Abstract
This review addresses both historical and recent investigations into viral contamination of marine waters. With the relatively recent emergence of molecular biology-based assays, a number of investigations have shown that pathogenic viruses are prevalent in marine waters being impacted by sewage. Research has shown that this group of fecal-oral viral pathogens (enteroviruses, hepatitis A viruses, Norwalk viruses, reoviruses, adenoviruses, rotaviruses, etc.) can cause a broad range of asymptomatic to severe gastrointestinal, respiratory, and eye, nose, ear, and skin infections in people exposed through recreational use of the water. The viruses and the nucleic acid signature survive for an extended period in the marine environment. One of the primary concerns of public health officials is the relationship between the presence of pathogens and the recreational risk to human health in polluted marine environments. While a number of studies have attempted to address this issue, the relationship is still poorly understood. A contributing factor to our lack of progress in the field has been the lack of sensitive methods to detect the broad range of both bacterial and viral pathogens. The application of new and advanced molecular methods will continue to contribute to our current state of knowledge in this emerging and important field.
INTRODUCTION
All of the known pathogenic viruses that pose a significant public health threat in the marine environment are transmitted via the fecal-oral route. This group, known collectively as enteric viruses, is a continually growing list. These viruses belong primarily to the families Adenoviridae (adenovirus strains 3, 7, 40, and 41), Caliciviridae (Norwalk virus, astroviruses, caliciviruses, Snow Mountain agent, and small round structured viruses), Picornaviridae (poliovirus, coxsackieviruses, echoviruses, enteroviruses, and hepatitis A virus [HAV]), and Reoviridae (reoviruses and rotaviruses). The enteric viruses are associated with a variety of diseases in humans (Table 1), from ocular and respiratory infections to gastroenteritis, hepatitis, myocarditis, and aseptic meningitis. The elderly, the very young, and the immunocompromised are the most susceptible and are more likely to develop severe infections (40). While diarrhea has been one of the primary manifestations of infection, it is now recognized that more serious chronic diseases are associated with viral infections and that the risks of such infections need to be better defined. The primary site of viral infection and replication is the intestinal tract. Virus particles are shed in feces for weeks at levels as high as 1010 g−1 (rotaviruses), with average levels between 106 g−1 (enteroviruses) and 108 g−1 (HAV) (32, 36, 166). While person-to-person and surface-to-person transmission can occur, viruses in wastewater that contaminate drinking water sources, recreational waters, and shellfish harvesting waters pose the greatest risk to the public (14, 42, 90, 99, 107, 122; C. P. Gerba and C. E. Enriquez, Proc. First Ann. Chem. Symp., 1997).
TABLE 1.
Examples of viral waterborne pathogens of concern
| Pathogen | Diseases | Yr first described (reference) | Methods of detection (references) |
|---|---|---|---|
| Coxsackievirus (members of the enterovirus group belonging to picornaviridae) | Aseptic meningitis, herpangina, paralysis, exanthema, hand foot and mouth disease, common cold, hepatitis, infantile diarrhea, acute hemorrhagic conjunctivitis | 1947a (22) | Tissue culture methods, molecular methods (65, 96) |
| Hepatitis viruses | Fever, nausea, abdominal pain, anorexia and malaise, associated with mild diarrhea, arthralgias, scleral icterus; cytologic damage, necrosis and inflammation of the liver (HAV) | 1950b (12) | Tissue culture methods, molecular methods, Immunofluorescence (70, 117, 150, 152) |
| Norwalk-like viruses (belonging to Caliciviridae) | Diarrhea, vomiting, abdominal pain, cramping, low fever, headache, nausea, tiredness (malaise), muscle pain (myalgia) | 1968,a 1968b (40) | Transmission EM, ELISA, molecular methods (69, 143) |
| Rotavirus (belonging to Reoviridae) | Vomiting, abdominal distress, diarrhea, dehydration, fever | 1973a (9) | Tissue culture methods, molecular methods (49) |
Human pathogen.
Waterborne transmission.
The majority of the world's population is found along the coasts. Often, wastewater is disposed directly or indirectly into coastal waters. Approximately 9.3 × 107 people (37%) of the total U.S. population reside in coastal areas and discharge about 1.0 × 1010 gallons of treated wastewater day−1 (101). In 2000, there were over 11,000 beach closings or advisories (freshwater and marine beaches) in the United States, a number that had almost doubled from the previous year, and a majority of these closings were due to wastewater pollution (102). On a global scale, coastal development is twice that of inland sites, with ∼90% of the generated wastewater being released untreated into marine waters (20, 60).
The discharge of viral pathogens in treated sewage is not regulated, and monitoring relies on bacterial indicator detection to predict virus contamination (54). Virus levels in wastewater, measured by cell culture assay, range from 1.82 × 102 to 9.2 × 104 liter−1 in untreated sewage and from 1.0 × 10−3 to 1.0 × 102 liter−1 in treated wastewater depending on the level of treatment (101, 131, 133). Some of the wastewater that is discharged into the marine environment is only partially treated and is not disinfected (examples of partially treated discharges where only the undissolved solids are removed before release of the sewage effluent from the plant can be found in Los Angeles, San Diego, and Hawaii). Combined sewer overflows, which are systems that receive rainwater and untreated wastewater and overflow during high precipitation events, have been sources of coastal pollution in areas like Puget Sound, Wash. (130). Finally, communities with high-density septic tanks (on-site, individual disposal systems) also contribute to poor water quality and increased viral pollution of coastal waters (52, 87).
Prior to 1950, little was known about human viral infections except for those associated with the more widespread diseases such as hepatitis. The first isolations of viruses from water were reported in the late 1930s and early 1940s and focused on poliovirus and other enteroviruses in feces and wastewater (22, 114). The method employed a gauze pad to adsorb the virus as the water passed through it and the subsequent use of cell culture (monkey kidney cells) to cultivate and identify the virus. Improvements in the filtration methods to recover viruses from water and the emergence of newly discovered pathogenic human enteric viruses associated with drinking water and shellfish outbreaks (the discovery of rotavirus and Norwalk virus) occurred in the 1970s and 1980s (42, 90).
The occurrence of pathogenic human enteric viruses in marine waters is not well characterized, and our understanding has been hampered by the limited number of scientific studies, lack of available and accurate detection assays, and erroneous assumptions in regard to virus viability and infectivity. The contamination of marine waters with viruses has been, and will continue to be, an important public health issue. The spread of viral diseases through recreational water exposure and ingestion of contaminated shellfish is a primary public health concern. The key to understanding and controlling viral contamination of our coastal environments is the application of new tools for monitoring and studying these microorganisms.
PUBLIC HEALTH RISK
Health Effects
The enteric viruses cause a wide range of diseases and symptoms. Gastroenteritis, otitis, and respiratory diseases are most often reported in studies of exposure to contaminated marine waters. Viral etiology is rarely identified, even though viruses are believed to cause a majority of waterborne marine illnesses.
Adenoviruses, which were first described in 1953 (134), have been identified as a significant cause of acute upper respiratory tract infections. While most infections caused by these viruses are asymptomatic, respiratory, gastrointestinal, and ocular infections are commonly reported. These viruses can cause significant problems for immunosuppressed or immunocompromised persons (146, 154). There are over 48 serotypes of adenoviruses, that comprise six subgroups (A to F), which have been identified as causative agents of human disease. Adenoviruses can cause a wide range of disease types, including respiratory, ocular, and gastrointestinal infections (35). The enteric adenoviruses are types 40 and 41 and are responsible for the majority of adenovirus-mediated cases of gastroenteritis (19, 28). Other types of adenoviruses which can cause gastroenteritis are types 3 and 7 in systemic infections. Most individuals become infected with adenoviruses before they reach 20 years of age. The adenoviruses are commonly found in wastewater-impacted marine environments (68, 79, 118, 120, 160).
Coxsackieviruses have emerged as important waterborne enteric pathogens. In a study of viruses in raw sewage and treated effluent in Puerto Rico, it was demonstrated that 95% of the enteroviruses detected were coxsackie B5 virus (21). Others have reported that approximately 30% of the isolates from untreated wastewater (Athens, Greece) were coxsackie B2, B4, and B5 virus, with an estimated overall coxsackievirus concentration of 35.8 to 172.8 cytopathic units per liter (79, 80). Coxsackieviruses have been associated with myocarditis, paralytic disease, aseptic meningitis, insulin-dependent diabetes, and cold-like illnesses (76, 77, 162).
Norwalk-like viruses (also called small round structured viruses) have been identified in drinking water (72, 84, 85), recreational water (73), and marine shellfish (81) tissue in a number of studies investigating their role in human disease (25, 29, 50, 55, 90). This diverse group of RNA viruses often causes gastroenteritis with diarrhea and/or vomiting, fever, and respiratory symptoms lasting approximately 2 days. These viruses were first identified by electron microscopy and include Norwalk virus, Snow Mountain agent, astroviruses and caliciviruses. These viruses are a major cause of shellfish-associated disease and may be the most significant cause of adult viral gastroenteritis (4, 5, 25, 29, 55, 63, 84, 86, 90, 91, 148).
Shellfish preferentially accumulate microorganisms during periods of low water temperatures (between 11.5 and 21.5°C), which results in a higher incidence of human viral gastroenteritis acquired through shellfish consumption during these periods (13). This preferential accumulation of microorganisms by shellfish, coupled with the enhanced survival of viruses at lower temperatures, may explain the seasonality of shellfish-borne viral disease. This group of viruses is heat stable and more resistant to chlorine disinfection than are bacteria. It has been suggested that caliciviruses are much more prevalent than were previously thought and have been reported in wastewater at 107 RNA-containing particles liter−1 (91).
Indigenous marine caliciviruses excreted into the water column by mammals may cause disease in swine (vesicular exanthema) and humans (149). These authors noted that infected whales may excrete an estimated 106 caliciviruses g of feces−1 as enumerated by electron microscopy (149).
Rotavirus was first identified as a causative agent of gastroenteritis in 1973 and is thought to be responsible for the deaths of 4 × 106 to 5 × 106 individuals annually worldwide (9). It is one of the major causes of infantile viral gastroenteritis worldwide, but adults are also susceptible. Several outbreaks of waterborne disease have been attributed to this organism. It is estimated that over one million cases of severe diarrhea in the 1- to 4-year-old age group are caused by rotaviruses annually in the United States, with up to 150 deaths (62). All of the rotavirus outbreaks have been associated with direct fecal contamination of an untreated or compromised water supply or suboptimal treatment of drinking water. Rotaviruses have been detected in surface waters worldwide, with average concentrations ranging from 0.66 to 29 liter−1 (43). The highest concentrations have been reported in surface waters receiving untreated wastewater effluent.
Other viruses may be of concern for non-industrialized countries. A review of hepatitis E virus (HEV) reported this virus to be a significant cause of hepatitis in tropical and subtropical environments, resulting in a high mortality (20%) of pregnant women (150). It has been suggested that swine feces may serve as a source of HEV contamination of recreational and shellfish-harvesting coastal waters (150).
Recreational Risks
In the United Kingdom, studies of marine recreational waters began in the 1970s. The European Economic Directive “Quality of Bathing Water” (76/160/EEC) required that cultivatable viruses be absent in 10 liters of water (<1 PFU/10 liters) At that time in Europe (unlike the United States), no mandate for wastewater treatment existed and untreated wastewater was discharged directly to the marine environment through outfalls. The approach to protect public health was to monitor bathing beaches in order to identify impact. At beaches associated with sewage outfalls, viruses were detected at levels between 1 and 520 PFU/10 liters (147). Illness was observed in adults and children using beaches where human viruses were detected (8).
Documented outbreaks in marine waters are rare; however, numerous epidemiological studies have found swimmers to be at increased risk of disease after swimming at polluted beaches (25, 34, 60, 105, 148). The etiology of illnesses in these studies was not identified, and the pollution was documented using bacterial indicator systems. Major epidemiological studies have been undertaken in the United Kingdom, United States, and China. In Hong Kong it was estimated that beach swimmers were five times more likely to develop gastroenteritis than nonswimmers, with no less than 400,000 illnesses attributed to exposure to polluted beach water in 1990 (16). Results from interviewing 25,000 beach users in Hong Kong in 1992 concluded that swimmers were 2 to 20 times more likely to exhibit eye, skin, and respiratory symptoms than were nonswimmers (81). In an effort to protect public health, Hong Kong developed a beach rating system with Escherichia coli as the water quality indicator for 41 of its recreational beaches (three samples per month with a geometric mean limit of 180 CFU/100 ml in March through October).
Research has correlated enterococcus (Enterococcus spp., formerly group D streptococci) levels with both gastrointestinal and respiratory disease (34, 75). Bathers swimming in waters containing ≥60 CFU of streptococci/100 ml of water were at risk of developing some form of illness compared to nonbathers (34). Significant gastrointestinal health effects were noted when enterococcus levels exceeded 32 CFU/100 ml measured in bathing water at chest depth (75). At a beach in Ramsgate, United Kingdom, 24% of 1883 individuals reported at least one symptom of illness after wading, swimming, or surfing at the beach and the relative risk was significant for bathers (relative risk = 1.31) versus nonbathers (8).
Based on epidemiological studies, the U.S. Environmental Protection Agency (UEPA) recommends the use of enterococci for monitoring the safety of marine bathing waters (26). Although epidemiological recreational water studies support the enterococcal standard, many states continue to rely on the coliform standard (119). An epidemiological study in Santa Monica Bay, Calif., found that 373 individuals of every 10,000 who swam near active storm drains were at risk of developing some symptom of disease (56). This led to a significant change in monitoring and to approaches to protect public health, including the closure of Huntington Beach in 1999. The 4-month closure, which was due to microbial standard violations, resulted in the loss of millions of dollars in tourism income to the business community and almost two million dollars in beach closure investigation fees (164). One of the investigation recommendations was that virus monitoring be implimented.
Despite knowledge of nearshore water pollution in Key West, Fla., a competitive race, Swim around Key West, was held in 1999. Surveys obtained from 160 swimmers who participated in the event reported that 31% had a least one symptom of disease following the event (105), with gastroenteritis being the most commonly reported symptom. It is difficult to relate outbreaks to epidemiological data after marine recreational exposure, and it is more feasible to monitor for pathogens of concern, including the viruses.
Risk assessment models can be used to evaluate health risks in the absence of epidemiological data by using pathogen-monitoring information. In a comprehensive study in Hawaii, an area that most would view as a pristine aquatic environment, enteroviruses were found in 6% of samples screened (18). Risk assessment models used to estimate the maximum risks from exposure to this level of viruses predicted 1.3 infections per 100 swimmers (18).
Viruses attached to particulate matter (inorganic soil particles or organic detritus) are more likely to cause disease than are free-floating viruses. Researchers used a model to predict the risk of infection from both unattached and particle-associated viruses in the Oder Lagoon (Southern Baltic). Unattached viruses were only a risk in a river and at the river mouth during the summer bathing period. Viruses attached to particles extended the range of risk from the river mouths out into associated regions of the lagoon (137). Risk modeling has also incorporated epidemiological data such as the number of individuals with symptoms, bacterial indicator data, pathogen concentrations, and dose-response functions to calculate the recreational risk in specific aquatic environments (27).
VIRUS OCCURRENCE AND FATE IN MARINE WATERS
Occurrence Studies
Studies performed during the last 5 years on the occurrence of viruses in coastal waters are from geographically diverse areas. Groups of researchers have conducted investigations in Greece, Italy, California, Florida, and Hawaii, using cell culture and/or molecular techniques (Table 2).
TABLE 2.
Occurrence of human viruses in marine environments
| Virus(es) | Location | Maximum % of samples positive via cell culture or PCR/RT-PCR | Virus concn/10 liters assayed where data available | Reference |
|---|---|---|---|---|
| Enteroviruses | Galveston Bay, Tex. | 40.0 (cell culture) | Data not included | 38 |
| Enteroviruses | Texas Gulf coast | 59.0 (cell culture) | 0.1-4.4 | 45 |
| Enteroviruses | Galveston Bay, Tex. | 72.0, 14.0 for sediment and water (cell culture) | 0.1-10.5 | 121 |
| Enteroviruses | Waikiki and neighboring beaches, Hawaii | 8.0 for beach samples, 50.0 for sewage outfall samples (cell culture) | <0.1-130,000 | 125 |
| Adenoviruses, enteroviruses, and HAV | Nearshore waters of Barcelona, Spain | 78.0, 44.0, and 33.0, respectively (PCR/RT-PCR) | Presence/absence only | 118 |
| Enteroviruses and adenoviruses | Beaches of Patras, Greece | 83.4.0 and 90.0, respectively, (RT-PCR/PCR) | 1.2-6.0 (via cell culture, enteroviruses only) | 160 |
| Enteroviruses, HAV, and Norwalk virus | Canals and nearshore waters of the Florida Keys | 79.0, 63.0, and 11.0 respectively, (RT-PCR) | Presence/absence only | 52 |
| Reoviruses | Beaches along the Catania coastline of Sicily | 27.0 (cell culture) | Presence/absence only | 7 |
| Enteroviruses and reoviruses | Beaches of Pesaro, Italy | 32.6 and 49.3, respectively (cell culture) | Presence/absence only | 116 |
| Reoviruses | Nearshore and canal waters around the mouth of the Tiber River, Italy | 3.0 (cell culture) | 2.0-5.0 | 6 |
| Enteroviruses | Nearshore waters, Florida Keys | 93.0 (RT-PCR) | 0.1 via cell culture for one water column sample; remaining presence/absence only via RT-PCR | 89 |
| Enteroviruses | Sarasota estuary, Fla. | 25.0 for samples (cell culture) 91.0 for stations positive (RT-PCR) | 17.0-77.0 (via cell culture) | 87 |
| Enteroviruses | Charlotte Harbor estuary, Fla. | 21.9 (cell culture), 75.0 during the 1997-1998 El Niño | 100.0-200.0 | 88 |
| Adenoviruses | Beaches from Malibu, Calif., to the Mexican border | 33.3 (PCR) | 8,800.0-75,000.0 (via MPN-nested PCR) | 68 |
| Reoviruses and enteroviruses | Fano Beach and Albani Channel Harbour, Italy | 30.0 and 0.0, respectively (RT-PCR) | Presence/absence only | 100 |
| Enteroviruses | Santa Monica Bay, Calif. | 32.0 (RT-PCR) | Presence/absence only | 103 |
In Italy, Pianetti et al. investigated nearshore waters along the beaches of Pesaro on the Adriatic Sea and found that 32% of 144 samples were positive for viable enteroviruses by cell culture (116). They concluded the viral pollution was originating from waste disposal systems (Foglia River discharge and regional public and resort wastewater disposal systems) being used in the region which periodically impact regional beach water quality. Previous studies by another group of researchers in the same area (estuarine waters of the Foglia River and Pesaro beach water samples) found poliovirus type 3 by reverse transcriptase polymerase chain reaction (RT-PCR) in cell culture-positive samples (98). Muscillo et al. surveyed wasters off of Fano's beaches and in Albani Channel Harbour (Adriatic coastline of Italy) and found that 30% of 72 samples were positive for the presence of reoviruses by RT-PCR (100). No enteroviruses were detected.
Aulicino et al. surveyed waters of the Catania coastline on Sicily (Ionian Sea) and nearshore sites around the mouth of the Tiber River (Tyrrhenian Sea) in Italy (6, 7). Reoviruses were detected in 27% (12 of 44) and 3% (12 of 58) of the samples in these two studies, respectively. No enteroviruses were detected in either of these studies. In the case of the Tyrrhenian Sea study, the authors attributed advanced wastewater treatment and disinfection as reasons for good water quality at a majority of the monitored sites. In Southwest Greece along the beaches of Patras (beaches on both the Gulf of Patras and the Corinthian Gulf), enteroviruses and adenoviruses were detected in 17% (21 of 120) and 28% (34 of 120) of the samples by nested PCR, respectively (160). When these samples were screened via cell culture, the enterovirus concentration was 290 PFU/100 liters. Only 9% (11 of 120) of the samples were positive for enteroviruses by cell culture, in contrast to the 17% positive sample rate via nested PCR.
A Hawaiian water quality study found that 8% of beach samples were positive for enteroviruses by cell culture (125). Concentrations were on average 3.7 PFU/100 liters, with the highest level detected at 10 PFU/100 liters. This study of Mamala Bay, Oahu, that included Waikiki and other beaches, suggested the viral pollution was originating from a wastewater outfall that received only primary treatment. This work led to recommendations that better sewage treatment be implemented.
Extensive studies in Florida have shown that enteroviruses and HAV can be routinely detected in nearshore marine environments. In Charlotte Harbor (important recreational and shellfish waters) on the Gulf Coast of Florida, enteroviruses were detected by cell culture assay in 75% of the sites screened (88). This study also found that combined surface water temperature, rainfall data, and regional river discharge rates could predict virus presence 98% of the time. In Sarasota, Fla., just north of Charlotte Harbor, enteroviruses were detected in 25% of the samples and at 81% of the sites by cell culture, with concentrations ranging from 0.17 and 0.59 PFU/100 liters (87). Using RT-PCR, 91% of the sites were shown to be positive for the presence of enteroviruses. Enteroviruses identified in these samples included echovirus types 20 and 24 and poliovirus. Their presence was attributed to the high density of septic tanks in this coastal community (87).
Over the last 20 years, extensive residential development in the Florida Keys, without the development of municipal wastewater treatment and disposal systems, has led to the majority of canal front homes using septic tanks for waste disposal. In these canals and in nearshore water sites of the Florida Keys, 79% of sample sites were positive for enteroviruses, 63% were positive for HAV, and 11% were positive for Norwalk viruses when samples were screened by RT-PCR (52). A total of 95% of sites were positive for at least one of the viral groups overall. Studies conducted in nearshore water of the Florida Keys showed that 93% of coral mucus samples were positive for the presence of enterovirus nucleic acid sequences as confirmed by PCR plus dot blot probing (89). The research in Florida has been able to demonstrate that: (i) the bacterial indicator levels used as recreational standards were not predictive of viral pollution; (ii) bacterial indicator levels were often under the standard limit, despite the presence of human viruses; and (iii) all sites were under the direct influence of either septic tank drain fields or compromised sewage treatment systems.
In Southern California, 33% (4 of 12) of marine samples were positive for adenoviruses. Most-probable-number (MPN) concentration estimates indicated that there were 880 to 7,500 adenoviruses per liter of water (68). These marine sites were located outside of river discharge points, and the authors noted that as in the Florida studies, bacteria indicators did not correlate with the presence of viruses. The authors did note that F+-specific RNA coliphages (RNA bacteriophages which require pilus production by strains of pilus-producing E. coli for attachment, i.e., F+ E. coli) did correlate with the presence of adenovirus (68). A study conducted in Santa Monica Bay, Calif., found enterovirus RNA in 32% of 50 samples by RT-PCR (103). These authors noted that while no correlation could be established between the presence of individual microbial water quality indicators and enterovirus RNA, there was a significant correlation between a recently adopted combined microbial indicator standard (total coliforms, fecal coliforms, and enterococci) and enterovirus RNA (103). In a 1979 study, 40% of the samples which passed bacterial indicator water quality standards contained viable enteroviruses (38). Other studies have shown that enterovirus contamination cannot be assessed by using bacterial indicators (46, 160).
Survival and Transport
A number of studies have investigated the ability of viruses to survive in marine systems and be transported from their sources to marine beaches or shellfish waters. The use of viral tracers was particularly successful in the study of septic tanks and package plants utilizing 90-ft injection wells in the Florida Keys. In Key Largo, Fla., bacteriophage were flushed down a toilet in a home with a septic tank and drain field adjacent to Port Largo Canal. The bacteriophage appeared in the canal in 11.2 h and in nearshore waters in 23 h. Estimated migration rates were as high as 24.2 m h−1, a rate 500-fold greater than that seen using subsurface flow meters (113). Bacteriophage flushed down two package plant injection wells located in Key Largo and Long Key appeared in groundwater monitoring wells in 8 h and in surface waters in 10 and 53 h, respectively. Migration rates from the Key Largo canal ranged from 2.5 to 3.5 m h−1, and the rates in Long Key were 0.1 to 2.0 m h−1. The higher rates in Key Largo were due to different influences of tidal pumping from site to site (111). In a similar study, viral tracer was observed in an adjacent canal in 3.25 h in a septio-tank study conducted in Marathon Key (Boot Key Harbor, the middle Keys). Another injection well study on Saddlebunch Key (lower Keys) found migration rates as high as 141.0 m h−1. In both studies, movement was toward the reef tract and Atlantic Ocean, with rapid movement being facilitated via tidal channels (112). All of the bacteriophage tracer studies demonstrated that septic tanks and wastewater treatment plants utilizing shallow-water injection wells do contribute to the deterioration of regional coastal surface water quality. This is of particular concern when waters such as those off the Florida Keys are heavily utilized for recreational activity in addition to being ecologically sensitive to poor water quality, a common trait of all coral reef ecosystems. Several physical factors such as tidal pumping and the porous nature of limestone contribute to the rapid groundwater migration rates observed in many of Florida's coastal communities (145).
Along the Catania coastline of Italy, reoviruses were detected only during the autumn and winter months, indicating that surface water temperature may play a key role in the survival of enteric viruses in marine environments (7). Another study noted that reoviruses were found in 95% of samples during the winter and in only 15% of the samples during the summer (59). In Charlotte Harbor, Fla., enteroviruses were detected by cell culture only in December and February, when lower surface water temperatures coincided with an increase in the number of water quality indicators relative to warmer months (88). This study also noted that an increase in rainfall and river discharge due to the 1997 to 1998 El Niño season decreased estuarine salinity and increased the probability of detecting viable enteroviruses (88). A 3-year study of French coastal waters indicated that the presence of viruses (enteroviruses, HAV, Norwalk-like virus, astrovirus and rotavirus) in shellfish usually coincided with incidence of human disease and episodes of rain. These authors hypothesized that short, heavy winter rains caused an overload of sewage treatment facilities, resulting in contamination of shellfish beds (92). Whether the source of feces in a given region is due to indigenous wildlife or human populations, precipitation events have been shown to significantly influence microbial water quality by increasing the bioload (33, 53, 88, 109, 129, 161, 165).
In a marine virus survival study, 90% inactivation of poliovirus and parvovirus was observed in 1 to 3 days at the highest temperature compared to 10 days at the lowest temperatures. The temperatures used in this study ranged from 6 to 28°C (163). In the Florida Keys, where 79% of the samples were positive for enterovirus presence by RT-PCR in the summer (the average water temperature ranged from 29 to 33°C), none of the samples were positive by cell culture (52). In a follow-up study to determine if water temperature influenced the viability of viruses being released into nearshore waters of the Florida Keys, six of the original samples were screened for the presence of viable viruses when surface water temperatures averaged ∼25°C. Two of the six sites were positive for enteroviruses by cell culture (J. L. Jarrell, E. K. Lipp, D. Griffin, J. Lukasik, D. Wait, M. Sobsey, and J. B. Rose, submitted for publication). This study further noted in a seeded marine study that viruses survived longer at moderate (22°C) than at higher (30°C) temperatures while viral nucleic acid persisted for a longer period (≥60 days) than did infectious viruses (≤51 days). These data emphasize the need to couple PCR with a viability assay (if one exists) to account for the detection of inactivated versus infectious human viruses.
A study at an offshore sewage sludge dumping site noted that viable enteric viruses could be detected in the sediments 17 months after dumping was halted (46). Enteroviruses associated with marine solids were shown to remain infectious for 19 days compared to 9 days for unassociated enteroviruses in the water column (121). The increase in the survival of enteroviruses associated with particulate matter has been observed by others (17, 82, 83). A 3-log-unit reduction in viable HAV and poliovirus type 1 at 5 days occurred in the water column (using a cell culture assay for a seeded estuarine study) (108). Marine mammal caliciviruses remained viable for over 14 days when the water temperature was 15°C (149). In a cell culture-based assay of feline caliciviruses, infectious viruses were detected for 30 days at or below 10°C (71).
This work has shown that temperature, rainfall, type of disposal system, and coastal processes such as tides and currents are responsible for the survival and transport of viruses in marine systems. The rates of survival and transport may be geographically dependent. Further research on comparative studies on coastal processes and particularly on the role of sediments is needed.
EVOLUTION OF DETECTION ASSAYS
In 1970, the Committee on Environmental Quality Management of the Sanitary Engineering Division of the American Society of Civil Engineers Committee Report stated that the methods for detecting, identifying, and enumerating viruses in water samples were inadequate (61). In 1971, when reference 61 was written by Hill et al., cell culture was the only method of viral detection for environmental samples. Many techniques such as radioimmunoassay, immunofluorescence (IF), complement fixation, and enzyme-linked immunosorbent assay (ELISA) were routinely used to identify viruses in clinical specimens but were either cost prohibitive or not sensitive enough for use with environmental samples. Today the standard method of virus detection continues to be cell culture.
Modern viral detection assays hold the greatest promise for current and future studies. These include molecular based techniques based on PCR and other amplification technologies. Regardless of the detection assay employed, research has demonstrated the need for efficient concentration from large volumes of water when attempting to detect viruses in all but the most polluted waters. Historically, the use of filter cartridges and the manipulation of water chemistry have been employed to accomplish this.
Sample Collection
Viral collection protocols evolved from the early use of gauze pads for capturing viruses. In the mid-1970s, the concentration of enteroviruses from 400 liters of marine water to volumes as low as 10 ml, with a recovery rate of 60 to 80% using cartridge filters, was demonstrated (30). It was shown that elution solutions at pH 9.0 were efficient at recovering viruses adsorbed to membrane filter surfaces (31). These methods used a pump to move marine water through a membrane filter located in a filter housing. After filtration, the viruses which were attached to the filter surface were released by using an eluate (1 to 3 liters) at a basic pH. Viruses were then concentrated in the eluate to 10 to 70 ml by using a series of chemical and pH manipulations and centrifugation. Concentrated viruses were then stored under refrigeration until an aliquot was used for cell culture assay. Fiberglass filters were utilized in a study in which rotaviruses were detected in large volumes of seawater (47). Another study investigated a microporous filtration method with recovery rates of 41% from 400 liters of water (155). This basic membrane filtration approach to collecting viruses from large volumes of water, although tedious, is still used today in many environmental virus studies (3). Variations of the original method exist for efficient recovery of viruses from freshwaters or marine waters by using a variety of filter types. A recently developed protocol which utilizes negatively charged membranes for filtration of 2 liters of marine water followed by real-time PCR for detection demonstrated recovery rates greater than 61% for poliovirus in seeded samples (74). This protocol is rapid and efficient and was used successfully to detect Norwalk virus and enteroviruses in Tokyo Bay (74).
An alternate method to membrane filtration, which has been successfully used to detect viruses in marine water, is vortex flow filtration (VFF). This technology utilizes a flow pattern (toroidal vortices) that keeps cell-sized particles from contacting the filter surface as water is removed from the sample. Typically 20-liter samples are concentrated to ∼50 ml over 2.5 to 3 h. The benefit to this technology is that it is automated and requires minimal supervision. The drawbacks are the cost of the equipment, the limitation on the volume of sample that can be concentrated relative to the existing membrane filtration protocol, and the time required for filtration. Samples seeded with T2 phage reported a recovery rate of 72% for T2 bacteriophage (110). Pathogenic human viruses have been detected in a number of marine water quality studies using this concentration method (52, 157). A similar ultrafiltration protocol known as tangential-flow filtration (TFF) is widely used in the biotechnology sector and has been successfully used to detect viruses in marine water (68, 159). While TFF has shown promise, some investigators have noted problems in regard to quantitative work with marine waters and a lower recovery rate in comparison to VFF (68, 128).
In an assay that was developed using silica particles and centrifugation, enteroviruses were detected in seeded and unseeded samples from an original marine water volume of 10 liters. This assay, like the outlined membrane filtration protocol, utilized pH manipulation to alternately capture and release viruses from a matrix (106).
Cell Culture
Until the 1990s, cell culture assay was the most widely used protocol for the detection of viruses in water (30, 37, 38, 46, 47, 121, 141). It had long been recognized that cell culture for the detection of viruses in waters and sediments has numerous shortcomings, such as long turnaround time, low sensitivity, and high cost. In addition, cell culture can be labor-intensive and host cells for many viruses such as the Norwalk-like viruses have not been identified (44, 151). Although the limitations of cell culture are clear, there is no alternative protocol to determine if viruses present in a given volume of water are viable and thus capable of causing infection. In a typical cell culture protocol, an aliquot of sample is exposed to host cells and incubated over time. If the viable viruses are present, destruction of host cells occurs, a process known as the cytopathic effect (115). Incubation periods vary depending on the protocol used, but the typical range is 2 to 4 weeks and can be double that depending on confirmation steps. Given the length of time required to complete the assay, it is clearly not a proactive monitoring protocol. To date, cell culture is used in parallel or in conjunction with emerging detection assays to address the issue of viability and to compare the sensitivity of assays (2, 48, 78, 87, 139; M. Abbaszadegan, P. Stewart, M. LeChevallier, M. Yates, and C. Gerba, Proc. Water Qual. Technol. Conf., 1995). The only alternatives to the cell culture assay prior to the mid- to late 1980s were the use of electron microscopy (EM) and hybridization or antibody-based assays to enumerate or detect viruses (11, 47, 58, 64).
Immunology
In the 1980s, several researchers tried some of the immunological methods used in clinical laboratories on environmental samples. Steinmann (151) used enzyme-linked immunosorbent assay (ELISA) and EM to detect rotavirus in concentrated sewage. Dowell et al. (25) used a nitrocellulose enzyme immunosorbent assay technique (NC-EIA) to monitor viruses in environmental waters. Both NC-EIA and ELISA are fast and easy and can be adapted for instrumentation, large-scale studies, and quantitative analysis. However, these methods can detect only high concentrations of viral particles. Ten million virus particles per ml of sample must be present to be detected by EM, and viral concentrations in coastal environments would probably be several orders of magnitude lower than this. Additionally, immunological techniques such as ELISA, radioimmunoassay, and IF can identify only viruses for which a specific antibody has been produced and cannot distinguish between infectious and non-infectious particles.
However, if IF is used in conjunction with cell culture, it can increase sensitivity, provide information on infectivity of the virus, and yield results in 24 to 48 h. One of the first uses of IF with cell culture for the detection of rotaviruses was described by Gerba et al. (39). However, again, only viruses for which appropriate cell lines and specific antibodies have been produced can be detected by this method.
PCR Amplification
A few of the most recent studies utilizing PCR and RT-PCR, nested PCR, and integrated cell culture PCR for marine samples are described in Table 3. Of the 10 studies, 5 focused on virus detection in shellfish and the other 5 examined viral contamination of marine waters. This compilation of work demonstrates that a diverse group of enteric viruses can be detected, including Norwalk and Norwalk-like viruses, HAV, small round structured viruses, enteroviruses, rotavirus, and adenoviruses. Some studies reported the sensitivity in regard to PFU as measured in cell culture. As few as 0.1 PFU could be detected, but other studies reported the sensitivity of the assay based on viral particles (PCR units), and only Jiang et al. (68) reported the sensitivity per volume of water concentrated. A variety of methods and primers and probes were used, and thus far no standardization of methods has been forthcoming. The American Academy of Microbiology has strongly recommended to the EPA, European Union, World Health Organization, and other organizations that they financially support the round-robin testing of PCR methods for detection of viruses as well as other microorganisms. This is needed so that a consensus method and standardization for the application of PCR for virus detection in all types of water can be developed (132).
TABLE 3.
Compilation of select viral studies in marine systems by PCRa
| Author (yr) | Amt and sample type | Concn method | Viral extraction or elution | Nucleic acid extraction | Method | Virus isolated | Primers (5′ to 3′) | Probe | Amplicon, target | Sensitivity and range detected |
|---|---|---|---|---|---|---|---|---|---|---|
| Atmar (1995) | 1.5 g of oyster (Crassostrea virginica) and hard-shell clam (Mercenaria mercenaria) tissue (stomach and diverticula) | Homogenization, centrifugation | PEG 6000 centrifugation | Proteinase K, phenol-chloroform extraction, ethanol precipitation | RT-PCR confirmation by Southern hybridization | HAV NV | GGAAATGTCTCAGGTACTTTCTTTG GTTTTGCTCCTCTTTATCATGCTATG CTTGTTGGTTTGAGGCCATAT ATAAAAGTTGGCATGAACA | CTCCAGAATCATCTCC GGCCTGCCATCTGGATTGCC | VP1, 248 bp Polymerase gene region, 470 bp | 100 PFU Oysters (5-200 PCR units), clams (10-100 PCR units) (1 PCR unit is ca. 42 viral genomes) |
| Green (1998) | 50 g of shellfish flesh | homogenization, sonification centrifugation | Freon TF centrifugation | Reaction mix of glass powder matrix and guanidine isothiocyanate | Nested RT-PCR | SRSV group I | TCNGAAATGGATGTTGG CGATTTCATCATCACCATA Internal: GAATTCCATCGCCCACTGGCT ATCTCATCATCACCATA | NA | 4679-4871, 192 bp | 100- to 10,000-fold greater than single round RT-PCR |
| SRSV group II | AGCCNTNGAAATNATGGT | NA | 4338-4607, 269 bp | |||||||
| CGATTTCATCATCACCATA | ||||||||||
| Internal: GAATTCCATCGCCCACTGGCT | ||||||||||
| Griffin (1999) | 110 liters of marine water | Filterite DFN 0.45-10UN | 1.5% beef extract-0.05 M glycine, concentrated using organic flocculation | RNEasy minikit (Qiagen, Santa Clarita, Calif.) | RT-PCR confirmation by dot blotting | EV HAV | CCTCCGGCCCCTGAATG ACCGGATGGCCAATC CAGCACATCAGAAAGGTGAG CTCCAGAATCATCTCCAAC | TACTTTGGGTGTCCGTGTTTC TGCTCCTCTTTATCATGCTATG | 5′-untranslated region, 197 bp VP1 and VP2 capsid protein protein interphase, 192 bp | 103 PFU 0.1 PFU |
| NV | CAAATTATGACAGAATCCTTC | ATGTCATCAGGGTCAAAGAGG | Viral polymerase, 260 bp | ∼106 virions | ||||||
| GAGAAATATGACATGGATTGC | ||||||||||
| SRSV (Ando) | TGTCACGATCTCATCATCACC TGGAATTCCATCGCCCACTGG | ATGTCAGGGGACAGGTTTGT ATGTCGGGGCCTAGTCCTGT ACATCGGGTGATAGGCCTGT | RNA polymerase region, 123 bp | ND | ||||||
| Green (1999) | ≥50 g of shellfish tissue (stomach and digestive gland) | Homogenization in 1:7 (wt/vol) 10% TPB-0.05 M glycine (pH9), centrifugation | Chloroform extraction, PEG 6000 precipitation | Proteinase K digestion, phenol-chloroform extraction, ethanol precipitation | RT-PCR and cell culture RT-PCR | EV RV | TCCGGCCCCTGAATGCG CACCGGATGGCCAATCC AAACGAAGTCTTCAACATGG | NA NA | 5′ nontranslated region, 196 bp 5′ terminus of gene 6, 154 bp | ND ND |
| TGAAACTCATTTCCATTCAT | ||||||||||
| RT-PCR | HAV | CTTGGTTTCATGAATCTCT AGGCCCCAAAGAACAGTCCA | NA | 5′ nontranslated region, 314 bp | ND | |||||
| Le Guyader (2000) | <1.5 g oyster (Crassostrea gigas) and mussel (Mytilus galloprovincialis) (stomach and diverticula) | Homogenization and centrifugation | PEG 6000 centrifugation | Proteinase K, phenol-chloroform extraction, ethanol precipitation | RT-PCR and hybridization | AV | GCTTCTGATTAAATCAATTTT CGAGTAGGATCGAGGGTA | ACCATTTAAAATTGATTTAATC | 6727-6797, 89 bp | ND |
| EV | TCCGGCCCCTGAATGCGG CACCGGATGGCCAATCCAAT CAAGCACTTCTGTTTCCCCGG | ACACGGACACCCAAAGTAGTCGGTTGG | 446-642, 196 bp 162-596, 434 bp | |||||||
| ATTGTCACCATAAGCAGCCA | TCAACAACAGTTTCTACAGA | 2389-2192, 247 bp | ||||||||
| HAV | GGAAATGTCTCAGGTACTTTCTTTG GTTTTGCTCCTCTTTATCATGCTATG | |||||||||
| NLV | GTAAAACGACGGCCAGACDATYC CAGGAAACAGCTATGACATAAAAG GGCCTGCCATCTGGATTGCC GAATTCCATCGCCCACTGGCT GTGAACAGCATAAATCACTGG GTGAACAGTATAAACCACTGG GTGAACAGTATAAACCATTGG | CTCTGTGCACTTTCTGAAGT ACCTTGTGTGCCATGTCTGA CTATGTGCACTGTCAGGAGT ATGTCAGGGGACAGGTTTGT ATGTCGGGGCCTAGTCCTGT | See reference | |||||||
| RV | GGCTTTAAAAGAGAGAATTTCCGTCTGG GATCCTGTTGGCCATCC | GTATGGTATTGAATATACCAC GTCACCATCATTGATTGAGTACTT TTCATCATCTGAAATCTCATTTTTA TGAATTATCATTTATTTCTGTTGCT TTCGTGTCACTAATTTGAGTTGGA | 1-392, 392 bp | |||||||
| Schwab (2001) | 1.5 g of oyster (Crassostrea virginica) and hard-shell clam (Mercenaria mercenaria) tissue (stomach and diverticula) | Homogenization, centrifugation | PEG 6000 centrifugation | Proteinase K, phenol-chloroform extraction, ethanol precipitation | RT-PCR-DEIA and Southern hybridization | HAV NV | GGAAATGTCTCAGGTACTTTCTTTG GTTTTGCTCCTCTTTATCATGCTATG CTTGTTGGTTTGAGGCCATAT ATAAAAGTTGGCATGAACA | GTGATAGCTCCCACAGGTGC | VP1 region, 248 bp | Comparable to Southern blot |
| Lipp (2001) | 100L estuarine surface water | Filterite DFN 0.45-10 UN | 1.5% beef extract-0.05 M glycine (pH 9.5) concentration using organic flocculation | NA | Cell culture CPE enumerated by MPN | EV | NA | NA | NA | 1.0-2.0 MPN 100 liters−1 |
| Jiang (2001) | 20-40 liters of coastal marine water | TFF (30-kDa spiral filter cartridge) VFF (100-kDa filter cartridge) | Centriprep-30 ultracentrifugation unit NA | NA | Nested PCR | AV | GCCGCAGTGGTCTTACATGCACATC CAGCACGCCGCGGATGTCAAAGT Internal: GCCACCGAGACGTACTTCAGCCTG TTGTACGAGTACGCGGTATCCTCGCGGTC | NA | 143 bp | 1 purified viral particle |
| Donaldson (2001) | 100 μl | NA | NA | RNeasy minikit | Real-time RT-PCR | EV: CX A9, CX A16, PV1 | GGCCCCTGAATGCGGCTAAT | CGGACACCCAAAGTAGTCGGTTCCG | 5′-untranslated region, 192 bp | 9.3 viral particles/ml to 155 viral particles/g of sponge |
| 20 l | VFF (100-kDa filter cartridge) | NA | CACCGGATGGCCAATCCAA | |||||||
| Reynolds (2001) marine water | Filterite electronegative filter | 1.5% beef extract-0.05 M glycine (pH 9.5) concentration and flocculation | NA Heat extraction for direct PCR | ICC-PCR PCR, cell culture | EV | TGTCACCATAAGCAGCC TCCGGCCCCTGAATGCGGCT Internal: CCCAAAGTAGTCGGTTCCGC | NA | 149 bp | EV ICC/PCR (0.1 PFU) HAV ICC/PCR (10 MPN/flask), PCR 12 (PFU/10 μl) | |
| HAV | CAGCACATCAGAAAGGTGAG CTCCAGAATCATCTCAAC Internal: GCTTCCCATGTCAGAGTG | 192 bp | Cell culture (0.002 MPN) |
Abbreviations: AV, adenoviruses; EV, enterovirus; CPE, cytopathic effect; CX, coxsackieviruses; DEIA, DNA enzyme immunoassay; HAV, Hepatitis A virus; ICC-PCR, integrated cell culture PCR; MPN, most probable number; NA, not applicable; ND, not done; NLV, Norwalk-like viruses; NV, Norwalk virus; PV, polioviruses; RT-PCR, reverse transcription-PCR; RV, rotaviruses; VP, viral capsid protein.
Since the late 1980s, PCR has been used for the detection and quantitation of viral pathogens in the environment. PCR was first developed and employed in laboratory-based studies (95, 135). Some of the earliest studies employing RT-PCR for the detection of enteroviruses included laboratory and field studies (15; R. DeLeon, C. Shieh, S. Baric, and M. D. Sobsey, Proc. Water. Qual. Technol. Conf., 1990). Standard RT-PCR has since been used in a number of marine-based water quality monitoring studies (52, 87-89, 118, 122).
A enterovirus primer set first used in environmental monitoring in the late 1980s is currently one of the most widely used primer-probe combinations employed in environmental monitoring due to good sensitivity, specificity, and reliability (DeLeon et al., Proc. Water Qual. Technol. Conf., 1990). An evaluation of this primer set against four other existing or new sets demonstrated that the original was superior or equivalent in sensitivity and specificity (unpublished data). This primer-probe set was used by Griffin et al. (52) and the sequences are listed in Table 3.
One of the central obstacles to the use of PCR in environmental monitoring is the susceptibility of the assay to inhibitors (divalent cations, humic acids, etc.) commonly found in aquatic samples. The traditional approach of separating viral nucleic acids from inhibitors was through the use of phenol-chloroform extraction protocols. The drawback to this protocol is time to completion and loss of viral genomes during the repetitive washing steps, which limited the ability to detect small numbers of viruses in water samples. A more rapid and sensitive assay was developed in the late 1980s which utilized heat to liberate viral nucleic acids (127). Several protocols involved using polyethylene glycol (PEG) and/or Pro-Cipitate (Bionostics Inc., Devens, Mass.) to concentrate and purify viral nucleic acids from water samples (67, 138, 156). Freon has been used to isolate Norwalk viruses from stool specimens followed by heat release of the Norwalk virus genomes from their capsids (140). One of the most widely used methods, due to outstanding reliability and simplicity, for purifying viral nucleic acids from environmental water samples was first described in a clinical setting (10). This assay uses silica beads to capture nucleic acids, which is the basis for many of the commercially available nucleic acid capture and purification kits. Other assays that used antibodies to capture viruses or genetic sequences include immunomagnetic or genomagnetic separation. Numerous studies have used antibody-coated vesicles or antibodies labeled with iron particles (immunomagnetic separation) to separate and purify viruses from particulate-laden samples (5, 51, 93, 139, 142).
Variants of the standard RT-PCR assay have been used to detect human viruses in laboratory and environmental studies. A nested RT-PCR assay which employs two primer sets (the second internal to the first used in successive reactions) was developed for sensitive (15 to 21 viruses) detection of Norwalk-like viruses in shellfish tissue (50). Nested multiplex RT-PCR was used for simultaneous detection of enteroviruses and herpesviruses in a clinical study (153). A seminested RT-PCR using the same primer on one end of the gene target and two more primers, one internal to the other, in a successive-PCR protocol was developed to detect HAV, enteroviruses, rotavirus, and Norwalk-like viruses in seafood samples (55). Booster nested RT-PCR, an assay which uses two primer sets, one internal to the other, in a single RT-PCR run was developed for sensitive detection of the Norwalk-like Sapporo virus. This assay proved more sensitive than standard RT-PCR followed by Southern hybridization (63). Triplex RT-PCR, which used three primer sets (each for a specific virus), was developed and used for simultaneous detection of poliovirus, HAV, and rotavirus in sewage and marine water samples (157).
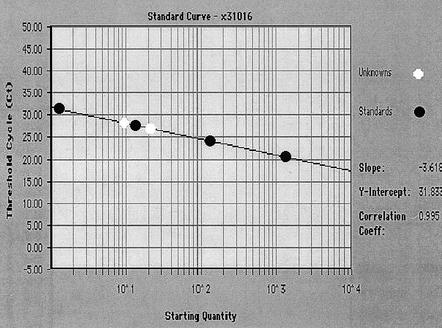
Quantification of viral particles is difficult when using molecular detection assays. An MPN PCR which used multiple amplification reactions to estimate viral numbers was successful in enumerating adenoviruses in marine waters along the coastline of Southern California (68). Real-time PCR was first developed for clinical quantitative PCR and has recently been adapted for environmental monitoring of enteroviruses in sewage and marine samples (24, 94, 158). Real-time PCR relies on the detection of a fluorescent signal produced during the amplification reaction that is proportional to the amount of amplicon being produced (Fig. 1). The benefit of this assay is that it is quantitative and the target amplicon is verified by using a fluorescently labeled probe which recognizes internal amplicon sequences. A detection limit of 13 viral particles in marine waters has been demonstrated (24).
FIG. 1.
Real-time PCR. A TaqMan poliovirus standard curve generated by plotting threshold cycle (CT) against the log of the virus concentration. The CT occurs where the sequence detection software begins to detect an increase in signal due to the exponential growth of PCR amplicons. Standards are shown here in black, two natural-water samples seeded with poliovirus are shown in white. A 2-ml volume of a poliovirus Sabin type 1 cell culture was lysed by vigorous shaking, and extracellular DNA was digested with pancreatic DNase I. Viral particles were purified using a glycerol step gradient (136), stained with SYBR Gold, and directly counted by epifluorescence microscopy (104). The stock dilution contained 9.1 × 103 viruses per μl. A 10-μl sample of the stock was purified and brought up in 70 μl of water. Four dilutions ranging from 1.3 × 100 to 1.3 × 103 were made using UV-treated 0.02-μm-pore-size-filtered deionized water to generate an RNA standard curve. RT-PCR on all standards was performed in triplicate. Two natural-water samples (white dots) were seeded with poliovirus and quantitated using this standard curve. The quantities of the two natural-water samples were 7.2 × 100 and 1.7 × 101 viruses per μl.
In addition to the susceptibility of PCR to environmental inhibitors, the remaining limitation is the inability of the assay to determine virus viability. While some investigations which utilize docking proteins of host cells for capture of viruses particles, with the assumption being that if the virus can still recognize its host cell docking protein it must be viable, are under way, the only recognized viability assay is cell culture. For viruses such as the enteroviruses and HAV, for which cell culture assays exist, the integration of cell culture with PCR was a means of addressing this dilemma.
Integrated cell culture-PCR (ICC-PCR) is rapid and specific and yields information on the infectivity of a virus (123). ICC-PCR allowed the detection of infectious enteroviruses within 48 h with detection limits as low as 0.001 MPN/liter of sample (126). ICC-PCR sensitivity was later shown to be 1 PFU for enteroviruses at 20 h of incubation and 1 to 10 PFU for HAV at 72 to 96 h (124). This research also demonstrated that 11 samples in the sample set were positive via ICC-PCR in comparison to 3 samples via straight RT-PCR. A modification of this assay using seminested RT-PCR has been published (97). Drawbacks to this method include the difficulty in adapting it for large-scale studies and automation. As in standard cell culture, maintenance of cell lines can be cost prohibitive. However, this technique can be faster and more sensitive than traditional cell culture, and it is one of the few techniques that can determine viral infectivity.
CONCLUSION AND PERSPECTIVES
It has been recognized that the observed decrease in coastal marine water quality occurring in areas impacted by human waste and refuse is negatively affecting both human and ecosystem health (57, 60, 144). A majority of pathogens responsible for outbreaks of human illnesses acquired from marine recreational exposure have not been identified but are thought to be viruses. Contrary to popular belief, many cell culture-based assays have shown that viable pathogenic human viruses can readily be detected in marine water being impacted by human sewage. From the literature cited in this review, it is clear that this has been demonstrated repeatedly since the 1970s. A common trend among many of the cited occurrence studies is that bacterial indicator occurrence did not correlate with viral occurrence. In addition, in a majority of the studies that monitored marine waters for both bacterial indicators and pathogenic viruses, viruses were detected when indicator levels were below public health water quality threshold levels (54). This creates a significant dilemma for public health officials who are responsible for marine water quality monitoring. The data obtained from three decades of occurrence studies warrant investigating the use of human viruses as indicators of marine water quality and public health risk.
Although progress has been made in the search for an ideal viral detection method, problems remain. Although most molecular biology-based assays are sensitive, specific, rapid and cost efficient, there has been no development of a consensus method or standardization. The use of docking proteins, antibodies, and genetic sequences for viral capture holds promise in isolating a particular virus from inhibitory particulate matter found in water samples. Gene chips, with their theoretical ability to detect individual viral genomes and to simultaneously detect multiple viral types, should prove useful in monitoring assays when combined with target capture protocols, cell culture, and PCR.
In the not too distant future, it may be that climate and marine conditions will be used in models to predict the risk to human health in marine environments. As cited studies have shown, precipitation, salinity, and water temperature have been correlated with viral occurrence and infectivity. The current limitations to the accurate development of public health risk models is the lack of integrated occurrence studies which characterize factors such as soil type, sewage disposal system density, pathogen and indicator occurrence, and the influence of climate. Tools and techniques are now available to conduct these types of studies to make the global assessment of viral pollution in marine waters achievable.
REFERENCES
- 1.Reference deleted.
- 2.Abbaszadegan, M., P. Stewart, and M. LeChevallier. 1999. A strategy for detection of viruses in groundwater by PCR. Appl. Environ. Microbiol. 66:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 4.Ando, T., S. S. Monroe, J. R. Gentsch, Q. Jin, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando, T., S. S. Monroe, J. S. Noel, and R. I. Glass. 1997. A one-tube method of reverse transcription-PCR to efficiently amplify a 3-kilobase region from the RNA polymerase gene to the poly(A) tail of small round-structured viruses (Norwalk-like viruses). J. Clin. Microbiol. 35:570-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aulicino, F. A., P. Orsini, M. Carere, and A. Mastrantonio. 2001. Bacteriological and virological quality of seawater bathing areas along the Tyrrhenian coast. Int. J. Environ. Health Res. 11:5-11. [DOI] [PubMed] [Google Scholar]
- 7.Aulicino, F. A., L. Mauro, M. Marranzano, M. Biondi, A. Ursino, and M. Carere. 2000. Microbiological quality of the Catania coastal sea water. Ann. Ig. 12:533-541. [PubMed] [Google Scholar]
- 8.Balarajan, R., V. Soni Raleigh, P. Yuen, D. Wheeler, D Machin, and R. Cartwright. 1991. Health risks associated with bathing in sea water. Br. Med. J. 303:1444-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bern, C., J. Martinew, I. deZoysa, and R. I. Glass. 1992. The magnitude of the global problem of diarrhoeal disease: a ten year update. Bull. W. H. O. 70:705-714. [PMC free article] [PubMed] [Google Scholar]
- 10.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-Dillen, and J. Van Der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:981-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsheim, K. Y., G. Bratbak, and M. Heldal. 1990. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl. Environ. Microbiol. 56:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley, D. 1992. Hepatitis E—epidemiology, etiology and molecular-biology. Rev. Med. Virol. 2:19-28. [Google Scholar]
- 13.Burkhardt, W., III, W. D. Watkins, and S. R. Rippey. 1992. Seasonal effects on accumulation of microbial indicator organisms by Mercenaria mercenaria. Appl. Environ. Microbiol. 58:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecuk, D., V. Kruzic, B. Turkovic, and M. Gree. 1993. Human viruses in the coastal environment of a Croatian harbor. Rev. Epidemiol. Santé 41:487-493. [PubMed] [Google Scholar]
- 15.Chapman, N. M., S. Tracy, C. J. Gauntt, and U. Fortmueller. 1990. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J. Clin. Microbiol. 28:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung, W. H. S., K. C. K. Chang, and R. P. S. Hung. 1990. Health effects of beach water pollution in Hong Kong. Epidemiol. Infect. 105:139-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung, H., and M. D. Sobsey. 1993. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci. Technol. 27:425-428. [Google Scholar]
- 18.Colwell, R. R., G.T. Orlob, and J. R. Schubel. 1996. Mamala Bay study report, vol. 1. City of Honolulu.
- 19.Crabtree, K. D., C. P. Gerba, J. B. Rose and C. N. Haas. 1997. Waterborne adenovirus: a risk assessment. Water Sci. Technol. 35:2548-2553. [Google Scholar]
- 20.Crossette, B. 1996. Hope, and pragmatism, for U.N. cities conferences, p. A3. New York Times, New York, N.Y.
- 21.Dahling, D. R., R. S. Safferma, and B. A. Wright. 1989. Isolation of enterovirus and reovirus from sewage and treated effluents in selected Puerto Rican communities. Appl. Environ. Microbiol. 55:503-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalldorf, G., and G. M. Sickles. 1948. An unidentified, filterable agent isolated from the feces of children with paralysis. Science 108:61-62. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Donaldson, K. A., D. W. Griffin, and J. H. Paul. 2001. Detection, quantitation and identification of enteroviruses from surface waters of the Florida Keys using real-time RT-PCR. Water Res. 36:2505-2514. [DOI] [PubMed] [Google Scholar]
- 25.Dowell, S. F., C. Groves, K. B. Kirkland, H. G. Cicirello, T. Ando, Q. Jin, J. R. Gentsch, S. S. Monroe, C. D. Humphrey, C. Slemp, D. M. Dwyer, R. A. Meriwether, and R. I. Glass. 1995. A multistate outbreak of oyster-associated gastroenteritis: implications for interstate tracing of contaminated shellfish. J. Infect. Dis. 171:1497-1503. [DOI] [PubMed] [Google Scholar]
- 26.Dufour, A. P., T. H. Ericksen, R. K. Ballentine, V. J. Cabelli, M. Goldberg, and W. E. Fox. 1986. Bacteriological ambient water quality criteria for marine and fresh recreational waters. Ambient water quality criteria for bacteria EPA44075-84-002. U.S. Environmental Protection Agency, Washington, D.C.
- 27.Eisenberg, J. N., E. Y. W. Seto, A. W. Olivieri, and R. C. Spear. 1996. Quantifying water pathogen risk in an epidemiological framework. Risk Anal. 16:549-563. [DOI] [PubMed] [Google Scholar]
- 28.Enriquez, C. E., C. J. Jurst, and C. P. Gerba. 1995. Survival of the enteric adenovirus 40 and 41 in tap, sea and waste water. Water Res. 29:2548-2560. [Google Scholar]
- 29.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 30.Farrah, S. R., S. M. Goyal, C. P. Gerba, C. Wallis, and J. L. Melnick. 1977. Concentration of enteroviruses from estuarine water. Appl. Environ. Microbiol. 33:1192-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrah, S. R., and G. Bitton. 1978. Elution of poliovirus adsorbed to membrane filters. Appl. Environ. Microbiol. 36:982-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feachem, R. G., D. J. Bradley, H. Garelick, and D. D. Mara. 1983. Sanitation and disease: health aspects of excreta and wastewater management. John Wiley & Sons, Inc., New York, N.Y.
- 33.Ferguson, C. M., B. G. Coote, N. J. Ashbolt, and I. M. Stevenson. 1996. Relationships between indicators, pathogens and water quality in an estuarine system. Water Res. 30:2045-2054. [Google Scholar]
- 34.Fleisher, J. M., D. Kay, R. L. Salmon, F. Jones, M. D. Wyer, and A. F. Godfree. 1996. Marine waters contaminated with domestic sewage: nonenteric illnesses associated with bather exposure in the United Kingdom. Am. J. Public Health 86:1228-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foy, H. M. 1997. Adenoviruses, p. 119-138. In A. Evans and R. Kaslow (ed.), Viral infections in humans: epidemiology and control, 4th ed. Plenum Press, New York, N.Y.
- 36.Gerba, C. P. 2000. Assessment of enteric pathogen shedding during recreational activity and its impact on water quality. Quant. Microbiol. 2:55-68. [Google Scholar]
- 37.Gerba, C. P., and S. M. Goyal. 1978. Detection and occurrence of enteric viruses in shellfish: a review. J. Food Prot. 41:743-754. [DOI] [PubMed] [Google Scholar]
- 38.Gerba, C. P., S. M. Goyal, R. L. LaBelle, I. Cech, and G. F. Bodgan. 1979. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health 69:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerba, C. P., B. H. Keswik, H. L. Dupont, and H. A. Fields. 1984. Isolation of rotavirus and hepatitis A virus from drinking water. Monogr. Virol. 15:119-125. [Google Scholar]
- 40.Gerba, C. P., J. B. Rose, and C. N. Haas. 1996. Sensitive populations: who is at the greatest risk? Int. J. Food Microbiol. 30:113-123. [DOI] [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.Gerba, C. P., and J. B. Rose. 1990. Viruses in source and drinking water, p. 381-396. In G. A. McFeters (ed.), Drinking water microbiology. Springer-Verlag, New York, N.Y.
- 43.Gerba, C. P., J. B. Rose, C. N. Haas, and K. D. Crabtree. 1996. Waterborne rotavirus: a risk assessment. Water Res. 12:2929-2940. [Google Scholar]
- 44.Gilgen, M., D. Germann, J. Lüthy, and P. H. Hüber. 1997. Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. Int. J. Food Microbiol. 37:189-199. [DOI] [PubMed] [Google Scholar]
- 45.Goyal, S. M., C. P. Gerba, and J. L. Melnick. 1979. Human enteroviruses in oysters and their overlying waters. Appl. Environ. Microbiol. 37:572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goyal, S. M., W. N. Adams, M. L. O'Malley, and D. W. Lear. 1984. Human pathogenic viruses at sewage sludge disposal sites in the Middle Atlantic region. Appl. Environ. Microbiol. 48:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goyal, S. M., and C. P. Gerba. 1983. Viradel method for detection of rotavirus from seawater. J. Virol. Methods 7:279-285. [DOI] [PubMed] [Google Scholar]
- 48.Grabow, W. O. K., K. D. Botma, J. C. de Villiers, C. G. Clay, and B. Erasmus. 1999. Assessment of cell culture and polymerase chain reaction procedures for the detection of polioviruses in wastewater. Bull. W. H. O. 77:973-980. [PMC free article] [PubMed] [Google Scholar]
- 49.Gratacap-Cavallier, B., O. Genoulaz, K. Brengel-Pesce, H. Soule, P. Innocenti-Francillard, M. Bost, L. Gofti, D. Zmirou, and J. M. Seigneurin. 2000. Detection of human and animal rotavirus sequences in drinking water. Appl. Environ. Microbiol. 66:2690-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green, J., K. Henshilwood, C. I. Gallimore, D. W. G. Brown, and D. N. Lees. 1998. A nested reverse transcriptase PCR assay for detection of small round-structured viruses in environmentally contaminated molluscan shellfish. Appl. Environ. Microbiol. 64:858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greening, G. E., L. Woodfield, and G. D. Lewis. 1999. RT-PCR and chemiluminescent ELISA for detection of enteroviruses. J. Virol. Methods 82:157-166. [DOI] [PubMed] [Google Scholar]
- 52.Griffin, D., C. J. Gibson III, E. K. Lipp, K. Riley, J. H. Paul, and J. B. Rose. 1999. Detection of viral pathogens by reverse transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Appl. Environ. Microbiol. 65:4118-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin, D. W., R. Stokes, J. B. Rose, and J. H. Paul. 2000. Bacterial indicator occurrence and the use of an F+ specific RNA coliphage assay to identify fecal sources in Homosassa Springs, Florida. Microb. Ecol. 39:56-64. [DOI] [PubMed] [Google Scholar]
- 54.Griffin, D. W., E. K. Lipp, M. McLaughlin, and J. B. Rose. 2001. Marine recreation and public health microbiology: quest for the ideal indicator. Bioscience 51:817-825. [Google Scholar]
- 55.Hafliger, D., M. Gilgen, J. Luthy, and P. Hubner. 1997. Seminested RT-PCR systems for small round structured viruses and detection of enteric viruses in seafood. Int. J. Food Microbiol. 37:27-36. [DOI] [PubMed] [Google Scholar]
- 56.Haile, R., J. Witte, J. Alamillo, K. Barrett, R. Cressey, J. Dermond, C. Ervin, A. Glasser, N. Harawa, R. Harmon, J. Harper, C. McGee, R. Millikan, and M. Nides. 1996. An epidemiological study of possible health effects of swimming in Santa Monica Bay. Santa Monica Bay restoration Project.
- 57.Hallock, P., F. E. Muller-Karger, and J. C. Halas. 1993. Coral reef decline. Natl. Geogr. Res. 9:358-378. [Google Scholar]
- 58.Hara, S., K. Terauchi, and I. Koike. 1991. Abundance of viruses in marine waters: assessment by epifluorescence and transmission electron microscopy. Appl. Environ. Microbiol. 57:2731-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havelaar, A. H., M. van Olphen, and Y. C. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henrickson, S. E., T. Wong, P. Allen, T. Ford, and P. R. Epstein. 2001. Marine swimming-related illness: implications for monitoring and environmental policy. Environ. Health Perspect. 109:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill, W. F. J., E. W. Akin, and W. H. Benton. 1971. Detection of viruses in water: a review of methods and application. Water Res. 5:967-995. [Google Scholar]
- 62.Ho, M. S., R. I. Glass, P. F. Pinshy, and L. L. Anderson. 1988. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J. Infect. Dis. 158:1112-1116. [DOI] [PubMed] [Google Scholar]
- 63.Honma, S., S. Nakata, Y. Sakai, M. Tatsumi, K. Numata-Kinoshita, and S. Chiba. 2001. Sensitive detection and differentiation of Sapporo Virus, a member of the family Caliciviridae, by standard and booster nested polymerase chain reaction. J. Med. Virol. 65:413-417. [DOI] [PubMed] [Google Scholar]
- 64.Hurst, C. J., W. H. Benton, and R. E. Stetler. 1989. Detecting viruses in water. J. Am. Water Works Assoc. Sept.:71-79.
- 65.Hurst, C. J. 1997. Sampling viruses from soil, p. 400-412. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 66.Reference deleted.
- 67.Jaykus, L.-A., R. De Leon, and M. D. Sobsey. 1996. A virion concentration method for detection of human enteric viruses in oysters by PCR and oligoprobe hybridization. Appl. Environ. Microbiol. 62:2074-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang, S., R. Noble, and W. Chu. 2001. Human adenoviruses and coliphages in urban runoff-impacted coastal waters of Southern California. Appl. Environ. Microbiol. 67:179-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang, X., N. Wilton, W. M. Zhong, T. Farkas, P. W. Huang, E. Barrett, M. Guerrero, G. Ruiz-Palacios, K. Y. Green, J. Green, A. D. Hale, M. K. Estes, L. K. Pickering, and D. O. Matson. 2000. Diagnosis of human caliciviruses by use of enzyme immunoassays. J. Infect. Dis. 181:S349-S359. [DOI] [PubMed] [Google Scholar]
- 70.Jothikumar, N., K. Aparna, S. Kamatchiammal, R. Paulmurugan, S. Saravanadevi, and P. Khanna. 1993. Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl. Environ. Microbiol. 59:2558-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadoi, K., and B. K. Kadoi. 2001. Stability of feline caliciviruses in marine water maintained at different temperatures. New Microbiol. 24:17-21. [PubMed] [Google Scholar]
- 72.Kaplan, J. E., R. A. Goodman, L. B. Schonberger, E. C. Lippy, and G. W. Gary. 1982. Gastroenteritis due to Norwalk virus: an outbreak associated with municipal water system. J. Infect. Dis. 146:190-197. [DOI] [PubMed] [Google Scholar]
- 73.Kappus, K. D., J. S. Marks, R. C. Holman, J. K. Bryant, C. Baker, G. W. Gary, and H. B. Greenberg. 1982. An outbreak of Norwalk gastroenteritis associated with swimming in a pool and secondary person-to-person transmission. Am. J. Epidemiol. 116:834-839. [DOI] [PubMed] [Google Scholar]
- 74.Katayama, H., A. Shimasaki, and S. Ohgaki. 2002. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 68:1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kay, D., J. M. Fleisher, R. L. Salmon, F. Jones, M. D. Wyer, A. F. Godfree, Z. Zelenauch-Jacquotte, and R. Shore. 1994. Prediction likelihood of gastroenteritis from sea bathing: results from randomised exposure. Lancet 344:905-909. [DOI] [PubMed] [Google Scholar]
- 76.Kiode, H., Y. Kitaura, H. Deguchi, A. Ukimura, K. Kawamura, and K. Hirai. 1992. Genomic, detection of enteroviruses in the myocardium studies on animal hearts, with coxsackievirus B3 myocarditis and endomyocardial biopsies from patients with myocarditis and dilated cardiomyopathy. Jpn. Circ. J. 56:1081-1093. [DOI] [PubMed] [Google Scholar]
- 77.Klingel, K., C. Hohenadl, A. Canu, M. Albrecht, M. Seemann, G. Mall, and R. Kandolf. 1992. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage and inflammation. Proc. Natl. Acad. Sci. USA 89:314-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kopecka, H., S. Dubrou, J. Prevot, J. Marechal, and J. M. Lopez-Pila. 1993. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl. Environ. Microbiol. 59:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krikelis, V., N. Spyrou, P. Markoulatos, and C. Serie. 1985. Seasonal distribution of enteroviruses and adenoviruses in domestic sewage. Can. J. Microbiol. 31:24-25. [DOI] [PubMed] [Google Scholar]
- 80.Krikelis, V., P. Markoulatos, and N. Spyrou. 1986. Viral pollution of coastal waters resulting from the disposal of untreated sewage effluents. Water Sci. Technol. 18:43-48. [Google Scholar]
- 81.Kueh, C. S. W., T.-Y. Tam, T. Lee, S. L. Wong, O. L. Lloyd, I. T. S. Yu, T. W. Wong, J. S. Tam, and D. C. J. Bassett. 1995. Epidemiological study of swimming-associated illnesses relating to bathing-beach water quality. Water Sci. Technol. 31:1-4. [Google Scholar]
- 82.Labelle, R., and C. P. Gerba. 1980. Influence of estuarine sediment on virus survival under field conditions. Appl. Environ. Microbiol. 39:749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labelle, R., and C. P. Gerba. 1981. Investigations into the protective effect of estuarine sediment on virus survival. Water Res. 16:469-478. [Google Scholar]
- 84.Lawson, H. W., M. M. Brayn, R. I. M. Glass, S. W. Stine, S. S. Monroe, J. K. Atrah, L. E. Lee, and S. J. Englander. 1991. Waterborne outbreak of Norwalk virus gastroenteritis at a Southwest U.S. resort; role of geological formations in the contamination of well water. Lancet 337:1200-1204. [DOI] [PubMed] [Google Scholar]
- 85.Le Guyader, F., E. Dubois, D. Menard, and M. Pommepuy. 1994. Detection of hepatitis A virus, rotavirus, and enterovirus in naturally contaminated shellfish and sediment by reverse transcription-seminested PCR. Appl. Environ. Microbiol. 60:3665-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis, D. C., A. Hale, X. Jiang, R. Eglin, and D. W. G. Brown. 1996. Epidemiology of Mexico Virus, a small round-structured virus in Yorkshire, United Kingdom, between January 1992 and March 1995. J. Infect. Dis. 175:951-954. [DOI] [PubMed] [Google Scholar]
- 87.Lipp, E. K., S. R. Farrah, and J. B. Rose. 2001. Assessment and impact of microbial fecal contamination and human enteric pathogens in a coastal community. Mar. Pollut. Bull. 42:286-293. [DOI] [PubMed] [Google Scholar]
- 88.Lipp, E. K., R. Kurz, R. Vincent, C. Rodriguez-Palacios, S. R. Farrah, and J. B. Rose. 2001. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries 24:238-258. [Google Scholar]
- 89.Lipp, E. K., J. L. Jarrell, D. W. Griffin, J. Lukasik, J. Jacukiewicz, and J. B. Rose. 2001. Preliminary evidence for human fecal contamination in corals of the Florida Keys. Mar. Pollut. Bull. 44:666-670. [DOI] [PubMed] [Google Scholar]
- 90.Lipp, E. K., and J. B. Rose. 1997. The role of seafood in foodborne diseases in the United States of America. Rev. Sci. Technol. 16:620-640. [DOI] [PubMed] [Google Scholar]
- 91.Lodder, W. J., J. Vinje, R. Van de Heide, A. M. de Roda Husman, E. J. Leenen, and M. P. G. Koopmans. 1999. Molecular detection of Norwalk-like caliciviruses in sewage. Appl. Environ. Microbiol. 65:5624-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miossec, L., F. Le Guyader, L. Haugarreau and M. Pommepuy. 2000. Magnitude of rainfall on viral contamination of the marine environment during gastroenteritis epidemics in human coastal population. Rev. Epidemiol. Santé 480398-7620:2S62-2S71. [PubMed] [Google Scholar]
- 93.Monceyron, C., and B. Grinde. 1993. Detection of hepatitis A virus in clinical and environmental samples by immunomagnetic separation and PCR. J. Virol. Methods 46:157-166. [DOI] [PubMed] [Google Scholar]
- 94.Monpoeho, S., A. Dehee, B. Mignotte, L. Schwartzbrod, V. Marechal, J. C. Nicholas, S. Billaudel, and V. Ferre. 2000. Quantification of enterovirus RNA in sludge samples using single tube real-time RT PCR. BioTechniques 29:88-93. [DOI] [PubMed] [Google Scholar]
- 95.Mullis, K., F. Faloona, S. Scharf, R. Saiki, G. Horn, and H. Erlich. 1986. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. L1:263-273. [DOI] [PubMed] [Google Scholar]
- 96.Murrin, K., and J. Slade. 1997. Rapid detection of viable enteroviruses in water by tissue culture and semi-nested polymerase chain reaction. Water Sci. Technol. 35:11-12. [Google Scholar]
- 97.Murrin, K. 1997. Rapid detection of viable enteroviruses in water by tissue culture and semi-nested polymerase chain reaction. Water Sci. Technol. 35:429-432. [Google Scholar]
- 98.Muscillo, M., G. La Rosa, A. Carducci, L. Cantiani, and C. Marianelli. 1999. Molecular and biological characterization of poliovirus 3 strains isolated in Adriatic seawater samples. Water Res. 33:3204-3212. [Google Scholar]
- 99.Muscillo, M., F. A. Aulicino, A. M. Patti, P. Orsini, L. Volterra, and G. M. Farra. 1994. Molecular techniques for the identification of enteric viruses in marine waters. Water Res. 28:1-7. [Google Scholar]
- 100.Muscillo, M., G. La Rosa, C. Marianelli, S. Zaniratti, M. R. Capobianchi, L. Cantiani, and A. Carducci. 2001. A new RT-PCR method for the identification of reoviruses in seawater samples. Water Res. 35:548-556. [DOI] [PubMed] [Google Scholar]
- 101.National Research Council. 1993. Managing wastewater in coastal urban Areas. Committee on Wastewater Management for Coastal Urban Areas National Academy Press. National Research Council, Washington, D.C.
- 102.National Resources Defense Council. 2001. Clean water and oceans: oceans: in brief: news: beach closings and advisories soar in 2000, increased monitoring and reporting reveals widespread pollution at U.S. beaches. National Resources Defense Council, Washington, D.C.
- 103.Noble, R. T., and J. A. Fuhrman. 2001. Enteroviruses detected by reverse transcriptase polymerase chain reaction from the coastal waters of Santa Monica Bay, California: low correlation to bacterial indicator levels. Hydrobiologia 460:175-184. [Google Scholar]
- 104.Noble, R. T., and J. A. Furman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 105.Nobles, R. E., P. Brown, J. Rose, and E. Lipp. 2000. The investigation and analysis of swimming-associated illness using the fecal indicator Enterococcus in Southern Florida's marine water. Fla. J. Environ. Health 169:13-19. [Google Scholar]
- 106.Pallin, R., A. P. Wyn-Jones, B. M. Place, and N. F. Lightfoot. 1997. The detection of enteroviruses in large volume concentrates of recreational waters by the polymerase chain reaction. J. Virol. Methods 67:57-67. [DOI] [PubMed] [Google Scholar]
- 107.Patti, A. M., F. A. Aulicino, A. L. Santi, M. Muscillo, P. Orsini, C. Bellucci, G. La Rosa, I. Mastroeni, and L. Volterra. 1996. Enteric virus pollution of Tyrrhenian areas. Water Air Soil Pollut. 88:261-267. [Google Scholar]
- 108.Patti, A. M., A. L. Santi, R. Gabrieli, S Fiamma, M Cauletti, and A. Pana. 1987. Hepatitis A virus and poliovirus 1 inactivation in estuarine water. Water Res. 21:1335-1338. [Google Scholar]
- 109.Paul, J. H., J. B. Rose, S. C. Jiang, P. London, X. Zhou, and C. Kellogg. 1997. Coliphage and indigenous phage in Mamala Bay, Oahu, Hawaii. Appl. Environ. Microbiol. 63:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paul, J. H., S. C. Jiang, and J. B. Rose. 1991. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl. Environ. Microbiol. 57:2197-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paul, J. H., J. B. Rose, S. C. Jiang, X. Zhou, P. Cochran, C. Kellogg, J. B. Kang, D. Griffin, S. Farrah, and J. Lukasik. 1997. Evidence for groundwater and surface marine water contamination by waste disposal wells in the Florida Keys. Water Res. 31:1448-1454. [Google Scholar]
- 112.Paul, J. H., M. R. McLaughlin, D. Griffin, E. K. Lipp, R. Stokes, and J. B. Rose. 2000. Rapid movement of wastewater from onsite disposal systems into surface waters in the Lower Florida Keys. Estuaries 23:662-668. [Google Scholar]
- 113.Paul, J. H., J. B. Rose, J. Brown, E. A. Shinn, S. Miller, and S. R. Farrah. 1995. Viral tracer studies indicate contamination of marine waters by sewage disposal practices in Key Largo, Florida. Appl. Environ. Microbiol. 61:2230-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paul, J. R., and J. D. Trask. 1941. The virus of poliomyelitis in stools and sewage. JAMA 116:493-497. [Google Scholar]
- 115.Payment, P. 1997. Cultivation and assay of viruses, p. 72-78. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 116.Pianetti, A., W. Baffone, B. Citterio, A. Casaroli, F. Bruscolini, and L. Salvaggio. 2000. Presence of enteroviruses and reoviruses in the waters of the Italian coast of the Adriatic Sea. Epidemiol. Infect. 125:455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pina, S., J. Jofre, S. U. Emerson, R. H. Purcell, and R. Girones. 1998. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl. Environ. Microbiol. 64:4485-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pruss, A. 1998. Review of epidemiological studies on health effects from exposure to recreational water. Int. J. Epidemiol. 27:1-9. [DOI] [PubMed] [Google Scholar]
- 120.Puig, M., J. Jofre, F. Lucena, A. Allard, G. Wadell, and R. Girones. 1994. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 60:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rao, V. C., K. M. Seidel, S. M. Goyal, T. G. Metcalf, and J. L. Melnick. 1984. Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments. Appl. Environ. Microbiol. 48:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reynolds, K. A., C. P. Gerba, and I. L. Pepper. 1995. Detection of enteroviruses in marine waters by direct RT-PCR and cell culture. Water Sci. Technol. 31:322-328. [Google Scholar]
- 123.Reynolds, K. A., C. P. Gerba, and I. L. Pepper. 1996. Detection of infectious enteroviruses by an integrated cell culture-PCR procedure. Appl. Environ. Microbiol. 62:1424-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reynolds, K. A., C. P. Gerba, M. Abbaszadegan, and L. L. Pepper. 2001. ICC/PCR detection of enteroviruses and hepatitis A virus in environmental samples. Can. J. Microbiol. 47:153-157. [PubMed] [Google Scholar]
- 125.Reynolds, K. A., K. Roll, R. S. Fujioka, C. P. Gerba, and I. L. Pepper. 1998. Incidence of enteroviruses in Mamala Bay, Hawaii using cell culture and direct polymerase chain reaction methodologies. Can. J. Microbiol. 44:598-604. [PubMed] [Google Scholar]
- 126.Reynolds, K. A., C. P. Gerba, and I. L. Pepper. 1997. Rapid PCR-based monitoring of infectious enteroviruses in drinking water. Water Sci. Technol. 35:423-427. [Google Scholar]
- 127.Richardson, K. J., A. B. Margolin, and C. P. Gerba. 1988. A novel method for liberation viral nucleic acid for assay of water samples with cDNA probes. J. Virol. Methods 22:13-21. [DOI] [PubMed] [Google Scholar]
- 128.Rodriquez, V., B. Bautista, F. Jimeniz-Gomez, F. Guerrero, J. M. Blanco, and J. Rodriguez. 1998. Loss of pico- and nanoplankton cells during concentration by tangential flow filtration. J. Plankton Res. 20:1087-1097. [Google Scholar]
- 129.Rose, J. B. 1997. Environmental ecology of Cryptosporidium and public health implications. Annu. Rev. Public Health 18:135-161. [DOI] [PubMed] [Google Scholar]
- 130.Rose, J. B., and J. Simonds. 1998. King County water quality assessment: assessment of public health impacts associated with pathogens and combined sewer overflows. Washington Department of Natural Resources, Olympia.
- 131.Rose, J. B. 1986. Microbial aspects of wastewater resuse for irrigation. Crit. Rev. Environ. Control 16:231-256. [Google Scholar]
- 132.Rose, J. B., and D. J. Grimes. 2001. Reevaluation of microbial water quality: powerful new tolls for detection and risk assessment. American Academy of Microbiology, Washington, D.C. [PubMed]
- 133.Rose, J. B., L. J. Dickson, S. R. Farrah, and R. P. Carnahan. 1996. Removal of pathogenic and indicator microorganisms by a full-scale water reclamation facility. Water Res. 30:2785-2797. [Google Scholar]
- 134.Rowe, W. P., R. J. Huebner, L. K. Gilmore, R. H. Parrot, and T. G. Ward. 1953. Isolation of a cytopathic agent from human adenoids undergoing spontaneous degradation in tissue culture. Proc. Soc. Exp. Biol. Med. 84:570-573. [DOI] [PubMed] [Google Scholar]
- 135.Saiki, R. K., S. Scharf, F. Faloona, K. D. Mullis, G. T. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 136.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 137.Schernewski, G., and W. D. Julich. 2001. Risk assessment of virus infections in the Oder estuary (southern Baltic) on the basis of spatial transport and virus decay simulations. Int. J. Hyg. Environ. Health 203:317-325. [DOI] [PubMed] [Google Scholar]
- 138.Schwab, K. J., R. De Leon, and M. D. Sobsey. 1995. Concentration and purification of beef extract mock eluates from water samples for the detection of enteroviruses, hepatitis A virus, and Norwalk virus by reverse transcription-PCR. Appl. Environ. Microbiol. 61:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwab, K. J., R. De Leon, and M. D. Sobsey. 1996. Immunoaffinity concentration and purification of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl. Environ. Microbiol. 62:2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwab, K. J., M. K. Estes, F. H. Neill, and R. L. Atmar. 1997. Use of heat release and an internal RNA standard control in reverse transcription-PCR detection of Norwalk virus from stool samples. J. Clin. Microbiol. 35:511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140a.Schwab, K. J., F. H. Neill, F. Le Guyader, M. K. Estes, and R. L. Atmar. 2001. Development of a reverse transcription-PCR-DNA enzyme immunoassay for detection of “Norwalk-like” viruses and hepatitis A virus in stool and shellfish. Appl. Environ. Microbiol. 67:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seidel, K. M., S. M. Goyal, V. C. Rao, and J. L. Melnick. 1983. Concentration of rotavirus and enteroviruses from blue crabs (Callinectus sapidus). Appl. Environ. Microbiol. 46:1293-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen, S., U. Desselberger, and T. A. McKee. 1997. The development of an antigen capture polymerase chain reaction assay to detect and type human enteroviruses. J. Virol. Methods 65:139-144. [DOI] [PubMed] [Google Scholar]
- 143.Shieh, C. Y.-S., S. S. Monroe, R. L. Fankhauser, G. W. Langlois, W. Burkhardt III, and R. S. Baric. 2000. Detection of Norwalk-like virus in shellfish implicated in illness. J. Infect. Dis. 181:S360-S366. [DOI] [PubMed] [Google Scholar]
- 144.Shinn, E. A., G. W. Smith, J. M. Prospero, P. Betzer, M. L. Hayes, V. Garrison, and R. T. Barber. 2000. African dust and the demise of Caribbean coral reefs. Geol. Res. Lett. 27:3029-3032. [Google Scholar]
- 145.Shinn, E. A., R. S. Reese, and C. D. Reich. 1994. Fate and pathways of injection-well effluent in the Florida Keys. Open-file report 94-276. U.S. Geological Survey, Department of the Interior, Washington, D.C.
- 146.Shirali, G. S., J. Ni, R. E. Chinnock, J. K. Johnston, G. L. Rosenthal, N. E. Bowles, and J. A. Towbin. 2001. Association of viral genome with graft loss in children after cardiac transplantation. N. Engl. J. Med. 344:1498-1503. [DOI] [PubMed] [Google Scholar]
- 147.Shoulder, A. 1982. Enteroviruses in seawater at bathing beaches, p. 84-87. In M. Butler, A. R. Medlen, and R. Morris (ed.), Viruses and disinfection of water and wastewater. University of Surrey Print Unit, Guildford, United Kingdom.
- 148.Simmons, G., G. Greening, W. Gao, and D. Campbell. 2001. Raw oyster consumption and outbreaks of viral gastroenteritis in New Zealand: evidence for risk to the publics health. Aust. N. Z. J. Public Health 25:234-240. [DOI] [PubMed] [Google Scholar]
- 149.Smith, A. W., D. E. Skilling, N. Cherry, J. H. Mead, and D. O. Matson. 1998. Calicivirus emergence from ocean reservoirs: zoonotic and interspecies movements. Emerg. Infect. Dis. 4:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith, J. L. 2001. A review of hepatitis E virus. J Food Prot. 64:572-586. [DOI] [PubMed] [Google Scholar]
- 151.Steinmann, J. 1981. Detection of rotavirus in sewage. Appl. Environ. Microbiol. 41:1043-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sunen, E., and M. D. Sobsey. 1999. Recovery and detection of enterovirus, hepatitis A virus and Norwalk virus in hardshell clams (Mercenaria mercenaria) by RT-PCR methods. J. Virol. Methods 77:179-187. [DOI] [PubMed] [Google Scholar]
- 153.Tenorio, A., J. M. Echevarria, P. E. Klapper, and G. M. Cleator. 1997. Detection of enteroviral RNA and specific DNA of herpesviruses by multiplex genome amplification. J. Virol. Methods 66:39-50. [DOI] [PubMed] [Google Scholar]
- 154.Thomas, P. D., R. C. Pollock, and B. G. Gazzard. 1999. Enteric viral infections as a cause of diarrhoea in the acquired immunodeficiency syndrome. HIV Med. 1:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Toranzos, G. A., and C. P. Gerba. 1989. An improved method for the concentration of rotaviruses from large volumes of water. J. Virol. Methods 24:131-140. [DOI] [PubMed] [Google Scholar]
- 156.Traore, O., C. Arnal, B. Mignotte, A. Maul, H. Laveran, S. Billaudel, and L. Schwartzbrod. 1998. Reverse transcriptase PCR detection of astrovirus, hepatitis A virus, and poliovirus in experimentally contaminated mussels: comparison of several extraction and concentration methods. Appl. Environ. Microbiol. 64:3118-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsai, Y.-L., B. Tran, L. R. Sangermano, and C. J. Palmer. 1994. Detection of poliovirus, hepatitis A virus, and rotavirus from sewage and ocean water by triplex reverse transcriptase PCR. Appl. Environ. Microbiol. 60:2400-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Elden, L. J. R., M. Nijhuis, P. Schipper, R. Schuurman, and A. M. Van Loon. 2001. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 39:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Reis, R., and A. Sydney. 2001. Membrane separations in biotechnology. Curr. Opin. Biotechnol. 12:208-211. [DOI] [PubMed] [Google Scholar]
- 160.Vantarakis, A. C., and M. Papapetropoulou. 1998. Detection of enteroviruses and adenoviruses in coastal waters of SW Greece by nested polymerase chain reaction. Water Res. 32:2365-2372. [Google Scholar]
- 161.Vonstille, W. T., W. T. Stille III, and Ralph C. Sharer. 1993. Hepatitis A epidemics from utility sewage on Ocoee, Florida. Arch. Environ. Health 48:120-124. [DOI] [PubMed] [Google Scholar]
- 162.Wagenknecht, L. E., J. M. Roseman, and W. H. Herman. 1991. Increased incidence of insulin-dependent diabetes mellitus following an epidemic of coxsackievirus B5. Am. J. Epidemiol. 133:1024-1031. [DOI] [PubMed] [Google Scholar]
- 163.Wait, D. A., and M. D. Sobsey. 2001. Comparative survival of enteric viruses and bacteria in Atlantic Ocean seawater. Water Sci. Technol. 43:139-142. [PubMed] [Google Scholar]
- 164.Weisberg, S., A. Dufour, F. Galt, M. Gold, M. Noble, R. Noble, E. Reichard, P. Roberts, J. Rose, and D. Rosenblatt. 2000. Huntington Beach closure investigation. Technical review USCSG-TR-01-2000. Sea Grant, University of Southern California, San Diego.
- 165.Wyer, M. D., D. Kay, G. F. Jackson, H. M. Dawson, J. Yeo, and L. Tanguy. 1995. Indicator organism sources and coastal water quality: a catchment study on the Island of Jersey. J. Appl. Bacteriol. 78:290-296. [DOI] [PubMed] [Google Scholar]
- 166.Yates, M. V., and S. R. Yates. 1988. Modeling microbial fate in the subsurface environment. Crit. Rev. Environ. Control 17:307-344. [Google Scholar]