical squamous cells of undetermined significance (ASC-US)” subcategory includes lesions that have cellular abnormalities suggestive of SIL, and the “atypical squamous cells, cannot exclude HSIL (ASC-H)” subcategory was separated out because it was felt that most of these cases would be referred to colposcopy. The LSIL and HSIL categories present in the previous version of the Bethesda System were retained. Low-grade lesions (LSIL) include mild CIN and other HPV-associated lesions generally considered to be due to transient HPV infection. High-grade lesions (HSIL) consist of moderate and severe cervical intraepithelial dysplasia and carcinoma in situ. There are some lesions in the HSIL category that are suspicious for invasion but are not definitive. These cases are reported as “HSIL, cellular features suspicious for invasion”. The invasive “squamous cell carcinoma” category was not changed.
TABLE 2.
The Bethesda Classification System for cervical squamous cell dysplasia
| Bethesda System 1999 | Bethesda System 1991 | CIN System | Interpretation |
|---|---|---|---|
| Negative for intraepithelial lesions or malignancy | Within normal limits | Normal | No abnormal cells |
| ASC | |||
| ASC-US (atypical squamous cells of undetermined significance) | ASCUS (atypical squamous cells of undetermined significance) | Squamous cells with abnormalities greater than those attributed to reactive changes but that do not meet the criteria for a squamous intraepithelial lesion | |
| ASC-H (atypical squamous cells, cannot exclude HSIL) | |||
| LSIL (low-grade squamous intraepithelial lesions) | LSIL (low-grade squamous intraepithelial lesions) | CIN 1 | Mildly abnormal cells; changes are almost always due to HPV |
| HSIL (high-grade squamous intraepithelial lesions) with features suspicious for invasion (if invasion is suspected) | HSIL (high-grade squamous intraepithelial lesions) | CIN2/3 | Moderately to severely abnormal squamous cells |
| Carcinoma | Carcinoma | Invasive squamous cell carcinoma Invasive glandular cell carcinoma (adenocarcinoma) | The possibility of cancer is high enough to warrant immediate evaluation but does not mean that the patient definitely has cancer |
The Pap smear procedure has some limitations. Inadequate samples constitute about 8% of specimens received. False-negative rates as high as 20 to 30% have been reported. False-negative results can occur from clumping of cells when the cells are not spread evenly and uniformly on the microscope slide. Sometimes, other contents of the cervical specimen such as blood, bacteria, or yeast contaminate the sample and prevent the detection of abnormal cells. If exposed to air too long before being fixed on the slide, cervical cells can become distorted. Human error is probably the primary threat to accurate interpretation. The average Pap smear slide contains 50,000 to 300,000 cells that must be examined. If the sample contains only a few abnormal cells within a crowded background of healthy cells, the abnormal cells can be missed, particularly by overworked readers. In 1988, the Clinical Laboratory Improvement Act (CLIA) established national guidelines that restricted technicians from reading more than 100 slides per day (25). Some experts think that this number is still too large. Also, CLIA mandated a manual rescreening of 10% of negative satisfactory smears to reduce the number of false-negative results.
Monolayer Cytology
New methods of collection and processing of specimens for Pap smears have recently been developed to help reduce the number of false-negative results. In these methods, the specimen is collected in a preservative solution rather than being spread directly on the microscope slide by hand. Cellular structure is better preserved because the cells are immediately fixed. In addition, a cervical brush is used to collect the specimen, which provides almost twice as many epithelial cells as do other collection devices (51). Slides are prepared under the control of the Cytology Laboratory, avoiding uneven manual smearing. The uniform monolayer created by these methods is easier for a technician to read, and the process prevents drying artifacts and removes most contaminating mucus, protein, red blood cells, bacteria, and yeast (Figure 4). There are currently two Food and Drug Administration (FDA)-approved liquid-based monolayer cytology methods: the PrepStain system (formerly the AutoCyte PREP system) (TriPath Imaging Inc., Burlington, N.C.) and the ThinPrep Pap Smear method (Cytyc Corp., Boxborough, Mass.). The methods used in the preparation of the monolayer slides differ somewhat, but the underlying principles of the two systems are similar. In the PrepStain system, cervical samples are collected in an ethanol-based preservative solution. The preserved sample is enriched using density gradient centrifugation to remove inflammatory cells and nondiagnostic debris. The enriched cellular sample is sedimented by gravity dispersion onto an adhesive-coated microscope slide within a 13-mm diameter circle. The slide is automatically stained with a modified Papanicolaou stain, using a separate stain for each slide, thereby eliminating potential carryover and providing consistent staining. In the ThinPrep Pap Smear method, the cell sample is collected in a buffered alcohol preservative solution. The preserved sample is mixed and gently dispersed by high-speed rotation to ensure uniform sampling of the material removed from the cervix. A vacuum is applied to draw the suspension through a polycarbonate filter. A microprocessor controls the number of cells deposited on the filter. The filtered cells are then automatically transferred in a 20-mm monolayer by touching the filter to a glass microscope slide. Papanicolaou staining is then performed manually using standard laboratory practices.
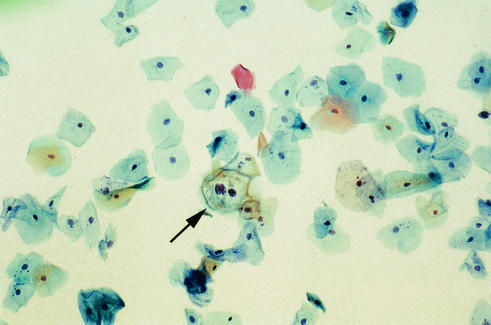
FIG. 4.
ThinPrep Pap Smear showing abnormal squamous cells with HPV cytopathic effect (arrow), consistent with LSIL.
Monolayer cytology methods add to the cost of a standard Pap smear, but more than 50 peer-reviewed publications promote the use of the these methods to improve the detection of precancerous lesions. In several of these publications, split samples collected using cervical brushes were used to compare monolayer cytology to conventional Pap smear cytology. Overall, the results showed statistically significant improvement in the diagnostic sensitivity of monolayer cytology in all categories of disease, with increased detection of epithelial cell abnormalities from 4 to 117% depending on the patient population (7, 49, 63, 109, 119). Specimen adequacy was greatly improved in all studies, with an 11% increase in the number of satisfactory slides and a 29% reduction in the number of satisfactory but limited slides reported in one study (63). When monolayer cytology and conventional Pap smear cytology are compared to results of “gold standard” colposcopically directed biopsies, monolayer cytology is significantly better at predicting the presence of dysplasia (101).
The FDA has also approved two new devices, the AutoPap 300QC (NeoPath, Redmond, Wash.) and the PapNet (Neuromedical Systems, Suffern, N.Y.), which are designed to help ensure consistent and objective evaluation of Pap smears without fatigue. These are computerized systems which display potentially abnormal cells on a screen for review and analysis. Either conventionally prepared or monolayer Pap smear slides can be screened by using these computer-assisted systems. The AutoPap 300QC has been FDA approved for both primary screening and rescreening of Pap smears. The PapNet system is approved for rescreening only. There has been some dispute about how much accuracy is gained through the use of these automated systems, especially in light of the additional expense (62, 97, 103).
Another way to improve the Pap smear diagnosis is to stain directly for HPV. The BenchMark (Ventana Medical Systems, Tucson, Ariz.) is an automated modular system that performs immunohistochemistry stains on ThinPrep Pap Smear cytology samples. A high-risk HPV probe set (INFORM HPV liquid-based prep high-risk probe) that detects genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, and 70 and a low-risk probe set (INFORM HPV liquid-based prep low-risk probe) that detects genotypes 6, 11, 42, 43, and 44 are available. The INFORM probes are analyte-specific reagents. HPV infection is indicated by pale blue (low copy number) to blue-black (high copy number) staining of the nucleus of infected cells.
Histopathology
Patients with abnormal Pap smear findings who do not have a gross cervical lesion are usually evaluated by colposcopy and colposcopy-directed biopsy. Following application of a 3% acetic acid solution, the cervix is examined with a bright filtered light under 10- to 15-fold magnification. Acetowhitening and the vascular patterns characteristic of dysplasia or carcinoma can be seen. Colposcopy can detect low-grade and high-grade dysplasia but does not detect microinvasive disease. If no abnormalities are found or if the entire squamocolumnar junction cannot be visualized, a cervical cone biopsy is done. Biopsy can be used to confirm most diagnoses by observing characteristic pathologic features of HPV infection such as epithelial hyperplasia (acanthosis) and degenerative cytoplasmic vacuolization (koilocytosis) in terminally differentiated keratinocytes with atypical nuclei. In addition, stains can be used which detect HPV antigens or HPV nucleic acids. Monoclonal and polyclonal antibodies are available (Dako Corp., Carpinteria, Calif.) that detect HPV common antigen, a linear epitope in the middle of the major capsid protein, which is broadly expressed among the different HPV subtypes. Bound antibody is detected by peroxidase-antiperoxidase immunocytochemical staining. Staining is usually confined to the nucleus of infected cells but is also occasionally seen in the cytoplasm of koilocytic cells.
HPV DNA or RNA can be demonstrated in biopsy tissues by in situ hybridization with probes labeled with either radioisotopes or chemically reactive ligands which are detected by autoradiography, fluorescence, or a detection of color reaction. In situ hybridization can localize HPV nucleic acid sequences inside individual cells while preserving cell and tissue morphology to allow simultaneous assessment of the morphological alterations associated with the lesion. In situ methods can be nonamplified, target amplification by PCR, or signal amplified. For HPV detection, nonisotopic probes are recommended and enzymatic methods are preferred over fluorescence methods for ease of interpretation. Characteristics of the signal (confluent versus punctate) may reflect either the episomal or integrated form of the viral target DNA. Intensity of the signal may reflect copy number. Target-amplified or signal-amplified in situ techniques have been developed to immunoenzymatically detect a small number of HPV nucleic acid sequences with high sensitivity by using bright-field microscopy. The GenPoint system (Dako, Trappes, France) is an automated, catalyzed signal amplification system using a biotinylated probe for immunohistochemical detection of HPV in formalin-fixed, paraffin-embedded biopsy tissue sections. Automated processing includes baking, deparaffinization, cell conditioning, staining, and counterstaining. This assay is able to detect as few as 1 to 2 copies of target sequence per nucleus and is more sensitive than the one-step (detects 20 to 50 HPV copies) or three-step (10 to 15 copies) nonamplified immunoenzymatic procedures that are more routinely used (64).
HPV DNA Detection
Type-specific PCR
Type-specific PCR assays are based on the sequence variations present in the E6 and E7 genes of HPV subtypes. Fourteen type-specific PCRs for high-risk HPV types (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, and -68) that target approximately 100 bp in the E7 ORF have been developed (124). Internal control primers are included to detect inhibitory substances. The analytical sensitivity of these assays is between 10 and 200 HPV copies per sample, depending on the HPV type. Type-specific PCRs are currently used primarily in research applications since throughput is limited by the need to use multiple PCR amplifications for each sample.
General primer PCR
The majority of studies using PCR to date have used consensus primers to amplify a broad spectrum of HPV types in a single PCR amplification. These primers target conserved regions of the HPV genome such as the L1 capsid gene. The MY09 plus MY11 primers target a 450-bp fragment within the HPV L1 ORF (15). The GP5+ plus GP6+ primers target a fragment within the region targeted by MY09 and MY11 with an analytical sensitivity of 0.5 to 10 fg (10-200 copies) (51). The MY09 plus MY11 primers failed to detect HPV DNA in 7% of cervical cancers in one study (122). This may have been due to absence of HPV DNA in the carcinoma cells or a false-negative PCR result due to integration of HPV DNA in the cervical carcinoma which may have disrupted PCR primer target sequences or resulted in loss of the L1 ORF.
Various methods have been used to identify HPV genotypes after amplification with general and consensus primers. Among them are sequence analysis, restriction fragment length polymorphism, and hybridization with type-specific probes using dot blot or microtiter plate formats (88, 130).
A PCR-based detection system has recently been developed which uses a general primer set, designated SPF10 that that amplifies a 65-bp segment of the L1 region of the HPV genome. Since smaller amplicons are more efficiently amplified, this assay is thought to be especially suited for formalin-fixed, paraffin-embedded tissue samples which often yield poorly amplifiable DNA. Amplicons are detected in an enzyme-linked immunosorbent assay using a mixture of HPV-specific probes that recognize a broad range of genotypes. The specific genotype of positive samples is then determined using a line blot assay, in which oligonucleotide probes are immobilized in parallel lines on nitrocellulose strips. Amplicons are hybridized to the probes on the strip and detected in a colorimetric reaction, which results in a purple precipitate at the positive probe lines (59).
Liquid hybridization
The Hybrid Capture (Digene, Beltsville, Md.) assay is the only kit currently approved by the FDA for the detection of HPV DNA in cervical samples. The Hybrid Capture assay has been used in many studies, and the second-generation Hybrid Capture II version of the assay is now widely used in clinical diagnostic laboratories. It is an antibody capture/solution hybridization/signal amplification assay that uses chemiluminescence detection to qualitatively detect the presence of HPV. In this assay, the DNA in the patient samples is first denatured and mixed with an RNA probe pool in a buffered solution in a tube. Two RNA probe pools are used. The assay can be performed using both probe pools together or separately. The probe A pool recognizes low-risk HPV-6, -11, -42, -43, and -44, and the probe B pool recognizes high-risk HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68. The assay does not distinguish among the HPV types within these groups. Further typing is not clinically significant and is used largely in epidemiology and research studies. The hybridization reaction between specimen target DNA and the RNA probe produces DNA-RNA complexes. The DNA-RNA complexes are immobilized onto wells of a microtiter plate which has been coated with antibody directed against DNA-RNA hybrids. The immobilized hybrids are recognized by a second anti-DNA-RNA antibody conjugated to alkaline phosphatase. Several alkaline phosphatase molecules are conjugated to each antibody, and multiple conjugated antibodies bind to each captured hybrid, resulting in substantial amplification of the signal. Light is emitted as a chemiluminescent substrate is cleaved by the bound alkaline phosphatase. The light is measured as relative light units on a luminometer. The relative light units obtained for each sample are compared to the cutoff value. The analytical sensitivity of this assay ranges from 6.6 to 17.6 pg/ml depending on the HPV type (Digene Corp., Digene HPV test Hybrid Capture II product insert, 1997). The microwell plate format allows the assay to be automated.
Several specimen types can be used in the Hybrid Capture assay. Cervical swabs and cervical biopsy specimens are transported in Digene specimen transport medium. Cervical samples collected in Cytyc ThinPrep PreservCyt solution for making ThinPrep Pap smears can also be used.
The Hybrid Capture assay is not intended as a screening assay for the general population. Indications for performing the Hybrid Capture assay include (i) to aid in the diagnosis of sexually transmitted HPV infections and to distinguish between infection with low-risk and high-risk HPV types, (ii) to screen patients with ASCUS Pap smear results so as to determine the need for referral for colposcopy, and (iii) to supplement the Pap smear in women with LSIL or HSIL results to assist in assessing the risk for development of cervical cancer. The HPV test should not be used independently of Pap smears. A positive HPV result alone does not confirm the presence of premalignant or malignant disease and potentially yields more false-positive results if used on its own (100, 126).
As with any assay, there are some limitations to the Hybrid Capture assay. Cross-reactivity may be observed in rare instances and may lead to false-positive results with the high-risk probe pool. Cross-reactivity may occur in the presence of HPV-13 since both probes cross-react with HPV-13. This finding, however, is not clinically relevant for cervical specimens since HPV-13 is associated with lip lesions in certain ethnic groups and is rarely, if ever, detected in the anogenital tract. A small amount of cross-hybridization can occur between HPV-6 and HPV-42 (low-risk) and the High Risk probe pool. Specimens with high levels of HPV-6 or HPV-42 DNA (4 ng/ml or higher) may be positive when tested with both probes. Cross-reactivity between both HPV probes and high levels of bacterial plasmid pBR322 which can be found in cervical samples is possible. The false-negative rate is estimated to be 1.1 to 7.5%. False-negative results can occur due to low levels of infection, sampling error, or the presence of interfering substances such as antifungal cream, contraceptive jelly, or douche.
HPV mRNA Detection
Rather than relying on the detection of different HPV serotypes, the In-Cell (Invirion, Frankfurt, Mich.) viral load test for HPV detects mRNA of the E6 and E7 transforming genes. In this way, the assay actually determines if the HPV genes that cause malignant changes are present and active. The assay can be automated on any analytic instrument that detects fluorescence. Flow cytometry instruments are readily adaptable for this assay by using liquid-based cytology specimens. The assay can also be done directly on Pap smear slides and visualized using a fluorescence microscope. The manufacturer reports that the sensitivity of this assay is 100% and the specificity is 70% compared to Pap smear. Apparent false-positive results account for the reduced specificity; however, these false-positives may in fact not be false but may be due to early up-regulation of the E6 and E7 genes.
CLINICAL UTILITY OF HPV DNA TESTING
It has been established that there is variation in interpretation of ASCUS Pap smears even among expert cytopathologists (104). In some women, ASCUS indicates real pathology and in others it represents only a vigorous reactive change that is benign. In the United States, about 2.5 million ASCUS Pap results are reported each year (61). A survey of U.S. laboratories found that a median of 2.9% of all Pap smears are reported as ASCUS, with 10% of laboratories reporting more than 9% ASCUS results (30). Several strategies are currently in use to manage patients with ASCUS Pap smear results. Some clinicians repeat the Pap smear in 4 to 6 months. Many ASCUS patients directly undergo colposcopy to detect the 10 to 20% who prove to have an underlying higher-grade lesion (e.g., LSIL or HSIL). Identifying women at high risk by testing for HPV DNA avoids unnecessary colposcopy procedures (Table 3). Patients with ASCUS who are positive for high-risk HPV DNA are referred for colposcopy. Those who are negative for HPV DNA undergo a repeat Pap smear at 6 months and 12 months. If these are also negative, the woman is returned to a routine screening schedule.
TABLE 3.
Interpretation of Hybrid Capture results in patients with ASCUS Pap smeara
| Digene Hybrid Capture HPV result | Refer to colposcopyb | Result interpretation | |
|---|---|---|---|
| Probe A | Probe B | ||
| Negative | Negative | No | There is a high probability that a higher disease stage will not be found at colposcopy |
| Positive | Negative | ||
| Negative | Positive | Yes | Progression to high-grade disease is probable |
| Positive | Positive | ||
Data from the Digene Corporation HPV Test Hybrid Capture II product insert.
Results are not meant to deter women from colposcopy.
Several smaller studies and two recent large studies have shown the utility of HPV DNA testing for management of women with ASCUS results on Pap smears (75, 107). The Kaiser Permanente study enrolled 995 women in Northern California with ASCUS on cervical Pap smear screening (75). Colposcopy was performed on all study participants to obtain cervical tissue for histologic examination for detection of underlying SIL in patients with an initial cytologic test result of ASCUS. Just prior to colposcopy, specimens were taken for repeat ThinPrep Pap Smear and HPV DNA testing using the Digene Hybrid Capture II assay. The sensitivity of the Hybrid Capture II HPV DNA assay was 89.2% compared with colposcopically directed biopsy for detection of SIL. This was significantly higher than repeat ThinPrep Pap Smear testing, which had a sensitivity of 76.2%. These findings suggest that incorporating HPV DNA testing into a triage scheme for ASCUS would result in fewer women referred for colposcopy or repeat Pap smear while still maintaining the sensitivity necessary to detect higher-grade SIL.
Results of the ASCUS/LSIL Triage Study (ALTS) conducted by the National Cancer Institute were reported in March 2000 and February 2001 (107, 115). The ALTS trial is an ongoing, multicenter, randomized clinical trial designed to determine the optimal management regimen for women with equivocal (ASCUS) and low-grade (LSIL) cytology results. All cytological specimens in the ALTS trial were prepared using the ThinPrep Pap Smear fluid-based system. The presenting cytologic diagnoses of all patients enrolled in the ALTS study were reevaluated by the Pathology Quality Control Group for final case designation. The Digene Hybrid Capture II assay was used for all HPV DNA detection. The primary study end point was a histologic grade of CIN2/3+ since current clinical practice in the United States is to treat histologically confirmed high-grade disease of CIN2 or greater.
Women enrolled in the study with cytologic diagnoses of ASCUS (n = 3,488) were randomly assigned in equal proportions to one of three management options: (i) immediate colposcopy, (ii) conservative management (repeat Pap smear every 6 months with colposcopy only if HSIL results or above were found, or (iii) HPV triage (testing for high-risk HPV DNA followed by colposcopy only if high-risk HPV DNA was present). ThinPrep Pap Smear cytology and HPV DNA testing were done on all women enrolled in all arms of the study, but these results were unmasked only in the HPV triage arm to allow subsequent referral to colposcopy. The overall cytology and HPV DNA data were used to determine the statistical estimations of performance of the management options. All women, except those treated or removed from the trial, continue to be evaluated by Pap smear every 6 months to detect those who develop clinically significant cervical disease with time. Future data from the ALTS trial will address whether women considered low risk based on a negative HPV DNA result develop precancerous lesions.
The ALTS ASCUS data were analyzed under the assumption that immediate colposcopy would detect all cervical disease, and therefore the immediate-colposcopy arm of the study served as the standard against which the other two treatment strategies were compared. The mean age of the women enrolled in the study was 29 years and was similar for each arm of the study. In the immediate-colposcopy arm, the prevalence of lesions with histopathology grade CIN 2/3+ was 131 cases (11.3%). The HPV triage arm detected 136 cases (11.7%), and the conservative-management cytology arm detected 56 cases (4.8%). Since the colposcopy referral criterion of HSIL or higher was poor at detecting CIN 2/3+ in the conservative-management arm, all further analysis was done using the lower cytology threshold of ASCUS estimated by cross-sectional analysis of results in the immediate-colposcopy and HPV triage arms. Test sensitivities, colposcopy referral rates, and positive predictive values of the three management strategies were calculated using combined data from the immediate-colposcopy and HPV triage arms since both detected virtually all CIN2/3+ cases ascertainable at baseline. The sensitivities of the immediate-colposcopy, HPV triage, and conservative-management arms were 100, 95.5, and 85%, respectively. Differences between the immediate-colposcopy and conservative-management arms and between the HPV triage and conservative-management arms were statistically significant (P < 0.05); however, the true sensitivity of repeat cytology will not be known until the results of the longitudinal study with follow-up testing every 6 months are available.
Compared to the immediate-colposcopy arm (100%), just over half (56.1%) of the patients in the HPV triage arm were estimated to require referral to colposcopy. The estimated rate of referral to colposcopy in the repeat-cytology group was almost identical at 58.6%. Positive predictive values (women with positive test results who had CIN 2/3+ biopsy diagnoses) were 19.6% for the HPV triage arm and 16.7% for the conservative-management arm using ASCUS+ as the threshold. For the immediate-colposcopy arm, the comparable percentage is the 11.3% of women with ASCUS found to have CIN2/3+. Negative predictive values (women with negative test results who did not have disease) of the immediate-colposcopy, HPV triage, and conservative-management arms were 100, 98.9, and 95.8%, respectively. Evaluation of colposcopic biopsy diagnosis by study arm showed that of 857 women in the immediate-colposcopy arm, 15.3% had CIN2/3+, 19.5% had CIN1, and 65.2% were normal and that of 494 women in the HPV triage arm, 27.5% had CIN2/3+, 22.5% had CIN1, and 50% were normal. Comparable data are not available for the conservative-management arm using a cytology threshold of ASCUS or greater since HSIL+ was the criterion for referral; however, for the 93 women in the HSIL+ group who underwent biopsy, 60.2% had CIN 2/3+, 17.2% had CIN1, and 22.6% were normal. A summary of the ALTS study data showed that incorporation of HPV DNA testing into a triage scheme reduced the need for referral to colposcopy by 44% and that of those who were referred to colposcopy, a higher percentage had biopsy results of CIN 2/3+. Sensitivity was not significantly increased by adding a single repeat cytologic test to the triage scheme. The study therefore suggests that HPV DNA testing could be used in a triage scheme for women with ASCUS to determine if colposcopy is needed.
In patients with LSIL, HPV DNA testing was found to play a more limited role (115). High-risk HPV DNA was detected in 83% of women with LSIL. Since high-risk HPV was so prevalent in this group, all patients would be referred to colposcopy regardless of the HPV result.
AGUS (atypical glandular cells of undetermined significance) is a relatively rare cytologic finding. HPV DNA Testing may play a role in the management of patients with AGUS. In the Bethesda System, AGUS refers to endocervical or endometrial cells with cytological changes greater than reactive but not adenocarcinoma. Most cases of AGUS represent a reactive change. Nonreactive AGUS represents an intermediate cervical cancer precursor and is usually managed aggressively with colposcopy or cone biopsy. HPV DNA testing identified 94.1% of women with high-grade lesions. A negative result provided good assurance (99%) that a patient did not have a high-grade lesion (96).
HPV DNA testing may reduce costs by triaging patients into appropriate management strategies and reducing unnecessary colposcopy and less frequent screening in low-risk patients. Computer-based mathematical models that incorporate a simulated natural history of HPV carcinogenesis have been used to assess the cost-effectiveness of HPV screening strategies (57, 74). These studies showed small differences between ASC-US management strategies in terms of reducing the incidence of cervical cancer. However, there were considerable differences in costs associated with the management strategies. Immediate colposcopy was always more expensive than the other strategies. Reflex HPV DNA testing of a liquid-based cytology specimen or testing of a co-collected second specimen at the time of the initial Pap smear was less expensive than repeat cytology, partly because these strategies eliminate the need for an additional clinic visit and reduce the number of colposcopies by 40 to 60% (74). Stratifying ASC into the 2001 Bethesda System categories of ASC-US and ASC-H made little difference in terms of clinical benefit or costs (57). In addition to improving the management of women with ASC, the superior negative predictive value of HPV DNA testing may allow longer screening intervals. The computer models showed that a biennial or triennial cervical cancer screening program involving liquid-based cytology and reflex HPV DNA testing is more effective and less costly than annual screening by conventional cytology for women with ASC (57, 74). It is estimated that savings of more than $15 billion would be gained over the lifetime of a typical cohort of 18- to 24-year-old women by using biennial liquid-based cytology screening and reflex HPV DNA testing (57). In addition, termination of screening at 75 years of age would capture 97.8% of the benefits of lifetime biennial screening and would be less expensive (74). Using HPV DNA screening alone as a primary biennial screen becomes more cost-effective than biennial Pap screening only if the cost per HPV DNA test is $5 or less (74). Many insurance companies are supporting HPV DNA testing as an adjunct to Pap smear screening and are providing reimbursement for these tests. In the United States, 50 to 60% of women diagnosed with invasive cervical cancer have not had a Pap smear within the preceding 3 to 5 years or have never had a Pap smear (60). It is paramount to a clinically effective and cost-effective screening program that Pap smear testing and management of an abnormal result is consistently available to all women and that unscreened women are encouraged to use the Pap smear screening program.
Evidence-based consensus guidelines for the management of cervical cytological abnormalities and cervical cancer precursors were developed at the American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus Conference in September 2001. The new Bethesda 2001 terminology for reporting cervical cytology results, fluid-based cytology methods, molecular methods for detecting high-risk HPV types, data from the ALTS trial, and cost analyses were all taken into consideration in development of the guidelines. Recommended management schemes for all grades of cervical cytology abnormalities are posted on the ASCCP website at http://www.asccp.org.
Patients with ASC-US
Recommended management of women with ASC-US includes three options. (i) HPV DNA testing is the preferred approach if fluid-based cytology is used or if specimens are co-collected for HPV DNA testing. If HPV DNA testing is negative for high-risk HPV types, the patient undergoes repeat cytology testing at 12 months. For women who test positive for high-risk HPV types, referral to colposcopy is recommended. If biopsy confirms CIN, patients are treated as per standard practice for CIN. If biopsy does not confirm CIN, then (a) Pap smear should be repeated at 6 and 12 months with referral back to colposcopy if cytology results show ASC-US or greater or (b) HPV DNA testing should be repeated at 12 months with referral back to colposcopy if high-risk HPV types are found.
(ii) If a program of repeat cervical cytology is used, ASC-US patients should undergo Pap testing at 4- to 6-month intervals until two negative results are obtained. The patient can then be returned to routine cytologic screening. If any repeat Pap smear shows ASC-US or greater, referral to colposcopy is recommended.
(iii) When immediate colposcopy is used, women with biopsy-confirmed CIN are treated as per standard practice for CIN. If biopsy does not show CIN, patients undergo repeat Pap smear at 12 months.
In postmenopausal women with ASC-US and clinical or cytologic evidence of atrophy, a course of intravaginal estrogen is recommended if there are no contraindications to estrogen use. A Pap smear is performed about a week after completion of the estrogen regimen. If Pap smear cytology is negative, the test should be repeated in 4 to 6 months. If the repeat Pap smear shows ASC-US or greater, the patient is referred to colposcopy.
Immunosuppressed women with ASC-US should be directly referred to colposcopy. Pregnant women should be managed in the same manner as nonpregnant women.
Patients with ASC-H
Women with Pap smear results indicating ASC-H should be directly referred to colposcopy. If no lesions are identified by colposcopy, a review of the Pap smear, colposcopy, and histology results is recommended, if possible. If review yields a revised interpretation, management guidelines for the revised interpretation should be followed. If colposcopy confirms ASC-H, a Pap smear should be performed at 6 and 12 months with referral back to colposcopy if cytology results show ASC-US or greater. Alternatively, HPV DNA testing can be done at 12 months with referral back to colposcopy if high-risk HPV is found.
Patients with LSIL
Women with Pap smear results indicating LSIL should be directly referred to colposcopy. If colposcopy is satisfactory and fails to confirm CIN, a Pap smear should be performed at 6 and 12 months with referral back to colposcopy if cytology results show ASC-US or greater. Alternatively, HPV DNA testing can be done at 12 months with referral back to colposcopy if high-risk HPV is found. If colposcopy is unsatisfactory and fails to confirm CIN, management options include repeat cytologic testing 6 and 12 months with referral back to colposcopy if cytology results show ASC-US or greater or HPV DNA testing at 12 months with referral back to colposcopy if high-risk HPV is found.
TREATMENT
Most HPV-induced cervical cell changes are transient, and 90% regress spontaneously within 12 to 36 months as the immune system eliminates the virus (24, 47, 78, 85, 112). The primary immune response to HPV infection is a cell-mediated response induced at local lymph nodes. A humoral immune response also develops, but local levels of HPV-specific immunoglobulin G (IgG) and IgA in tissue do not correlate with clearance of virus (14). Systemic levels of HPV-specific IgA were correlated with virus clearance. Systemic levels of HPV-specific IgG were detected more frequently in patients with persistent HPV infection. The tendency toward regression of HPV infection correlates inversely with the severity of cervical disease. Only a small proportion of mild and moderate cervical diseases develop into invasive cancer, but the risk of progression from severe cervical cellular abnormality to invasive carcinoma is at least 12% (85, 112). Factors such as genetic predisposition, frequency of reinfection, intratypic genetic variation within HPV type, coinfection with more than one HPV type, hormone levels, and immune response may influence the ability to clear an HPV infection.
A number of factors such as size, stage, and histologic features of the tumor, lymph node involvement, risk factors for complications from surgery or radiation, and patient preference determine the course of treatment. In general, noninvasive intraepithelial lesions identified only microscopically are treated with superficial ablative procedures such as cryotherapy or laser therapy. These are outpatient office procedures, and fertility is maintained. With cryotherapy, abnormal tissue and the surrounding 5 mm is frozen with a supercooled probe. A single freeze is usually not adequate to induce necrosis, so the area is allowed to thaw and is frozen again. Ablation of tissue with a carbon dioxide laser beam is as effective as cryotherapy, and the tissue heals faster with less distortion, but the procedure is more expensive. Loop electrosurgical excision procedures are now considered to be the preferred treatment for noninvasive squamous lesions. In these procedures, an electrically charged wire is used to excise the transformation zone and distal endocervical canal. It is less expensive than laser therapy and preserves the excised tissue for histologic examination of margin status. Following treatment of noninvasive intraepithelial neoplasia lesions by any technique, there is always a potential risk of leaving dysplastic cells behind. Recurrence rates as high as 31% with a mean time to recurrence of 11.9 months have been reported following loop diathermy procedures in immunologically normal patients (43). Patients with positive margins had a higher recurrence rate (47%) than did those with clear margins (26%). Human immunodeficiency virus-infected women have a significantly higher recurrence rate (87%) than do uninfected women (18%), indicating the importance of an effective immune system in resolution of HPV-associated disease (19). Progression to invasive disease is rare (< 2% in most series). However, these data emphasize the importance of follow-up surveillance in treated patients. Preliminary evidence suggests that detection of HPV DNA using molecular techniques may be able to help detect residual lesions following treatment (81). Detection of high-risk HPV DNA at 6 months after treatment was more sensitive than abnormal cytology findings in patients with moderate or severe cervical disease prior to treatment. The negative predictive value of absence of high-risk HPV DNA and normal cytologic test results in these patients was 99%. The utility of HPV DNA testing for residual disease following treatment of lower grades of dysplasia remains to be evaluated.
Microinvasive cancers less than 3 mm in size are managed conservatively by excisional cone biopsy.
Early invasive cancers are managed with radical hysterectomy or external-beam high-energy (to 18 MV) radiotherapy and implants loaded with 192Ir. The goal of this therapy is to destroy malignant cells in the cervix, paracervical tissues, and regional lymph nodes. Selected patients also benefit from concurrent chemotherapy.
Locally advanced cancers are managed with radiotherapy to the primary tumor and potential sites of regional spread.
In addition to surgical and cytodestructive procedures, several antiviral and immunomodulatory agents have been evaluated as treatment for HPV-associated cervical lesions. Cidofovir is an acyclic nucleoside phosphonate derivative which has broad-spectrum activity against DNA viruses and is in use clinically for the treatment of CMV infections. Exposure of human carcinoma cell lines containing HPV-16 or HPV-18 and human cervical keratinocytes immortalized by HPV-33 to cidofovir resulted in inhibition of cell proliferation (4). The in vitro antiproliferative activity was shown to be selective for the rapidly proliferating HPV-infected cells when normal primary human cervical keratinocytes were treated similarly. A 1% cidofovir gel was used topically without side effects every other day for 1 month to treat 15 women with severe CIN (106). Complete or partial response was seen in 80% of patients as assessed by histology and detection of HPV DNA by PCR.
Podophyllin, a cytotoxic agent that arrests mitosis in metaphase (also used to treat genital warts), in combination with vidarabine, a DNA polymerase inhibitor, suppressed HPV gene expression and cell growth in cervical cancer cell lines (84). The expression of HPV-16 E6 and E7 gene products in normal cervical keratinocytes in vitro in the presence of either podophyllin or vidarabine sensitized these cells to apoptosis. Combined topical therapy with podophyllin and vidarabine ointments in 28 patients with mild to moderate CIN resulted in regression of lesions and successful eradication of HPV-16 or HPV-18 DNA in 81% of patients.
The IFNs and intravaginal 5-fluorouracil have shown variable response in clinical and in vitro studies. IFN-α is approved for treatment of genital warts. The effects of IFN-α, IFN-β, and IFN-γ in several human carcinoma cell lines containing HPV-16 or HPV-18 have been studied (56). Response was seen in some cell lines but not others. In HPV-18 HeLa cells, all IFNs suppressed the levels of HPV E6 and E7 gene transcripts. In HPV-18 C-411 cells, IFNs had no effect. In HPV-16 CaSki and HPK1A cells, only IFN-γ was effective. It is likely that, since IFN-responsive elements appear to be down-regulated by at least some oncogenic HPV types, the utility of IFN therapy in cervical disease will be limited (22).
PREVENTION
Primary approaches to prevent HPV infection include both risk reduction and development of HPV vaccines. Use of latex condoms and a spermicide may decrease the risk of contracting HPV. Condoms, however, are not totally reliable, since HPV may be contracted by contact with other parts of the body, such as the labia, scrotum, or anus, that are not protected by a condom.
Vaccines directed against HPV are in phase I and phase II clinical trials but are not currently commercially available. HPV vaccines are usually composed of virus-like particles (VLPs), which are empty virus capsids containing the major HPV capsid antigen and possibly the minor capsid antigen but lacking viral DNA. The vaccines are produced by expressing the L1 or L1 and L2 ORFs in eukaryotic cells. These proteins then self-assemble into VLPs which are highly immunogenic. Because of the high level of antigenic specificity of HPV capsid antigens, there is no cross-protection among subtypes, and protection against each subtype requires vaccination with VLPs of that subtype. Optimal vaccines would contain a cocktail of VLPs of the most common high-risk HPV subtypes. Preliminary reports indicate that animal papillomavirus vaccines have excellent immunogenicity and protection against experimental animal papillomavirus diseases.
A double-blind, randomized, placebo-controlled phase I safety and immunogenicity trial has been conducted using a subunit vaccine composed of VLP formed from the entire L1 major capsid protein of HPV-16 strain 114K (45). The vaccine was prepared by inserting the L1 capsid gene into a baculovirus vector. The gene was then expressed in transfected Sf9 insect cells. An optimal dose of 50 μg of HPV-16 L1 VLP vaccine was administered by injection into the deltoid muscle at 0, 1, and 4 months. The vaccine generated high titers of type-specific neutralizing antibodies without adjuvant and was well tolerated.
CONCLUDING REMARKS AND FUTURE DIRECTION
Screening for cervical cancer remains an important health and economic concern in the United States and throughout the world. Many studies over several decades have helped elucidate the natural history and pathogenesis of cervical neoplasia. The incidence of cervical cancer and its associated mortality have declined in recent years, largely due to the widespread implementation of screening programs that use Pap smear testing for detection of abnormal cervical cells. Methods such as fluid-based cytology have recently been developed that improve the ability to detect precursor lesions in Pap smears and allow detection at an earlier stage. The Bethesda System reporting of Pap smear results has also recently been updated to improve the utility and understandability of results. Molecular and epidemiologic studies have solidified the association between high-risk strains of HPV and cervical squamous cell carcinoma. Tests have been developed to detect high-risk HPV DNA with high sensitivity and specificity in cervical samples. These tests are now available in clinical laboratories at many medical centers and in reference laboratories. Large-scale studies such as the National Cancer Institute ALTS trial to evaluate management options for women with abnormal Pap smear results have been completed. Particularly problematic are women with ASC-US borderline or equivocal Pap smear results. These studies indicate the potential utility of HPV DNA testing in the management of women with ASC-US Pap smear results. Based on the results of these studies, new screening strategy options that include testing for high-risk HPV DNA as an adjunct to cytology have been developed to triage and monitor ASC-US patients. These strategies are meant to minimize unnecessary follow-up visits and invasive procedures without compromising the detection of disease. The improvements in cytologic screening as well as the introduction of HPV DNA testing greatly facilitate the identification of women at risk for cervical cancer. Early identification and intervention will probably have a significant impact on the reduction of cervical cancer morbidity and mortality.
Modifications to screening strategies will also probably be made in the future. Retrospective data analysis indicate that it may be advantageous to lengthen the Pap smear screening interval from annually to every 2 to 3 years (99). Results from ongoing studies will help clarify the benefits and risks of lengthened screening intervals. Several other studies to examine the role of HPV DNA testing as a primary screening method for cervical cancer are under way. In addition to changes in screening strategies, effective therapeutic and preventive vaccines may be developed that have the potential to contribute significantly to the control and prevention of cervical cancer.
REFERENCES
- 1.Adam, E., Z. Berkova, Z. Daxnerova, J. Icenogle, W. C. Reeves, and R. H. Kaufman. 2000. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am. J. Obstet. Gynecol. 182:257-264. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M., M. Kalantari, N. Ylitalo, B. Pettersson, B. Hagmar, L. Scheibenplug, B. Johansson, U. Petterson, and U. Gyllensten. 1996. HLA DQ-DR haplotype and susceptibility to cervical carcinoma: indications of increased risk for development of cervical carcinoma in individuals infected with HPV 18. Tissue Antigens 48:32-37. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, S., E. Rylander, B. Larsson, A. Strand, C. Silfversvard, and E. Wilander. 2001. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur. J. Cancer 37:246-250. [DOI] [PubMed] [Google Scholar]
- 4.Andrei, G., R. Snoeck, J. Piette, P. Delvenne, and E. DeClercq. 1998. Antiproliferative effects of acyclic nucleoside phosphonates on human papillomavirus (HPV)-harboring cell lines compared with HPV-negative cell lines. Oncol. Res. 10:523-531. [PubMed] [Google Scholar]
- 5.Apple R. J., T. M. Becker, C. M. Wheeler, and H. A. Erlich. 1995. Comparison of human leukocyte antigen DR-DQ disease associations found with cervical dysplasia and invasive cervical carcinoma. J. Natl. Cancer Inst. 87:427-436. [DOI] [PubMed] [Google Scholar]
- 6.Apt, D., R. M. Watts, G. Suske, and U. Bernard. 1996. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transcription correlate with the activation of the HPV-16 promoter. Virology 224:281-291. [DOI] [PubMed] [Google Scholar]
- 7.Austin, R. M., and I. Ramzy. 1998. Increased detection of epithelial cell abnormalities by liquid-based gynecologic cytology preparations. Acta Cytol. 42:178-184. [DOI] [PubMed] [Google Scholar]
- 8.Baker, T. S., W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown. 1991. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 60:1445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaudenon, S., D. Kremsdorf, O. Croissant, S. Jablonska, S. Wain-Hobson, and G. Orth. 1986. A novel type of human papillomavirus associated with genital neoplasias. Nature 321:246-249. [DOI] [PubMed] [Google Scholar]
- 10.Beaudenon, S., D. Kremsdorf, S. Obalek, S. Jablonska, G. Pehau-Amaudet, O. Croissant, and G. Orth. 1987. Plurality of genital human papillomaviruses: characterization of two new types with distinct biological properties. Virology 161:373-384. [DOI] [PubMed] [Google Scholar]
- 11.Bedell, M. A., J. B. Hudson, T. R. Golub, M. E. Turyk, M. Hosken, G. D. Wilbanks, and L. A. Laimins. 1991. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 65:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnez, W., and R. C. Reichman. 2000. Papillomaviruses, p. 1630-1640. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingston, Philadelphia, Pa.
- 13.Bontkes, H. J., M. Van Duin, T. D. deGruijl, M. F. Duggan-Keen, J. M. Walboomers, M. J. Stukart, R. H. Vereheijen, T. J. Helmerhorst, C. J. Meijer, R. J. Scheper, F. R. Stevens, P. A. Dyer, P. Sinnott, and P. L. Stern. 1998. HPV 16 infection and progression of cervical intraepithelial neoplasia: analysis of HLA polymorphism and HPV 16 E6 sequences variants. Int. J. Cancer 78:166-171. [DOI] [PubMed] [Google Scholar]
- 14.Bontkes, H. J., T. D. deGruijl, J. M. Walboomers, J. T. Schiller, J. Dillner, T. J. Helmerhorst, R. H. Vereheijen, R. J. Scheper, and C. J. Meijer. 1999. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J. Gen. Virol. 80:409-417. [DOI] [PubMed] [Google Scholar]
- 15.Bosch, F., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, and International Biological Study on Cervical Cancer (IBSCC) Study Group. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. [DOI] [PubMed] [Google Scholar]
- 16.Brisson, J, K. Morin, M. Fortier, M. Roy, C. Bouchard, J. Leclerc, A. Christen, C. Guimont, F. Penault, and A. Meisels. 1994. Risk factors for cervical intraepithelial neoplasia:differences between low and high-grade lesions. Am. J. Epidemiol. 40:700-710. [DOI] [PubMed] [Google Scholar]
- 17.Broder, S. 1992. The Bethesda System for reporting cervical/vaginal cytologic diagnoses—report of the 1991 Bethesda Workshop. JAMA 267:1892. [PubMed] [Google Scholar]
- 18.Burk, R. D., P. Kelly, J. Feldman, J. Bromberg, S. H. Vermund, J. A. Deltovitz, and S. H. Landesman. 1996. Declining presence of cervicovaginal human papilllomavirus infection with age is independent of other risk factors. Sex. Transm. Dis. 23:333-341. [DOI] [PubMed] [Google Scholar]
- 19.Calore, E. E., S. M. M. Pereira, and M. J. Cavaliere. 2001. Progression of cervical lesions in HIV-seropositive women: a cytological study. Diagn. Cytopathol. 24:117-119. [DOI] [PubMed] [Google Scholar]
- 20.Cates, W., and the American Social Health Association Panel. 1999. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex. Transm. Dis. 26:S2-S7. [DOI] [PubMed] [Google Scholar]
- 21.Chan, P. K., M. Y. Chan, W. W. Li, D. P. Chan, J. L. Cheung, and A. F. Cheung. 2001. Association of human beta-herpesviruses with the development of cervical cancer:bystanders or cofactors? J. Clin. Pathol. 54:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang, Y. E., and L. A. Laimins. 2000. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J. Virol. 74:4174-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, M., N. Popescu, C. Woodworth, Z. Berneman, M. Corbellino, P. Lusso, D. V. Ablashi, and J. A. DiPaolo. 1994. Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. J. Virol. 68:1173-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua, K. L., and A. Hjerpe. 1996. Persistence of human papillomavirus (HPV) infections preceding cervical carcinoma. Cancer 77:121-127. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Laboratory Improvement Amendments of 1988. 1988. P.L. 100-578. Congress. Rec. 134:3828-3863. [Google Scholar]
- 26.Coker, A. L., R. B. Russell, S. M. Bond, L. Pirisi, Y. Liu, M. Mane, N. Kokorina, T. Gerasimova, and P. L. Hermonat. 2001. Adeno-associated virus is associated with a lower risk of high-grade cervical neoplasia. Exp. Mol. Pathol. 70: 83-89. [DOI] [PubMed]
- 27.Conrad-Stöppler, M. C., K. Ching, H. Stöppler, K. Clancy, R. Schlegle, and J. Icenogle. 1996. Natural variants of the human papillomavirus type 16 E6 protein differ in their abilities to alter keratinocyte differentiation and to induce p53 degradation. J. Virol. 70:6987-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubie, H. A., A. L. Seagar, G. J. Beattie, S. Monaghan, and A. R. W. Williams. 2000. A longitudinal study if HPV detection and cervical pathology in HIV infected women. Sex. Transm. Infect. 76:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davey, D. D., S. Naryskin., M. L. Nielsen, and T. S. Kline. 1994. Atypical squamous cells of undetermined significance: interlaboratory comparison and quality assurance monitors. Diagn. Cytopathol. 11:390-396. [DOI] [PubMed] [Google Scholar]
- 30.DeVillez, R. L., and C. S. Stevens. 1980. Bowenoid papulosis of the genitalia. A case progressing to Bowen's disease. J. Am. Acad. Dermatol. 3:149-152. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Dong, X.-P., F. Stubenrauch, E. Beyer-Finkler, and H. Pfister. 1994. Prevalence of deletions of YY1-binding sites in episomal HPV 16 DNA from cervical cancers. Int. J. Cancer 58:803-808. [DOI] [PubMed] [Google Scholar]
- 33.Evander, M., I. H. Frazer, E. Payne, Y. M. Qui, K. Hengst, and N. A. McMillan. 1997. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favre, M. 1975. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J. Virol. 15:1239-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores, E. R., and P. F. Lambert. 1997. Evidence for a switch in the mode of human papillomavirus type 16 DNA replication during the viral life cycle. J. Virol. 71:7167-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores, E. R., B. L. Allen-Hoffman, D. Lee, C. A. Sattler, and P. F. Lambert. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344-354. [DOI] [PubMed] [Google Scholar]
- 37.Franco, E. L. 1995. Cancer causes revisited:human papillomavirus and cervical neoplasia. J. Natl. Cancer Inst. 87:779-780. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs, P. G., F. Girardi, and H. Pfister. 1988. Human papillomavirus DNA in normal, metaplastic, preneoplastic and neoplastic epithelia of the cervix uteri. Int. J. Cancer 41:41-45. [DOI] [PubMed] [Google Scholar]
- 39.Giannoudis, A., and C. S. Herrington. 2001. Human papillomavirus variants and squamous neoplasia of the cervix. J. Pathol. 193:295-302. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert, D. M., and S. N. Cohen. 1987. Bovine papillomavirus plasmids replicate randomly in mouse fibroblasts throughout S phase of the cell cycle. Cell 50:59-68. [DOI] [PubMed] [Google Scholar]
- 41.Giroglu, T., L. Florin, F. Schäfer, R. E. Streek, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gissmann, L., L. Wolnik, H. Ikenberg, U. Koldovsky, H. G. Schnurch, and H. zur Hausen. 1983. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc. Natl. Acad. Sci. USA 80:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez, D. I., C. M. Zahn, M. G. Retzloff, W. F. Moore, E. R. Kost, and R. R. Snyder. 2001. Recurrence of dysplasia after loop electrosurgical excision procedures with long-term follow-up. Am. J. Obstet. Gynecol. 184:315-321. [DOI] [PubMed] [Google Scholar]
- 44.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harro, C. D., Y.-Y. S. Pang, R. B. S. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 46.Hildesheim, A., M. Schiffman, C. Bromley, S. Wacholder, R. Herrero, A. C. Rodriuez, M. C. Bratti, M. E. Sherman, U. Scarpidis, Q.-Q. Lin, M. Terai, R. L. Bromley, K. Buetow, R. J. Apple, and R. D. Burk. 2001. Human papillomavirus type 16 variants and risk of cervical cancer. J. Natl. Cancer Inst. 93:315-318. [DOI] [PubMed] [Google Scholar]
- 47.Ho, G. Y., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:413-428. [DOI] [PubMed] [Google Scholar]
- 48.Holowaty, P., A. B. Miller, T. Rohan, and T. To. 1999. Natural dysplasia of the uterine cervix. J. Natl. Cancer Inst. 91:252-258. [DOI] [PubMed] [Google Scholar]
- 49.Howell, L. P., R. L. Davis, T. I. Belk, R. Agdigos, and J. Lowe. 1998. The AutoCyte preparation system for gynecologic cytology. Acta Cytol. 42:171-177. [DOI] [PubMed] [Google Scholar]
- 50.Hummel, M., J. B. Hudson, and L. A. Laimins. 1992. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J. Virol. 66:6070-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchinson, M. L., L. M. Isenstein, A. Goodman, A. A. Hurley, K. L. Douglass, K. K. Mui, F. W. Patten, and D. J. Zahniser. 1994. Homogeneous sampling accounts for increased diagnostic accuracy using the ThinPrep Processor. Am. J. Clin. Pathol. 101:215-219. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs, M. V., P. J. Snijders, A. J. van den Brule, T. J. Helmerhorst, C. J. Meijer, and J. M. Walboomers. 1997. A general primer P5+/GP6(+)-mediated PCR enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J. Clin. Microbiol. 35:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon, S., B. L. Allen-Hoffman, and P. F. Lambert. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 69:2989-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin, X. W., J. Cash, and A. W. Kennedy. 1999. Human papillomavirus typing and the reduction of cervical cancer risk. Cleveland Clin. J. Med. 66:533-539. [DOI] [PubMed] [Google Scholar]
- 55.Joyce, J. G., J.-S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 56.Kim, K. Y., L. Blatt, and M. W. Taylor. 2000. The effects of interferon on the expression of human papillomavirus oncogenes. J. Gen. Virol. 81:695-700. [DOI] [PubMed] [Google Scholar]
- 57.Kim, J. J., T. C. Wright, and S. J. Goldie. 2002. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA 287:2382-2390. [DOI] [PubMed] [Google Scholar]
- 58.Kiviat, N. B., and L. A. Koutsky. 1993. Specific human papillomavirus types as the causal agents of most cervical intraepithelial neoplasia: implications for current views and treatment. J. Natl. Cancer Inst. 85:934-935. [DOI] [PubMed] [Google Scholar]
- 59.Kleter, B., L. J. van Doorn, L. Schrauwen, A. Molijn, S. Sastrowijoto, J. TerSchegget, J. Lindeman, B. Ter Harmsel, M. Burger, and W. Quint. 1999. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 37:2508-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koopmanschap, M. A., G. A. van Oortmarssen, H. M. A. van Agt, M. van Ballegooijen, J. D. Habbema, and K. T. Lubbe. 1990. Cervical cancer screening: attendance and cost-effectiveness. Int J. Cancer 45:410-415. [DOI] [PubMed] [Google Scholar]
- 61.Kurman, R. J., D. E. Henson, A. L. Herbst, K. L. Noeller, and M. H. Schiffman. 1994. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Workshop. JAMA 271:1866-1869. [PubMed] [Google Scholar]
- 62.Lee, J. S. J., P. Wilhelm, L. Kuan, D. G. Ellison, X. Lei, S. Oh, and S. F. Patten. 1997. AutoPap system performance is screening for low prevalence and small cell abnormalities. Acta Cytol. 41:56-64. [DOI] [PubMed] [Google Scholar]
- 63.Lee, K. R., R. Ashfaq, G. G. Birdsong, M. E. Corkill, K. M. McIntosh, and S. L. Inhorn. 1997. Comparison of conventional Papanicolaou smears and a fluid-based, thin-layer system for cervical cancer screening. Obstet. Gynecol. 90:278-284. [DOI] [PubMed] [Google Scholar]
- 64.Lizard, G, M.-J. Démares-Poulet, P. Roignot, and P. Gambert. 2001. In situ hybridization detection of single-copy human papillomavirus on isolated cells using a catalyzed signal amplification system:GenPoint™. Diagn. Cytopathol. 24:112-116. [DOI] [PubMed] [Google Scholar]
- 65.Lonquet, M., S. Beaudenon, and G. Orth. 1996. Two novel genital papillomavirus (HPV) types: HPV68 and HPV70, related to the potentially oncogenic HPV39. J. Clin. Microbiol. 34:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorincz, A. T., W. D. Lancaster, and G. F. Temple. 1986. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J. Virol. 58:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lorincz, A. T., A. P. Quinn, W. D. Lancaster, and G. F. Temple. 1987. A new type of papillomavirus associated with cancer of the uterine cervix. Virology 159:187-190. [DOI] [PubMed] [Google Scholar]
- 68.Lorincz, A. T., G. F. Temple, R. J. Kurman, A. B. Jenson, and W. D. Lancaster. 1987. Oncogenic association of specific human papillomavirus types with cervical neoplasia. JNCI 79:671-677. [PubMed] [Google Scholar]
- 69.Lorincz, A. T., A. P. Quinn, M. D. Goldsborough, P. McAllister, and G. F. Temple. 1989. Human papillomavirus type 56: a new virus detected in cervical cancers. J. Gen. Virol. 70:3099-3104. [DOI] [PubMed] [Google Scholar]
- 70.Lorincz, A. T., A. P. Quinn, M. D. Goldsborough, B. J. Schmidt, and G. F. Temple. 1989. Cloning and partial DNA sequencing of two new human papillomavirus types associated with condylomas and low-grade cervical neoplasia. J. Virol. 63:2829-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorincz, A. T., and R. Reid. 1989. Association of human papillomavirus with gynecological cancer. Curr. Opin. Oncol. 1:123-132. [DOI] [PubMed] [Google Scholar]
- 72.Lorincz, A. T., R. Reid, A. B. Jenson, M. D. Greenberg, W. Lancaster, and R. J. Kurman. 1992. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet. Gynecol. 79:328-337. [DOI] [PubMed] [Google Scholar]
- 73.Magnusson, P. K. E., P. Lichtenstein, and U. B. Gyllenstein. 2000. Heritability of cervical tumors. Int. J. Cancer 88:698-701. [DOI] [PubMed] [Google Scholar]
- 74.Mandelblatt, J. S., W. F. Lawrence, S. M. Womack, D. Jacobson, B. Yi, Y.-T. Hwang, K. Gold, J. Barter, and K. Shah. 2002. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA 287:2372-2381. [DOI] [PubMed] [Google Scholar]
- 75.Manos, M. M., W. K. Kinney, L. B. Hurley, M. E. Sherman, J. Shieh-Ngai, R. J. Sherman, J. E. Ramsey, B. J. Fetterman, J. S. Hartinger, K. M. McIntosh, G. F. Pawlik, and R. A. Hiatt. 1999. Identifying women with cervical neoplasia: Using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 281:1605-1610. [DOI] [PubMed] [Google Scholar]
- 76.Matsukura, T., and M. Sugase. 1990. Molecular cloning of a novel human papillomavirus (type 58) from an invasive cervical carcinoma. Virology 177:833-836. [DOI] [PubMed] [Google Scholar]
- 77.Meyers, C., M. G. Frattini, J. B. Hudson, and L. A. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257:971-973. [DOI] [PubMed] [Google Scholar]
- 78.Moscicki, A. B., J. Palefsky, G. Smith, S. Siboshski, and G. Schoolnik. 1993. Variability of human papillomavirus DNA testing in a longitudinal cohort of young women. Obstst. Gynecol. 82:578-585. [PubMed] [Google Scholar]
- 79.Munoz, N., F. X. Bosch, K. V. Shah, and A. Meheus . 1992. The epidemiology of cervical cancer and human papillomavirus. Scientific Publication 119. International Agency for Research on Cancer, Lyon, France.
- 80.Naghashfar, Z. S., N. B. Rosenshein, A. T. Lorincz, J. Buscema, and K. V. Shah. 1987. Characterization of human papillomavirus type 45, a new type 18-related virus of the genital tract. J. Gen. Virol. 68:3073-3079. [DOI] [PubMed] [Google Scholar]
- 81.Nobbenhuis, M. A., C. J. Meijer, A. J. van den Brule, L. Rozendaal, F. J. Voorhoost, E. K. Risse, R. H. Verheijen, and T. J. Helmerhorst. 2001. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br. J. Cancer 84:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nuovo, G. J., C. P. Crum, E. M. de Villiers, R. U. Levine, and S. J. Silverstein. 1988. Isolation of a novel human papillomavirus (type 51) from a cervical condyloma. J. Virol. 62:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obalek, S., S. Jablonska, and G. Orth. 1985. HPV-associated intraepithelial neoplasia of external genitalia. Clin. Dermatol. 3:104-113. [DOI] [PubMed] [Google Scholar]
- 84.Okamoto, A., C. D. Woodworth, K. Yen, J. Chung, S. Isonishi, T. Nikaido, T. Kiyokawa, H. Seo, Y. Kitahara, K. Ochiai, and T. Tanaka. 1999. Combination therapy with podophyllin and vidarabine for human papillomavirus positive cervical intraepithelial neoplasia. Oncol. Rep. 6:269-276. [DOI] [PubMed] [Google Scholar]
- 85.Ostor, A. G. 1993. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 12:186-192. [PubMed] [Google Scholar]
- 86.Papanicolaou, G. N. 1949. A survey of actualities and potentialities of exfoliative cytology in cancer diagnosis. Ann. Intern. Med. 31:661-674. [DOI] [PubMed] [Google Scholar]
- 87.Philips, D. H., and M. NiShé. 1993. Smoking-related DNA adducts in human cervical biopsies. IARC Sci. Publ. 124:327-330. [PubMed] [Google Scholar]
- 88.Quint, W. G. V., G. Scholte, L. J. Van Doorn, B. Kleeter, P. H. M. Smits, and J. Lindeman. 2001. Comparative analysis of human papillomavirus infections in cervical scrapes and biopsy specimens by general SPF10 PCR and HPV genotyping. J. Pathol. 194:51-58. [DOI] [PubMed] [Google Scholar]
- 89.Razzaque, A., O. Williams, J. Wang, and J. S. Rhim. 1993. Neoplastic transformation of immortalized human epidermal keratinocytes by two HHV-6 DNA clones. Virology 195:113-120. [DOI] [PubMed] [Google Scholar]
- 90.Reid, R., M. Greenberg, A. B. Jenson, M. Husain, J. Willett, Y. Daoud, G. Temple, C. R. Stanhope, A. I. Sherman, G. D. Phibbs, and A. T. Lorincz. 1987. Sexually transmitted papillomavirus infections. 1. The anatomic distribution and pathogenic grade of neoplastic lesions associated with different viral types. Am. J. Obstet. Gynecol. 156:212-222. [DOI] [PubMed] [Google Scholar]
- 91.Rho, J., A. Roy-Burman, H. Kim, E. M. de Villiers, T. Matsukura, and J. Choe. 1994. Nucleotide sequence and phylogenetic classification of human papillomavirus type 59. Virology 203:158-161. [DOI] [PubMed] [Google Scholar]
- 92.Richart, R. M. 1973. Cervical intraepithelial neoplasia. Pathol. Annu. 3:301-328. [PubMed] [Google Scholar]
- 93.Ries, L. A. G., M. P. Eisner, C. L., Kosary, B. F. Hankey, B. A. Miller, L. Glegg, and B. K. Edwards. 2001. SEER cancer statistics review 1973-1998. National Cancer Institute, Bethesda, Md.
- 94.Roden, R. B., D. R. Lowy, and J. T. Schiller. 1997. Papillomavirus is resistant to dessication. J. Infect. Dis. 176:1076-1079. [DOI] [PubMed] [Google Scholar]
- 95.Romano, N., F. M. Romano, E. Viviano, F. Vitale, M. R. Viaalfrate, A. M. Perna, and F. Bonura. 1996. Rare association of human herpesvirus 6 DNA with human papillomavirus DNA in cervical smears of women with normal and abnormal cytologies. J. Clin. Microbiol. 34:1589-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ronnett, B. M., M. M. Manos, J. E. Ransley, B. J. Fetterman, W. K. Kinney, L. B. Hurley, J. S. Ngai, R. J. Kurman, and M. E. Sherman. 1999. Atypical glandular cells of undetermined significance (AGUS): cytopathologic features, histopathologic results, and human papillomavirus DNA detection. Hum. Pathol. 30:816-825. [DOI] [PubMed] [Google Scholar]
- 97.Ryan, M. R., J. F. Stastny, R. Remmers, M. A. Pedigo, L. A. Cahill, and W. J. Frable. 1996. PapNet-directed rescreening of cervicovaginal smears: a study of 101 cases of atypical squamous cells of undetermined significance. Am. J. Clin. Pathol. 105:711-718. [DOI] [PubMed] [Google Scholar]
- 98.Sapp, M., C. Volpers, M. Muller, and R. E. Streck. 1995. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J. Gen. Virol. 76:2407-2412. [DOI] [PubMed] [Google Scholar]
- 99.Sawaya, G. F., K. Kerlikowske, G. Gildengorin, and A. E. Washington. 2000. Incidence of Pap test abnormalities within 3 years of a normal Pap test—United States, 1991-1998. Morb. Mortal. Wkly. Rep. 49:1001-1003. [PubMed] [Google Scholar]
- 100.Schiffman, M., R. Herrero, A. Hildesheim, M. E. Sherman, M. Bratti, S. Wacholder, M. Alfaro, M. Hutcinson, J. Morales, M. D. Greenberg, and A. T. Lorincz. 2000. HPV DNA testing in cervical cancer screening. Results from women in a high-risk province of Costa Rica. JAMA 283:87-93. [DOI] [PubMed] [Google Scholar]
- 101.Sheets, E. E., N. M. Constantine, S. Sinisco, B. Dean, and E. S. Cibas. 1995. Colposcopically-directed biopsies provide a basis for comparing the accuracy of ThinPrep and Papanicoloau smears. J. Gynecol. Technol. 1:27-34. [Google Scholar]
- 102.Shen, C. Y., M. S. Ho, S. F. Chang, M. S. Yen, H. T. Ng, E. S. Huang, and C. W. Wu. 1993. High rate of concurrent genital infections with human cytomegalovirus and human papillomaviruses in cervical cancer patients. J. Infect. Dis. 168:449-452. [DOI] [PubMed] [Google Scholar]
- 103.Sherman, M. E., L. J. Mango, D. Kelly, G. Paull, V. Ludin, C. Copeland, D. Solomon, and M. H. Schiffman. 1994. PapNet analysis of reportedly negative smears preceeding the diagnosis of a high grade squamous intraepithelial lesion or carcinoma. Mod. Pathol. 7:578-581. [PubMed] [Google Scholar]
- 104.Sherman, M. E., M. H. Schiffman, A. T. Lorincz, M. M. Manos, D. R. Scott, R. J. Kurman, N. B. Kiviat, M. Stoler, A. G. Glass, and B. B. Rush. 1994. Toward objective quality assurance in cervical cytopathology: correlation of cytopathologic diagnoses with detection of high-risk human papillomavirus types. Am. J. Clin. Pathol. 102:182-187. [DOI] [PubMed] [Google Scholar]
- 105.Shimoda, K., A. T. Lorincz, F. Temple, and W. D. Lancaster. 1988. Human papillomavirus type 52: a new virus associated with cervical neoplasia. J. Gen. Virol. 69:2925-2928. [DOI] [PubMed] [Google Scholar]
- 106.Snoeck, R., J. C. Noel, C. Muller, E. De Clercq, and M. Bossens. 2000. Cidofovir, a new approach for the treatment of cervix intraepithelial neoplasia grade III (CIN III). J. Med. Virol. 60:205-209. [DOI] [PubMed] [Google Scholar]
- 107.Solomon, D., M. Schiffman, and R. Tarone. 2001. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance. Baseline results from a randomized trial. J. Natl. Cancer Inst. 93:293-299. [DOI] [PubMed] [Google Scholar]
- 108.Solomon, D., D. Davey, R. Kurman, A. Moriarity, D. O'Connor, M. Prey, S. Raab, M. Sherman, D. Wilbur, T. Wright, and N. Young. 2002. The 2001 Bethesda System. Terminology for reporting results of cervical cytology. JAMA 287:2114-2119. [DOI] [PubMed] [Google Scholar]
- 109.Spitzer, M. 1998. Cervical screening adjuncts: recent advances. Am. J. Obstet. Gynecol. 179:544-556. [DOI] [PubMed] [Google Scholar]
- 110.Su, P. F., S. Y. Chiang, C. W. Wu, and F. Y. Wu. 2000. Adeno-associated virus major Rep78 protein disrupts binding of TATA-binding protein to the p97 promoter of human papillomavirus type 16. J. Virol. 74:2459-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swan, D. C., R. A. Tucker, G. Tortolero-Luna, M. F. Mitchell, L. Wideroff, E. R. Unger, R. A. Nisenbaum, W. C. Reeves, and J. P. Icenogle. 1999. Human papilloma (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J. Clin. Microbiol. 37:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Syrjänen, K. J. 1996. Natural history of genital human papillomavirus infections, p. 189-206. In C. Lacey (ed.), Papillomavirus reviews. Leeds University Press, Leeds, United Kingdom.
- 113.Syrjänen, S. M., and K. J. Syrjänen. 1999. New concepts on the role of human papillomavirus in cell cycle regulation. Ann. Med. 31:175-187. [DOI] [PubMed] [Google Scholar]
- 114.Tewari, K. S., J. A. Taylor, S. Y. Liao P. J. DiSaia, R. A. Burger, B. J. Monk, C. C. Hughes, and L. P. Villarreal. 2000. Development and assessment of a general theory of cervical carcinogenesis utilizing a severe combined immunodeficiency murine-human xenograft model. Gynecol. Oncol. 77:137-148. [DOI] [PubMed] [Google Scholar]
- 115.The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. 2000. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions:baseline data from a randomized trial. J. Natl. Cancer Inst. 92:397-402. [DOI] [PubMed] [Google Scholar]
- 116.Thomas, M., D. Pim, and L. Banks. 1999. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 18:7690-7000. [DOI] [PubMed] [Google Scholar]
- 117.Torrisi, A., A. Del Mistro, G. L. Onnis, F. Merlin, R. Bertorelle, and D. Minucci. 2000. Colposcopy, cytology and HPV testing in HIV-positive and HIV-negative women. Eur. J. Gynecol. Oncol. 21:168-172. [PubMed] [Google Scholar]
- 118.VanRanst, M., R. Tachezy, and R. D. Burk. 1996. Human papillomaviruses: a neverending story, p. 1-20. In C. Lacey (ed.), Papillomavirus reviews. Leeds University Press, Leeds, United Kingdom.
- 119.Vassilaskos, P., J. Saurel, and R. Rondez. 1999. Direct-to-vial use of the AutoCyte PREP liquid-based preparation for cervical-vaginal specimens in three European laboratories. Acta Cytol. 43:65-68. [DOI] [PubMed] [Google Scholar]
- 120.Veress, G., K. Szarka, X.-P. Dong, L. Gergely, and H. Pfister. 1999. Functional significance of sequence variation in the E2 gene and the long control region of human papillomavirus type 16. J. Gen. Virol. 80:1053-1043. [DOI] [PubMed] [Google Scholar]
- 121.Villa, L. L. 1997. Human papillomaviruses and cervical cancer. Adv. Cancer Res. 71:321-341. [DOI] [PubMed] [Google Scholar]
- 122.Volpers, C., and R. E. Streek. 1991. Genome organization and nucleotide sequence of human papillomavirus type 39. Virology 181:419-423. [DOI] [PubMed] [Google Scholar]
- 123.Walboomers, J. M. M., and C. J. L. M. Meijer. 1997. Do HPV-negative cervical carcinomas exist? J. Pathol. 181:253-254. [DOI] [PubMed] [Google Scholar]
- 124.Walboomers, J. M. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. F. Snijders, J. Peto, C. J. L. M. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 125.Wright, T. C., and R. T. Kurman. 1994. A critical review of the morphologic classification system of preinvasive lesions of the cervix: the scientific basis for shifting the paradigm. Papillomavirus Rep. 5:175-182. [Google Scholar]
- 126.Wright, T. C., L. Denny, L. Kuhn, A. Pollack, and A. Lorincz. 2000. HPV DNA Testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 283:81-86. [DOI] [PubMed] [Google Scholar]
- 127.Wright, T. C., J. T. Cox, L. S. Massad, L. B. Twiggs, and E. J. Wilkinson. 2002. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 287:2120-2129. [DOI] [PubMed] [Google Scholar]
- 128.Yang, X., G. Jin, Y. Nakao, M. Rahimtula, M. M. Pater, and A. Pater. 1996. Malignant transformation of HPV-16 immortalized human endocervical cells by cigarette smoke condensate and characterization of multistage carcinogenesis. Int J. Cancer 65:338-344. [DOI] [PubMed] [Google Scholar]
- 129.Yoshinouchi, M., A. Hongo, K. Nakamura, J. Kodama, S. Itoh, H. Sakai, and T. Kudo. 1999. Analysis by multiplex PCR of the physical status of human papillomavirus type 16 DNA in cervical cancers. J. Clin. Microbiol. 37:3514-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zerbini, M., S. Venturoli, M. Cricca, G. Gallinella, P. De Simone, S. Costa, D. Santini, and M. Musiani. 2001. Distribution and viral load of type specific HPVs in different cervical lesions as detected by PCR-ELISA. J. Clin. Pathol. 54:377-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.zur Hausen, H. 1982. Human genital cancer: synergism between two virus infections and or synergism between a virus infection and initiating events? Lancet ii:1370-1372. [DOI] [PubMed] [Google Scholar]
- 132.zur Hausen, H. 1996. Papillomavirus infections - a major cause of human cancers. Biochim. Biophys. Acta 1288:55-78. [DOI] [PubMed] [Google Scholar]
- 133.zur Hausen, H. 1999. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111:581-587. [DOI] [PubMed] [Google Scholar]