Abstract
Damp buildings often have a moldy smell or obvious mold growth; some molds are human pathogens. This has caused concern regarding health effects of moldy indoor environments and has resulted in many studies of moisture- and mold-damaged buildings. Recently, there have been reports of severe illness as a result of indoor mold exposure, particularly due to Stachybotrys chartarum. While many authors describe a direct relationship between fungal contamination and illness, close examination of the literature reveals a confusing picture. Here, we review the evidence regarding indoor mold exposure and mycotoxicosis, with an emphasis on S. chartarum. We also examine possible end-organ effects, including pulmonary, immunologic, neurologic, and oncologic disorders. We discuss the Cleveland infant idiopathic pulmonary hemorrhage reports in detail, since they provided important impetus for concerns about Stachybotrys. Some valid concerns exist regarding the relationship between indoor mold exposure and human disease. Review of the literature reveals certain fungus-disease associations in humans, including ergotism (Claviceps species), alimentary toxic aleukia (Fusarium), and liver disease (Aspergillys). While many papers suggest a similar relationship between Stachybotrys and human disease, the studies nearly uniformly suffer from significant methodological flaws, making their findings inconclusive. As a result, we have not found well-substantiated supportive evidence of serious illness due to Stachybotrys exposure in the contemporary environment. To address issues of indoor mold-related illness, there is an urgent need for studies using objective markers of illness, relevant animal models, proper epidemiologic techniques, and examination of confounding factors.
INTRODUCTION
Damp buildings often have a moldy smell or obvious mold growth, and some molds are known human pathogens. This has caused concern regarding potential health effects of moldy indoor environments. As a result, there have been many studies of moisture- and mold-damaged buildings. More recently, there have been a growing number of articles in the media and of lawsuits claiming severe illness as a result of indoor mold exposure, particularly to Stachybotrys chartarum. However, while many authors report a clear relationship between fungal contaminated indoor environments and illness, close examination of the literature reveals a much more confusing picture.
In this review, we discuss indoor environmental mold exposure and mycotoxicosis, with an emphasis on S. chartarum and its toxins (due to the breadth of the topic, we will not discuss better understood areas such as invasive disease caused by Aspergillus). We also discuss specific organ effects, focusing on illnesses purportedly caused by indoor mold. These illnesses include pulmonary, immunologic, neurologic, and oncologic disorders. We discuss the Cleveland infant idiopathic pulmonary hemorrhage (IPH) reports in some detail, since they provided much of the fuel for current concerns about Stachybotrys exposure. As we will see, while there is cause for concern about the potential effects of indoor mold exposure, particularly to Stachybotrys species, there is no well-substantiated evidence linking the presence of this fungus to health concerns elaborated in the scientific and lay press.
As patients and society at large become increasingly concerned that illnesses may be due to the home or work environment, an understanding of mycotoxins by microbiologists and clinicians (especially infectious-disease subspecialists) is of growing importance. Such knowledge is critical to the diagnosis of potential fungus-related disease and is necessary to assuage fears instilled by extensive media coverage (34; J. MacFarlane, 1997, Beware the mold Stachybotrys, http://www.cnn.com/HEALTH/9711/05/deadly.mold; J. McKenzie, 2001, Hidden menace: insurers worry about toxic mold claims, http://more.abcnews.go.com/sections/wnt/dailynews?toxicmold_010626.html; E. Moriarty, 2000, Invisible killers, OBS News, New York, N.Y.; N. Morris, 2001, Moldy Schools: are your kids getting sick at school? http://more.abcnews.go.com/sections/wnt/WorldNewsTonight/wnt010418_moldyschools_feature.html). Finally, such knowledge may be important in the wake of recent terrorist events in the United States. Some toxins, particularly aflatoxins and trichothecenes, have the potential to be used as weapons. There is evidence that several countries are currently involved in mycotoxin weapon research (30, 239, 465). The latter point is beyond the scope of this article.
INDOOR AIR AND BUILDING-RELATED ILLNESS
It has long been postulated that exposure to damp, moldy home and workplace environments has detrimental health effects. At the beginning of the 18th century, Ramazzini, considered “the father of occupational medicine,” described an illness of workers inhaling ‘foul and mischievous powder' from handling crops (116, 302). More recently, Platt et al. (333), found that occupants of wet, moldy buildings had an increase in subjective complaints. Brunekreef et al. (47) found a similar pattern in >6,000 children in six states in the United States and reported home dampness was a strong predictor of respiratory and other illness in this age group. The list of putative symptoms generally consists of upper respiratory complaints, including headache, eye irritation, epistaxis, nasal and sinus congestion, cough, “cold and flu” symptoms, as well as generalized gastrointestinal complaints (240). Taskinen et al. (409, 410) reported an increased prevalence of asthma in moisture-affected schools, although there were no objective measurements of respiratory disease. A number of studies have reported a relationship between similar symptoms and damp housing or workplace environments, although the proposed etiologies have varied (117, 118, 164, 416, 419, 444).
The causal relationship between damp housing and illness is unclear. Establishing such a relationship is complicated since there are a variety of pollutants in the indoor environment (143) including volatile organic compounds such as toluene, benzene, alkenes, aromatic hydrocarbons, esters, alcohols, aldehydes, and ketones (208, 267, 277, 310, 332, 357, 450); radon (256); combustion gases, sulfur dioxide, nitrogen dioxide, carbon dioxide, ozone (16, 20, 357); and the essentially ubiquitous formaldehyde (205, 357). Other items (copy paper) and activities (photocopying and video terminal exposure) have been linked to symptoms (178). Other studies have suggested that shade, organic debris, landscaping quality, central electrostatic systems, ventilation rates, temperature, noise levels, dust control compliance, and patient gender may be important (23, 42, 77, 215, 264, 298, 308, 344, 437, 440), as well as the presence of tobacco smoke (81, 123, 276). Psychosocial issues may be playing a role in building-related complaints. Several studies have reported that the quality of the work environment, stress, and somaticization may all be significant (31, 42, 89, 232, 276, 307, 308, 413).
The indoor environment also contains a wide range of microorganisms including bacteria (e.g., Legionella and other gram-negative species) (85), mycobacteria (9, 415), and molds (161), as well as their products, including endotoxins and mycotoxins. There may often be a much higher bacterial load than fungal load (161, 416).
Most fungi are metabolically active over a broad temperature range (203); however, high moisture and relative humidity are required for optimal growth (69). The lowest relative humidity supporting mold growth is approximately 75%, although the requirements of Stachybotrys are much higher, around 93% at 25°C (142). Increasing temperature and nutritional status of the substrate can lead to lower moisture requirements. Surfaces that are soiled or have susceptible paint or paper do not need to be as damp for mold to develop. While promoting mold growth, moisture itself may be critical in “sick-building syndrome” (SBS) illnesses, since humidity affects mite and ozone levels, as well as off-gassing, salt, and acid formation (26). The links between moisture damage, any of these related cofactors, and building-related illnesses are not clear (24, 27, 50, 83, 260). For example, dust mites are notorious allergic agents and produce many of the upper airway symptoms ascribed to mold exposure or SBS; moreover, they are almost always found in association with mold species (90, 262, 359), confounding moisture- and mold-related findings. Gram-negative bacteria, endotoxin, and mycobacteria are found in water-damaged buildings in association with mold (9, 85). To our knowledge, only one paper has actually reported a lack of association between symptom prevalence and endotoxin, dust mites, or other nonfungal agents (85). In moldy office buildings there is an association between microbial contamination and repeated flooding or stagnant pools of water (280). Some geographic locales are obviously more likely to be affected than others. For example, 12% of English building stock suffer serious dampness; extrapolation suggested that there were 2.5 million affected dwellings in the United Kingdom but that 60% of these were from condensation rather than overt flooding (362; Anonymous, Bldg. Res. Estab. Semin. Proc., 1981). Readers interested in an in-depth review of these issues are referred to the recent comprehensive report by the Institute of Medicine (175).
FUNGI IN THE INDOOR ENVIRONMENT
Fungal Organisms in Damp Buildings
A host of mold species have been isolated from damp buildings: the most frequently isolated in one study were Penicillium (96%), Cladosporium (89%), Ulocladium (62%), Geomyces pannorum (57%), and Sistronema brinkmannii (51%) (142, 168). There were 66 species of filamentous fungi, and yeasts were found in 94% of dwellings and 13% of CFU on Anderson sampler plates. In contrast to the aforementioned species, Stachybotrys was less common, being found in 12.8% of dwellings and 4.5% of samples. Other studies have reported similar organism frequencies (Table 1) (72, 144, 215, 267, 420). In most studies, Stachybotrys has had a low prevalence, being present in less than 3% of samples (214, 267). However, some recent work has suggested that it may be more common than was initially thought (144, 405). Regardless, Stachybotrys is rarely found in isolation, nearly always occurring in the presence of other fungi (164, 419). This fact is critical, since many of the other species are capable of producing mycotoxins (70, 379, 416), and recent work suggests that volatiles from S. chartarum may represent a small fraction of the total amount present in problem buildings where other fungi exist (139).
TABLE 1.
Fungi found in dust and air samples- representative findingsa
| Species | % of houses where species was found in:
|
|
|---|---|---|
| Dust samples | Air samples | |
| Penicillium spp. | 80 | 47 |
| Rhizopus spp. | 73 | |
| Cladosporium cladosporioides (Fresen) de Vries | 67 | 8 |
| Alternaria alternata (fr.) Keissler | 57 | Xb |
| Sterile isolates | 55 | |
| Aspergillus niger van Tieghem | 53 | 6 |
| Penicillium viridicatum Westling | 39 | 6 |
| Mucor spp. | 31 | X |
| Trichoderma viride (Pers.) ex Gray | 25 | 6 |
| Ulocladium botrytis Preuss | 22 | 4 |
| Penicillium fellutinum Biourge | 20 | 14 |
| Penicillium decumbens Thom | 18 | 10 |
| Cladosporium herbarum (Pers.) Link | 16 | |
| Paecilomyces variotii Bainier | 10 | |
| Phoma spp. | 10 | |
| Arthrinium spp. | 8 | |
| Aspergillus fumigatus Fresen. | 6 | |
| Aureobasidium pullulans (deBary) Arnaud | 6 | |
| Monilia sitophila Sacc. | 6 | |
| Paecilomyces spp. | 6 | |
| Periconia spp. | 6 | |
| Aspergillus candidus Link | 4 | |
| Aspergillus ochraceus Wilhelm | 4 | X |
| Aspergillus spp. | 4 | 8 |
| Phialophora melinii (Nannf.) Conant | 4 | |
| Yeasts | 82 | 6 |
| Aspergillus versicolor (Vuill.) Tiraboschi | X | X |
| Basidiomycetes (clamp connections) | X | X |
| Cladosporium sp. | X | |
| Curvularia inaequalis (Shear) Boedijn | X | |
| Geotrichum candidum Link | X | X |
| Penicillium aurantiogriseum Dierckx | X | |
| Penicillium brevi-compactum Dierckx | X | 6 |
| Penicillium chrysogenum Thom | X | X |
| Penicillium digitatum Sacc. | X | X |
| Penicillium janthinellum Biourge | X | 8 |
| Penicillium lividum West | X | X |
| Penicillium purpurogenum Stoll | X | |
| Penicillium rugulosum Thom | X | X |
| Penicillium simplicissimum (Oud.) Thom | X | |
| Stachybotrys atra Corda | X | |
| Thamnidium elegans Link. | X | |
| Torula herbarum (Pers.) Link ex Gray | X | |
| Verticladiella sp. | X | |
Adapted from reference (267) (a survey of Canadian homes) with permission of the publisher.
X, found in one house only.
Stachybotrys has a fondness for cellulose (350). While cellulose (especially water damaged) may promote Stachybotrys growth, the same is true for Cladosporium, Penicillium, and Aspergillus species (144, 185, 202). The predilection for cellulose, moisture, and nutrient-poor settings explains the appearance of Stachybotrys in affected buildings, where it is a tertiary wall colonizer that comes after primary (Penicillium and A. versicolor) and secondary (Cladosporium) fungal colonizers (327). Stachybotrys can sometimes be isolated from other substrates including pipe insulation, gypsum, fiberglass wallpaper, and aluminum foil (144). The nutritional and growth requirements of the organism may also explain the lack of recovery from cultures and perhaps underreporting of Stachybotrys incidence. The fungus proliferates more slowly than other species, leading to overgrowth by other molds unless appropriate culture substrates (e.g., cellulose based) are used. Studies using cellulose-based agar techniques have reported a relatively high prevalence of Stachybotrys, with positive cultures in up to 30% of water-damaged homes (121). Similar issues may exist when trying to identify mycotoxin-producing Fusarium strains (420).
Technical Problems in Determining Fungal Exposure
Difficulties in measuring fungal organisms.
Although available studies provide information regarding which organisms are present in the indoor environment, there are significant concerns associated with sampling methods. While a detailed description of such techniques is beyond the scope of this article, several points are worth mentioning. Most traditional sampling methods (e.g., exposed agar plates) are incapable of adequately measuring either airborne or sedentary organisms, which necessitates the use of devices such as Anderson samplers (135, 142, 168, 267, 358, 416). Even using such quantitative devices, there can be huge variations (up to 1,000-fold) between essentially identical specimens (168). Thus, little can be deduced from single air samples, and protocols involving multiple samples from suspect houses versus single samples from control houses will probably disproportionately find fungus in case houses due to attrition (142). Furthermore, sampling needs to be done under normal room activity, since aggressive measures (e.g., vacuuming) will probably overestimate actual exposure levels (238, 359, 416). These last two points are critical to examination of the Cleveland IPH reports, discussed below. Hunter et al. (168) found that while large numbers of spores in the internal air were associated with surface mold growth and construction work, disturbance of surface growth and vacuum cleaning of carpets (techniques often involved in surveys) caused large temporary increases in the atmospheric spore count. An increase of 3,300% in the number of four categories of mold was observed after disturbing mural mold growth (e.g., by wiping with a hand). Other factors affecting apparent airborne fungal spore load are carpeting type (162), pets (394), dust control measures (215), and humidification (394). Finally, particle size may play a key role when attempting to quantitate some species; for example, the rapid settling of the large spores of Ulocladium species probably accounts for their being underrepresented in the airborne spore load (144, 171). Such culture difficulties may eventually be circumvented using new techniques such as PCR (80, 347).
A final problem in measuring fungal organisms in the indoor environment relates to selective sampling. As noted above, Stachybotrys species rarely exist in isolation. They are often present in settings which select for a host of other fungal species and their potential mycotoxins, as well as bacteria, mycobacteria, arthropods, and man-made organic chemicals. However, most studies cited below have used methods that preferentially select for Stachybotrys species and mycotoxins. Of more immediate scientific and medicolegal concern, many studies of purportedly affected housing are surveying only for Stachybotrys species while ignoring other organisms (our unpublished experience).
Difficulties in measuring mycotoxins.
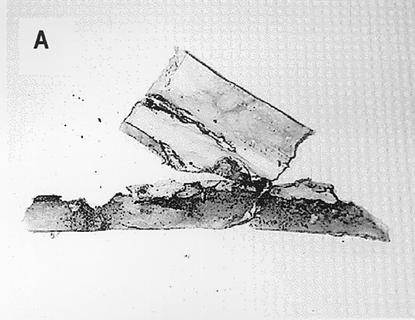
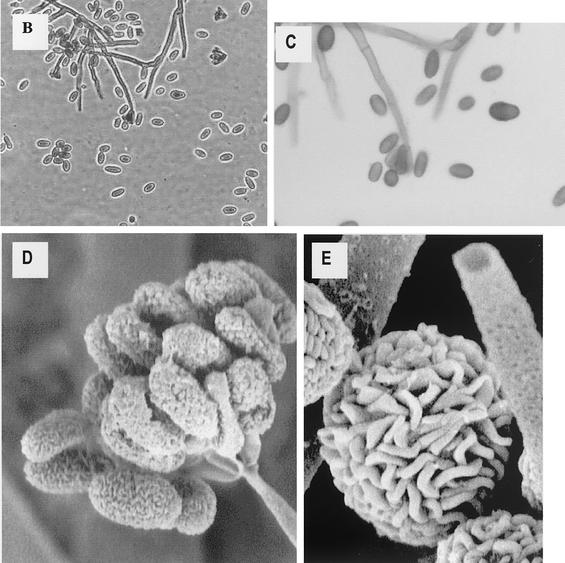
As discussed in detail below, similar problems exist regarding the detection and significance of indoor environmental mycotoxins. Many purported fungal volatiles are in fact common and are not unequivocally fungal in origin (267). While some true mycotoxins have been detected in indoor air, this has usually been in the context of heavy industrial contamination (240, 295). Although it is occasionally possible to collect mycotoxins by using air filters followed by extraction (318, 388), they are usually isolated from inert dust or building materials (9, 79, 111, 267, 294). This may misrepresent exposure, since the compounds are not volatile. In the case of Stachybotrys, toxin-bearing spores are produced in a slimy mass with high moisture content (Fig. 1), becoming airborne only when dry and disturbed or when attached to other particles such as dust (161). Serologic testing of potentially exposed individuals is not useful, since specific immunoglobulin levels do not correlate with exposure (419).
FIG. 1.
Morphology of S. chartarum. (A) Representative section of damaged wallboard. Note the areas of black discoloration. (B) Pure culture of S. chartarum, initially obtained from contaminated wallboard (40× objective). (C) Higher-power view of the same culture as in panel B (100× oil objective). (D) Scanning electron micrograph of conidia at the tip of a conidiaphore. (E) Scanning electron micrograph of mature conidia. Slime has been removed by scanning electron microscope processing. Panels D and E are reprinted from Nelson, http://www.apsnet.org/online/feature/stachybotrys/ with permission.
Most importantly, the presence of potentially toxigenic fungi does not imply the presence of mycotoxins, nor does the finding of mycotoxins prove that a particular species is, or was, present (267, 396, 420). Toxin production is dependent on substrates, nutrient levels, moisture, pH, and temperature (161, 283). While many species can produce toxins (Table 2), the ability to produce toxin varies under particular conditions, and often “known” toxin producers will not make the compounds (291). There are also extreme variations in toxin production between strains (11, 153), making culture insufficient as an indicator for the presence of mycotoxins. In addition, many unknown secondary metabolites, yet to be detected or identified, can be produced (291), and new compounds are constantly being identified (166). Fungal species identification is not a simple process but often requires the expertise of specialized medical mycologists. Toxins purportedly produced by a particular organism may suffer from misidentification of that organism (237). Therefore, specific tests for individual mycotoxins or biological assays (e.g., skin irritation) need to be performed as tools for mycotoxin screening (317, 420, 451). In this regard, newer analytic methods are being developed, including a protein translation (luciferase) assay for trichothecene toxicity in airborne particulates (457). This technique offers a greatly increased sensitivity compared with prior systems and may provide a novel way to measure environmental mycotoxins.
TABLE 2.
Toxigenic species of mold isolated from indoor air of housesa
| Species | Toxins produced |
|---|---|
| Aspergillus flavus | Aflatoxins |
| Aspergillus fumigatus | Fumiclavines, fumigatoxin, fumigillin, fumitrems, gliotoxin |
| Aspergillus versicolor | Sterigmatocystin, versicolorin, aspercolorin, averufin, cyclopiazonic acid |
| Penicillium brevicompactum | Brevianamide A, mycophenolic acid |
| Penicillium chrysogenum | Citrinin, penicillic acid, PR-toxin, roquefortin C |
| Penicillium citrinum | Kojic acid, citrinin, citreoviridin |
| Penicillium corylophilum | Gliotoxin |
| Penicillium cyclopium | Penicillic acid |
| Penicillium expansum | Patulin, citrinin |
| Penicillium fellutanum | Citreoviridin, citrinin |
| Penicillium spinulosum | Spinulosin |
| Penicillium viridicatum | Brevianamide A, citrinin, mycophenolic acid, penicillic acid, viomellein, xanthomegnin |
| Stachybotrys chartarum | Roridin E, satratoxin H, sporidesmin G, trichoverrins, verrucarol |
| Trichoderma viride | Gliotoxin, T-2 toxin, trichodermin, trichodermol, viridiol |
Adapted from reference 130 with permission of the publisher.
Problems with Clinical Studies
Most studies describing the health effects of indoor dampness and mold have relied on subjective and retrospective questionnaires. Remarkably few studies have included physical examinations or diagnostic testing. There are obviously potential problems with such an approach, and when study validity was examined, some notable conclusions were reached. To examine the validity of self-reported symptoms, one group compared parental reports of children's coughing, with overnight cough recording (88). The results showed there was extremely low agreement between the two measures. Additionally, parental smokers underreported their children's coughing, which biased the actual odds ratio (OR) of 3.1 (based on recording) down to 0.6 if their reports were relied on instead. The same group tested the validity of questions commonly used to indicate presence of indoor molds, compared to established objective measures of mold (e.g., airborne ergosterol) (86). They found that more mold was present if odor or water damage were reported and that twice as much Aspergillus and Penicillium was found when mold was mentioned. However, the presence of reported mold or water damage was unrelated to objective measures, and there was evidence of substantial reporting bias (e.g., allergy patients were more likely to report visible fungus despite low levels of viable fungus in dust, while smokers were less likely to report visible mold). Overall, while reported mold, water damage, and odors were associated with elevated levels of indoor fungi, inaccuracy was high and there was evidence of systemic bias, causing the authors to conclude that objective measures, not questionnaires, are appropriate. In another study of associations between residential mold growth and symptoms, the authors tried to confirm the findings by objective measures (87). Using the same group as in their previous work (n = 403 homes), they compared reported respiratory symptoms with objective measures including airborne ergosterol, dust, viable fungus counts, and nocturnal cough recordings. Despite a 25 to 50% relative increase in symptom prevalence when mold was reported, neither symptoms nor recorded cough were related to objective measures of mold. It is reasonable to conclude that retrospective subjective questionnaires are at best suspect. It is worth noting the authors of this work are in fact proponents of a mold-illness link, making their conclusions that subjective complaints are inadequate measures of pathology perhaps even stronger. Similar negative findings have been found when examining subjective neurologic complaints in the setting of SBS (310).
Such findings may explain the confusing results of earlier studies. For example, some authors have claimed links between childhood asthma and damp, moldy housing (401). While retrospective questionnaires reported more wheezing, cough, and chest cold symptoms in children from affected houses, the degree of bronchospasm was not different between groups. Thus, despite the claim that there was a causal association between moldy houses and wheezing, there was no supporting objective evidence. Some studies which claim that moisture and mold were associated with respiratory infections, cough, and wheezing (again with no objective measures) also fail to show differences in asthma prevalence between case and control schools (409, 410). Other authors report that despite claims of symptoms being more prevalent in case groups (reporting exposure to fungi, pets, mold odor, and dampness), actual asthma prevalence was no different (177).
ASSOCIATION OF STACHYBOTRYS SPECIES WITH “SICK BUILDINGS”
Because of concerns of mold-induced building-related illness and the particular characteristics of Stachybotrys species, there has been growing concern about the health of occupants of Stachybotrys-“damaged” buildings (9, 79, 91, 133, 150, 157, 184, 188, 197, 241, 318, 423, 424). Many authors have reported ill effects in relation to Stachybotrys, although it is critical to note these reports are often associations rather than proof of causation. Hodgeson et al. (164) reported building-related illness in Florida; this was described as symptoms consisting of mucosal irritation, fatigue, headache, and chest tightness that occurred within weeks of moving into the affected building. The symptoms were purportedly caused by S. chartarum and A. versicolor, although a number of other species were seen. The authors identified mycotoxins including satratoxins G and H (see below) in moldy ceiling tiles, although the significance of these findings is unclear. While they concluded the symptom outbreak was likely a result of inhalation of fungal toxins, there was in fact no clear evidence (e.g., laboratory parameters) to support the claim. Tuomi et al. (420) examined Finnish buildings with water damage and identified a host of fungal organisms and mycotoxins (satratoxins G and H, T-2 toxin, and the aflatoxin precursor sterigmonisin) in bulk samples, although the relationship between the organisms and toxins was unclear, as explored below. Examining buildings with building-related illness complaints, Johanning et al. (197) isolated satratoxin H and spirocyclic lactones from water damaged material. The authors implied these mycotoxins were the cause of respiratory and immune problems, although, as we discuss below, the claims are questionable. Other authors have reported anecdotal cases of illness in which S. chartarum and mycotoxins have been isolated from building materials, but again there are few objective measurements of illness or clear etiologic links to the fungus (79). While authors claim the health effects are similar to past cases of stachybotryotoxicosis, such effects are often vague, poorly described, and clearly not the same as the serious illnesses of equine stachybotryotoxicosis and alimentary toxic aleukia described below.
One of the best studies of building-related illness showed minimal relation to Stachybotrys. Miller et al. (267) examined 50 Canadian homes in which the occupants had complaints of respiratory or allergic symptoms for which there was no explanation, although at the time of the study, occupants of only 6 houses had “building-related illnesses.” Looking at air exchange rate, moisture levels, and analyzing air and dust for fungus and fungal products in 37 of the homes, they found S. chartarum in only one house; analysis of the 6 “sick” houses did not indicate fungus-related disease. During parts of the year when windows are open, indoor fungi are comparable to outdoor species (Cladosporium, Alternaria, and Aureobasidium) (391). However, in this study, outdoor air spores were negligible and Penicillium and other soil fungi were most important. Toxigenic fungi included P. viridicatum, Trichoderma viride, P. decumbens, and A. versicolor. House dust usually contained “appreciable” amounts of filamentous fungi and yeast, and so it was expected that spores could be found in air, depending on the activity in the room.
Recently, there has been a great concern regarding exposure of school children in “contaminated schools,” sometimes resulting in building closures (367, 409, 414; Norris, ABC News online article). In fact schools may have lower mean viable mold spore counts than the students' homes (105). In one 22-month study of 48 schools in which there were concerns regarding indoor air quality and health (rhinitis and congestion which improved when the students were away from school), fewer than 50% of affected schools had fungal CFUs higher than outdoor air (72). In 11 schools where complaint areas had samples with the same organisms as outdoors, Stachybotrys was found, but only on surface swabs and not air specimens. The researchers did not look for other etiologies, nor were there objective measures of illness. Taskinen et al. (409, 410) also reported an increase in asthma in moisture- and mold-affected schools but presented no objective measurements of asthma and very limited immune data, including surprisingly low incidences of positive skin prick tests. Other authors have presented similar findings, reporting that “exposed” children had a higher prevalence of respiratory symptoms and infection, doctor visits, and antibiotic use, and got better post renovation (367). However despite claiming “[exposure]…increased the indoor air problems of the schools and affected the respiratory health of the children,” the study was neither controlled nor blinded, and presented no physical diagnosis or objective measures.
Other evidence suggests that Stachybotrys exposure is not responsible for these building-related episodes. Sudakin (404) examined water-damaged buildings in the Pacific Northwest, due to occupants' neurobehavioral and upper respiratory health complaints (there were no objective pulmonary data) and found S. chartarum in only 1 of 19 cellulose agar cultures from building materials; the fungus was not detected in any of the above samples. While employees felt better after being relocated, there was no evidence that Stachybotrys was a causative agent. Even when large amounts of fungus are detected, analysis often fails to show direct links between symptomatic residents and fungal growth (41). In studies reporting that exposure to home dampness and mold may be a risk factor for respiratory disease, other factors such as smoking may be more contributory (84). In buildings with moisture problems where mycotoxins have been identified, a variety of species are identified, and links between a particular organism and toxin often cannot be established (420).
Despite these problems and an almost complete lack of objective evidence to support guidelines, broad recommendations have been made concerning indoor mold exposure, acceptable air contamination limits, and remediation goals. The sources range from individual authors (267, 292) to the American Academy of Pediatrics (7a) to government agencies (15). Nikodemusz et al. (292) declared that microbial monitoring of air is important even though the organisms the author found were not pathogens. While Miller et al. (267) admit that their “data seriously call into question any attempt to set arbitrary standards for fungal CFU values,” they proposed that some fungi should be considered unacceptable, e.g., pathogens and certain toxigenic species such as S. chartarum, even though complete elimination would be untenable. The same authors stated that it is reasonable to assume there is a problem if a single species predominates with >50 CFU m−3; that <150 CFU m−3 is acceptable if there is a mix of benign species; and that there is no problem when up to 300 CFU of Cladosporium or other common phylloplane fungi m−3 is isolated. Notably there is no source material to support these assertions. The American Association of Pediatrics produced guidelines in the wake of the Cleveland IPH story (7a), again without substantial evidence. More moderate recommendations (while recognizing that the presence of fungi does not necessarily imply illness) would appear reasonable (240). These could include maintaining heating, ventilation, and air conditioning (HVAC) systems, controlling humidity, inspecting and repairing water damage and other sources of contamination, regularly cleaning the home environment with dust removal, cleaning carpets, removing visible mold growth, and formulating guidelines to standardize the levels of fungal and bacterial contamination.
There have been a number of media reports on the abandonment or destruction of buildings contaminated with S. chartarum (34; McFarlane, CNN online article; Moriarty, CBS News program). It is unlikely that such extreme measures are warranted. Methods are discussed further below, but it is important to note that individuals get better with remediation efforts (6, 72, 79, 191, 321, 367), although perhaps not always (164). Simple methods, including removing damaged material and spraying affected areas with bleach, are generally effective in controlling contamination and result in “clean” air samples (430). In some cases, temperature and humidity control may be adequate (142).
FUNGI AND FUNGAL TOXINS
Mycotoxins
Perhaps the earliest recorded cases of mycotoxicosis date to the Middle Ages with the description of “St. Anthony's Fire” or ignis sacer (sacred fire) due to ergotism from Claviceps purpurea (which can also be produced by some species of Penicillium, Aspergillus, and Rhizopus) (322, 382). By the 17th century, it was recognized that moldy rye produced the disease, and ergot alkaloids from fungi were identified as toxins in the 18th century (53, 69, 157). The source of ergot affects both the type of alkaloid produced and the clinical syndrome. There are two types of toxicity: C. purpura produces gangrenous ergotism, while C. fusiformis causes convulsive ergotism (discussed below). The disease is rare today due to food hygiene and the lability of the alkaloid toxins. That ergotism was produced by oral consumption is important, reflecting the fact that historically, mycotoxicosis has usually been associated with oral consumption of moldy grain (157). As discussed below, other routes of instillation result in significantly different types and degrees of toxicity.
Mycotoxins are diverse secondary metabolites produced by fungi growing on a variety of foodstuffs consumed by both animals and humans (Table 2) (76). Clinical toxicological syndromes caused by ingestion of large amounts of mycotoxins have been well characterized in animals and range from acute mortality to slow growth and reduced reproductive efficiency. The effects on humans are much less well characterized (Table 3) (76, 329). Outbreaks of various types of animal mycotoxicosis have occurred worldwide in livestock, including sweet clover poisoning, moldy- corn toxicosis, cornstalk disease, bovine hyperkeratosis, and poultry hemorrhagic syndrome (76, 133).
TABLE 3.
Mycotoxin-related illnesses postulated to affect animals and humansa
| Toxin | Principal fungus | Primary disease |
|---|---|---|
| Probable or definite agents | ||
| Ergot alkaloids | Cladosporium purpurea | Ergotism (gangrenous and convulsive) |
| Aflatoxins | Aspergillus flavus, Aspergillus parasiticus | Aflatoxicosis, hepatitis, liver cancer, childhood cirrhosis |
| Trichothecenes (T-2 toxin, DAS)b | Fusarium, Myrothecium, Trichoderma, Cephalosporium spp. | ATA, stachybotryotoxicosis, others?b |
| Zearalenone | Fusarium spp. | Premature thelarchec |
| Vomitoxin, deoxynivalenol | F. graminearum | Anorexiac |
| Ochratoxin | Aspergillus ochraceus, Penicillium viridicatum | BEN, urinary tract tumors |
| Sterigmatocystin | Aspergillus versicolar | Cancerc |
| Ergovaline | Acremonium coenophialum | Fescuec |
| Amatoxins | Toxic mushrooms | Mushroom poisoning |
| Possible agents | ||
| Aflatoxin | Aspergillus flavus, Aspergillus parasiticus | Immunosuppressionc |
| Fusarium metabolites | Fusarium spp. | Akakabi-byo disease |
| Fusarium metabolites | Fusarium spp. | Onyalai disease |
| Fusarium metabolites | Fusarium equiseti | Kashin-Beck disease |
| Fumonisins | Fusarium moniliforme | Esophageal cancer, equine leukomalacia |
| 3-Nitropropionic acid | Arthrinium spp. | Pediatric neurotoxicity |
| Cyclopiazonic acid | Aspergillus, Penicillium | Kodua poisoning |
| Penitrem | Aspergillus, Penicillium | Tremors |
| Citrinin | Penicillium citrinin | Renal damagec |
| Rubratoxin | Penicillium rubrum | Hepatoxicityc |
| Secalonic acid D | Penicillium oxalicum | Teratogenicc |
| Patulin | Penicillium expansum | Tumorsc |
| Unlikely or wrong agents | ||
| Cyclosporins | Various species | AIDS |
| Gliotoxins | Aspergillus, Trichoderma spp. | AIDS |
| Zearalenone | Fusarium spp. | Cervical cancer |
| Citreoviridin | Penicillium spp. | Cardiac beriberi |
| T-2 toxin | Fusarium spp. | Pellagra |
| Aflatoxin | Aspergillus spp. | Reye's syndrome, kwashiorkor |
Mycotoxins are probably responsible for a range of acute and chronic effects that cannot be attributed to fungal growth within the host (301) or allergic reactions to foreign proteins (370). There are at least 21 different mycotoxin classes (71), with over 400 individual toxins produced by at least 350 fungi (38, 53, 192, 334, 335, 390, 420). They are all complex organic compounds of 200 to 800 kD and are not volatile at ambient temperatures. A number of these are plant disease virulence factors, while others kill other fungi and microorganisms and thus may represent spillover effects when causing disease in animals (322).
A variety of factors affect toxin occurrence (157). Many toxins are secondary metabolites, produced under suboptimal growth conditions (48) or in the presence of limited nutrients (161). (For reviews of toxin synthesis, see references 46 and 406.) Temperature, relative humidity, moisture, and growth rate all affect fungal mass as well as toxin synthesis. Aflatoxin production by Aspergillus is dependent on concentrations of O2, CO2, zinc, and copper, as well as physical location (A. fumigatus and A. flavus grow in trench silos, while upright silos favor Fusarium species) (402). Ochratoxin production relates to air exhaustion (69), patulin production relates to limiting nitrogen, ergot production relates to phosphate limitation (48), and A. parasiticus toxin production relates to temperature (35). These considerations are critical, since the recovery of toxigenic species from any environment does not substantiate the presence of a mycotoxin (mycotoxin production is not a necessary result of fungal growth) (48). Indeed, the conditions necessary for mycotoxin production are usually very different from those required for growth; for example, Fusarium tricintum produces a significant amount of T-2 toxin at 15°C but little at higher temperatures (69).
The most notorious and best described of the mycotoxins are the aflatoxins. In the early 1960s, an outbreak of turkey X disease in England, in which over 100,000 fowl died, was later traced to contaminated peanuts from Brazil (454). Aflatoxins were subsequently identified as the toxic agent. While made primarily by Aspergillus species, these toxins are also produced by Penicillium and Fusarium species (406). A. flavus makes aflatoxin B (AFB), while A. parasiticus produces both AFB and AFG. AFM1 and AFM2 are oxidative metabolic products made after ingestion and appear in milk, urine, and feces. The aflatoxins are toxic, immunosuppressive, mutogenic, teratogenic, and carcinogenic, and their main target is the liver. Most have been classified as type 1 carcinogens (172). AFB1 is probably the most potent liver carcinogen for a variety of species, including humans (102). Aflatoxin-related disease can occur in outbreaks, causing acute, often fatal, liver injury. The compounds have been best studied in veterinary practice, where they show the most potent effects. Toxicity is species, age, and route dependent; for example, farm animals ingest large quantities in feed (402). Species variability may relate to the ability to form epoxide derivatives in liver microsomes and endoplasmic reticulum.
The case of aflatoxin also illustrates the problems of elucidating clinically relevant levels of mycotoxins. Determining actual exposure levels is exceedingly difficult, even in known contaminated foodstuffs (334). While aflatoxin contaminates many imported goods (from almonds to melon seeds), there is a large variation in toxin distribution. Data validity is suspect when looking at small quantities, since aflatoxin is normally found in only very limited portions of a food lot and levels in such samples can range from 0 to >400,000 ng/g. For many mycotoxins, it becomes a matter of how hard one looks, and as more sensitive methods are developed, more toxins are found. Currently, at least 29 mycotoxins have been identified in commercially available foods or feeds (57), and in rare cases of high feed contamination they have been found in meat, milk, and eggs.
Stachybotrys Species as Pathogens
In the Ukraine in the early 1930s, a unique disease of horses was recognized that was characterized by lip edema, stomatitis, oral necrosis, rhinitis, and conjunctivitis. The symptoms often progressed through well-defined stages to pancytopenia, coagulopathy and hemorrhage, neurologic compromise (irritability, gait disturbance, and blindness), superinfections, and finally death (133). There was also a rare “atypical” or “shocking” form, which was primarily neurological and highly fatal, with areflexia (loss of sensorimotor reflexes), hyperesthesia (hypersensitivity to pain), hyperirritability, blindness, and stupor. In these latter cases, there were no blood dyscrasias. Pathologic examination of tissue from affected animals revealed diffuse hemorrhage and necrosis, with involvement of the entire alimentary tract. Pulmonary changes consisted of lung congestion and edema (429). Pathologic changes in tissue appeared unique, since there was no zone of demarcation around necrotic foci and since the tissue appeared to be in a “nonreactive state.” In retrospect, these findings were perhaps the first indication that the entity had a toxigenic origin rather than being due to direct tissue infection. In the wake of the equine outbreak, it was soon realized the disease was not limited to horses. However, there were marked species and age effects on susceptibility: cattle were less affected than horses, and younger animals fared better than older ones (134).
While in 1938 this animal disease was associated with Stachybotrys species, it was nearly a decade before the etiologic organism was identified in contaminated grain as S. alternans var. jateli (133, 429). In 1837, Corda first defined the Stachybotrys genus, from a strain growing on domestic wallpaper in Prague (75). S. alternans and S. atra are obsolete species names, and the organism has been renamed S. chartarum (www.doctorfungus.org/imageban/synonyms/stachybotrys.htm). S. chartarum is dematiaceous and is a member of the Fungi Imperfecti. Interested readers should refer to the article by Jong and Davis (200) as well as the recent on-line review by Nelson (B. D. Nelson, 2001, Stachybotrys chartarum: the toxic indoor mold, APSnet, http://www.apsnet.org/online/feature/stachybotrys).
In the 1940s Drobotko et al. (103, 104) reported finding the fungus on straw and fed it to horses, which developed the characteristic equine illness. At the same time, scientists recognized that the disease was toxin mediated and that exposure to isolated toxin could produce symptoms (133). Subsequently, Drobotko coined the term “stachybotryotoxicosis.” By the late 1940s, similar outbreaks of livestock disease had been reported in the rest of the USSR and Eastern Europe, as well as among nonequine species (134, 387, 434, 455). More recently, animal disease has been seen from Europe to South Africa (150, 212, 224, 225, 373; P. Le Bars and J. Le Bars, Proc. 5th Int. Working Conf. Stored Product Prot., 1990). In South Africa, a case of sheep disease was seen in the 1990s after animals consumed heavily contaminated grain cubes (374). The affected animals had fever, listlessness, oral lesions, pancytopenia, hemorrhage, opportunistic infections, and a significant mortality rate.
Members of the Stachybotrys genus exist worldwide, from Finland to the South Pacific Islands (133, 434), and were identified in the United States in the 1940s (442). In general, the organism is found in soil and strata rich in cellulose (hay, straw, grain, hemp, plant debris, dead roots, wood pulp, cotton, fabrics, paper, book bindery glue, plant fiber-processing plants, etc.) (133). It has also appeared in cigarette tobacco (1, 115). The organism can survive a wide temperature range, only dying at temperatures of >60°C. The fungus can survive over winter, spores stay viable for years to decades, and conidia retain viability despite passage through the gastrointestinal tract (but are killed in composting and degradation of manure). These factors, combined with their geographic range, suggest that Stachybotrys species are essentially ubiquitous. While disinfectants kill conidia and mycelia, cell walls are quite stable.
Associations with Human Disease
The possible association of Stachybotrys species with human disease became apparent coincident with the equine epidemics. In areas of enzootic equine disease, humans, especially fodder-handlers and others who had close contact with musty straw, developed a dermatologic and respiratory syndrome (103, 104, 196, 229, 429, 434). Occasionally, individuals who used straw for fuel or bedding became ill (133). Close family members without such exposures, and even workers protected by clothing, did not become ill. Primary disease manifestations appeared on the skin, with dermatitis on the scrotum, medial thighs, axilla, and, less frequently, the hands and other areas. Lesions progressed from hyperemia to crusting exudates to necrosis, with subsequent resolution (429). Lesion location suggested that rather than direct contact, the lesions were due to aerosolization of the offending substances, with primary effects in dermal areas with abundant moisture and skin-to-skin contact. Some patients suffered erosions on the oral and gingival mucosa (7, 229). Respiratory symptoms were described, including catarrhal angina, bloody rhinitis, cough, throat pain, chest tightness, and occasional fever. Some patients experienced transient leukocytopenia. Subsequently, straw yielded S. alternans isolates that were toxic in a rabbit dermal toxicity test, producing areal fructifications. When applied to the skin of volunteers, the isolates produced the same local and systemic responses (104). A striking aspect of these observations is their significant difference from animal disease, both in symptoms (dermatologic versus systemic illness) and in the route of exposure (ingested versus aerosolized contact) (7, 360, 361).
Despite the association of Stachybotrys with animal and human disease, early researchers were unable to fulfill Koch's postulates with the fungus. There was no evidence that the fungus itself was a pathogen, and scientists could not transmit infection or disease by injection of tissue from affected animals (104). At most, injection of the fungus caused a local response but no systemic invasion (429). It was only with the identification and application of Stachybotrys toxins that the nature of the disease process was understood.
Mycotoxins from Stachybotrys
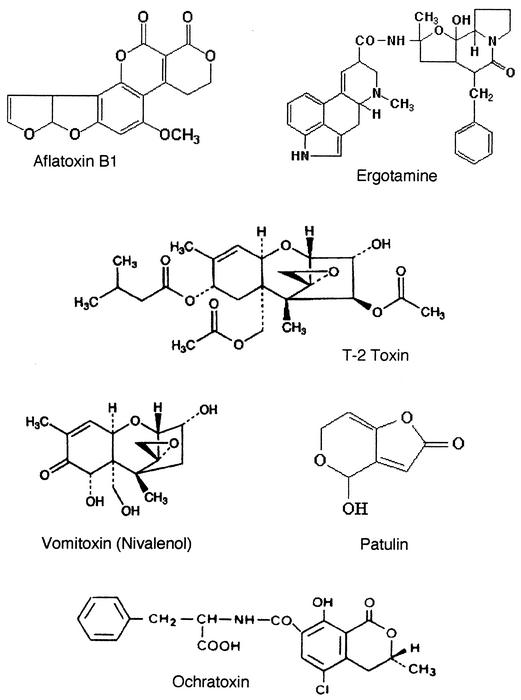
The mycotoxins responsible for many of the described effects of Stachybotrys were isolated in the 1940s during the aforementioned Russian equine outbreak and were found to have an empiric formula of C25H34O6 or C26H38O6, consistent with the trichothecene class of compounds (103, 104, 133). There are 148 natural trichothecenes alone (147). At least 40 of these are mycotoxins, produced mainly by Fusarium species (71). While trichothecenes are chemically diverse, they are all tricyclic sesquiterpenes with a 12,13-epoxy-trichothec-9-ene ring (187, 322), some of the best described of which are satratoxins F, G, and H, roriden E, verrucarin J, and trichoverrols A and B. The toxins have been isolated from a variety of substrates, including dust (satratoxins, trichoverrols, verrucarol, verrucarins, trichoverrins) and grain (T-2 toxin, nivalenol, and derivatives of others) (68). The most potent of these are T-2 toxin, diacetoxyscirpenol (DAS or anguidine), deoxynivalenol (vomitoxin), and fusarenon-X (Fig. 2). Sites of action include initiation of protein synthesis (scirpentriol, 15-acetoxyscirpendiol, DAS, verucarin A, and T-2 toxin) and elongation or termination (trichodermin, trichodermol, crotocol, trichothecolone, trichothecin, and verrucarol) (187, 258). Because of their potency in affecting protein synthesis, they may cause a predilection to other diseases, masking the underlying toxicosis (322, 337). As a result, many diagnoses were entertained before alimentary toxic aleukia (see below) was correctly linked to fusarial toxins (Table 4) (194). Stachybotrys species can produce spirolactams and spirolactones related to anticomplement components (188), phenylspirodrimanes which inhibit complement activation (275), cyclosporins (354), and endothelin receptor antagonists (287). There is also a beneficial trichothecene complex of antibiotics exerting phytotoxic, cytotoxic, and cytostatic properties (186) and recently described stachyflin compounds with potent antiviral activity (269). Trichothecenes resist sunlight, UV light, X-rays, heat (up to 120°C), and acids. They are readily destroyed by alkali, which allows for detoxification with sodium, potassium, calcium, or ammonium hydroxide or gaseous ammonia (28, 336). This has important ramifications for building remediation.
FIG. 2.
Structures of some representative mycotoxins.
TABLE 4.
Trichothecene mycotoxicosesa
| Toxicosis | Geographical location | Affected species | Symptoms | Fungus |
|---|---|---|---|---|
| Alimentary toxic aleukia (ATA) | USSR | Human, horse, pig | Vomiting, diarrhea, skin inflammation, leukopenia, angina | Fusarium sporotrichioides |
| Bean hull toxicosis | Japan | Horse | Convulsions, cyclic movement | Fusarium solani |
| Dendrodochiotoxicosis | Europe | Horse | Skin inflammation | Dendrochium toxicum |
| Moldy corn toxicosis | USA | Pig, cow | Vomiting, hemorrhage, refusal of feed | Fusarium tricinctum |
| Red-mold toxicosis | Japan | Human, horse, pig, cow | Vomiting, diarrhea, abortion | Fusarium graminearum |
| Stachybotryotoxicosis | Europe | Horse | Shock, stomatitis, dermal necrosis, leukopenia | Stachybotrys chartarum |
| “Taumelgetreide” toxicosis | USSR (Siberia) | Human, horse, pig, fowl | Headaches, chills, nausea, vomiting | Giberella saubinetti |
Experimentally, two-thirds of Stachybotrys isolates produce stachybotryotoxins. S. chartarum is the species most closely associated with trichothecene mycotoxicosis, although it should be noted that other main producers of trichothecenes are Fusarium, M. verrucaria, and M. roridum. Other fungi capable of synthesizing these compounds include Trichothecium, Trichoderma, Cephalosporium, Verticimonosporum, and Cylindrocarpon (186). As noted above the presence of potentially toxigenic strains does not imply the production of toxin (either in the environment from which the organism was isolated, nor the laboratory), nor does the appearance of a toxin in environmental samples mean that Stachybotrys (or another relevant fungus) is present. While S. chartarum produces several very toxic macrocyclic trichothecenes (32, 150-152, 183, 188), the levels at which these toxins are produced in laboratory cultures have never appeared sufficient to cause such profound toxic effects as have been observed in animals (188). Levels are also low in environmental samples. Chemical analysis of such samples is difficult due to intrinsic compound properties and secondary metabolite production; despite much work, most potential products are uncharacterized (e.g., due to irreversible column gel binding) (188), and even experienced investigators can obtain different toxin results from identical strains (e.g., using differing culture media or pH). Toxicity can also vary widely during culture (134). Matters are further complicated by the fact that more than one compound is often involved in field outbreaks, and little research has been performed to examine combined toxicity.
The most famous purported case of mass human trichothecene toxicity was in fact due to Fusarium. The illness was initially dubbed “septic sore throat” and subsequently called alimentary toxic aleukia (ATA) (7, 193-195, 252, 253, 274, 369, 380, 408, 429). It occurred in Russia in the early 20th century, most notably prior to and during World War II, and was characterized by several stages. Initially, there was oral mucosal ulceration and gastroenteritis. Subsequently, there was pancytopenia accompanied by fatigue, vertigo, and hypotension. The illness had a substantial mortality rate, at least in part due to opportunistic bacterial infections developing in the later stages of the disease (53, 69, 370). One-third of family members who ate contaminated grain became ill, and one-third of those died; this was responsible for thousands of deaths. Where nutrition was good, morbidity and mortality were much lower (7, 274). As in the case of the equine stachybotryotoxicosis, Koch's postulates could not be fulfilled during research into the disease's etiology. The illness was subsequently attributed to trichothecene mycotoxins in overwintered grain infected with F. sporotrichioides or F. poae (194). The grain had been left in the fields due to the severe conditions prior to and during the war.
The precise toxic cause of the illness was never fully identified. A number of mycotoxins were obtained from laboratory cultures of F. sporotrichioides and F. poae: trichothecenes (T-2, HT-2, and neosolaniol), sterols (sporofusarin, sporofusariogenin, poaefusarin, and poaefusariogenin), and fatty acids (29, 195, 273, 382, 452, 453). The relevance of these mycotoxins to the observed human illness is still not clear. T-2 toxin was commonly produced and may be responsible (69), and T-2 toxin produces an ATA-like illness in cats (233, 234); however, many of the other compounds also produced toxic effects in cats (195). ATA has not been seen since the last Soviet reports in the late 1940s, and so there have been no recent episodes to study by modern analytical techniques. (In 1970, Saito and Tatsuno [353] described four human cases of Akakabi-byo, or red mold disease, which had some similarity to ATA. Analysis of fungus-contaminated grain revealed F. graminanum and mycotoxins including nivalenol, fusarenone-X, and diacetoxyscirpenol.) The reasons for the disappearance of ATA are probably multifactorial, including improved grain handling and nutrition. Several important points are worth mentioning. First, while the clinical picture of ATA is similar to that of equine stachybotryotoxicosis, the former disease is almost certainly caused by Fusarium rather than Stachybotrys species. Second, the exact toxicologic mechanism has not been determined. Third, the population affected by ATA also suffered severe nutritional deficiency (252, 253), which can produce similar symptoms.
While the effects of large amounts of orally ingested mycotoxins on animals have been well described, systemic effects of exposure to inhaled mycotoxins have been much less characterized. As noted above, workers exposed to aerosolized mold or mycotoxins during the equine stachybotryotoxicosis outbreak had a very different constellation of symptoms from animals ingesting affected grain. Only a few mycotoxins have been conclusively shown in aerosols, including aflatoxin, some trichothecenes (157), zearalenone (69), and secalonic acid D (112). This is because purified mycotoxins are not volatile (370), due to their high molecular weights (71). Therefore, inhalation exposure probably occurs through inhalation of airborne particulates containing mycotoxins, such as dust and fungal components (314, 443; H. B. Schiefer, Proc. 5th Int. Conf. Indoor Air Qual. Climate, 1990). Moreover, since mycotoxins have been postulated to normally be confined in spores, it is doubtful that they frequently reach the lower airways due to size limitations. The depth of particle penetration is inversely proportional to size; the upper airways trap particles of 10 to 60 μm, while particles 2 to 4 μm in diameter can reach the alveoli (141). Mold spore size depends on the organism; e.g., Alternaria spores are more than 7 μm in diameter, while thermophillic actinomycetes are less than 1 μm (141). This point will become important below, when discussing models of stachybotryotoxicosis.
SPECIFIC TOXICITIES
Pulmonary
Rationale for concerns regarding Stachybotrys.
It is only recently that the idea that Stachybotrys can cause significant disease has risen to national prominence (137), although the documented pulmonary effects of exposure to some fungal species date to the 18th century. The transient acute upper respiratory symptoms in workers exposed to contaminated materials during the equine Stachybotrys outbreak (312), as well as anecdotal reports (11, 423), suggested that this fungus could exert at least minor pulmonary effects. Concerns in the United States regarding Stachybotrys developed primarily due to reports linking an unusual cluster of pediatric IPH and mold exposure in the Cleveland area (65, 123, 278). Coincidentally, S. chartarum, and in some cases its mycotoxins, was isolated in building materials and air samples in buildings associated with moisture problems and complaints of SBS-like illnesses (41, 79, 164, 168, 267; E. Johanning, P. Morey, and M. Goldberg, Proc. Sixth Int. Conf. Indoor Air Qual. Climate, 1993).
Possible links between mold and respiratory disease have been recognized for more than a century; literature describing connections between indoor air and pulmonary disease is cited above. Initial study of the problem came about due to work-related problems in several areas, including farming and industry (73, 376). Early Soviet literature described a toxicosis associated with inhalation of dust heavily contaminated with spores of a variety of fungi (e.g., A. fumigatus, A. niger, Dendrodochium toxicum, and Stachybotrys), which was subsequently dubbed respiratory mycotoxicosis or pneumomycotoxicosis (360, 361). This illness was considered an occupational disease in many professions, including those involving work in binder twine factories, cottonseed oil processing plants, grain elevators, and mills (133, 360). However, occupational lung disease occurs in many industries, generally in the absence of an infectious agent (73), so that it is often unclear if fungi are the responsible agents or epiphenomenona.
With the dissemination of the concept of toxigenic indoor mold, scientific reports (see below) and legal claims (13, 14, 17; C. Kingdollar, 2001, Pollution litigation review—August 2001, http://www.facworld.com/FACworld.nsf/doc/pollitrev0801) of mold-induced respiratory complaints have become commonplace. For example, Johanning et al. (197) reported that exposure to toxigenic S. chartarum and other atypical fungi was associated with disorders of the respiratory system, although pulmonary disease was never documented beyond subjective complaints. However, it must be noted that many different conditions can produce essentially the same respiratory symptoms (129). These range from the benign, such as congestion and cough from rhinitis, to reactive airways disease to more serious syndromes including alveolitis, bronchiectasis, and pulmonary fibrosis. Within the rubric of subjective complaints subsequently described as “asthma,” there may be a variety of actual syndromes ranging from asthma to allergic bronchopulmonary aspergillosis, hypersensitivity pneumonitis, emphysema, pulmonary fibrosis, and pulmonary hemosiderosis (73, 128). It is also critical to distinguish conditions which are readily reversible from those which produce permanent damage: most mold-related respiratory diseases in fact appear reversible (72). This is especially important given legal claims of permanent lung injury and “lung scarring” (Anonymous, 2000, New details from mold investigation: news comes too late for former employee, ChannelCincinnati, http://www.channelcincinnati.com/cin/news/investigations/stories/investigations-20000126-221100.html; Kingdollar, Pollution litigation review—August 2001 online article; M. McConnell, 2001, Awareness of mold problem increasing among homeowners, hvac industry, http://www.snipsmag.com/CDA/ArticleInformation/features/BNP_Features_Item/0,3374,20041,00.html). Although these conditions can be diagnosed by methods ranging from radiology to pulmonary function tests (PFTs) to biopsy, most studies have not done so, relying only on subjective reports instead. A number of papers have made claims regarding asthma and interstitial and emphysematous lung disease with no data beyond subjective questionnaires, which cannot diagnose many of these conditions (164). As noted above, objective studies often reveal poor correlation between complaints (on retrospective questionnaires) and actual pathology. Thus, a main concern lies in determining if there is actually disease beyond mild upper airway inflammatory responses and in whether these symptoms are due to fungus as opposed to other contaminants (73, 319).
Organic toxic dust syndrome, hypersensitivity pneumonitis, and allergic lung disease.
Exposure to massive amounts of fungus can cause a significant, but transitory, acute lung injury. Anecdotal reports have documented disease, generally in farmers, who inhaled large quantities of organisms (116, 286, 324). All describe an acute organic dust toxic syndrome or silo unloaders syndrome (also called atypical farmer's lung or pulmonary mycotoxicosis). This illness differs from hypersensitivity pneumonitis (HP) in that it is transient, occurs in naive patients, needs intense exposure, neutrophils and not lymphocytes are found on brochoalveolar lavage (BAL), and fungal precipitin testing is negative (377). The episodes described occurred shortly after inhalation of unusually massive amounts of fungus and resulted in cough, respiratory distress (sometimes requiring ventilatory support), fever, fatigue, alveolar and interstitial infiltrates on chest X-ray, and leukocytosis. These cases are relatively unusual since lung biopsy alveolar specimens showed fungus, including A. niger (286), Fusarium, Penicillium (324), and in one case five different organisms (116). In addition to fungal organisms, histopathologic testing showed acute and organizing diffuse alveolar damage or bronchopneumonia (324). Serologic studies for antigens capable of causing hypersensitivity pneumonitis were negative (286). Most importantly, all patients recovered, with no residual chest X-ray abnormalities or residual deficits (324). While one author claimed that these cases were due to fungal toxins (116), there was nothing to suggest that these responses were more than an acute pneumonitis or near-drowning-type response. To our knowledge, these cases are the only ones where fungi have been demonstrated in lung tissue (aside from the quite different diseases of frank pulmonary fungal infection with organisms such as Aspergillus), with one exception noted below. This is an important point, since as discussed below, most animal models of Stachybotrys pulmonary toxicity rely on direct inoculation of organisms into the airway, in concentrations high enough that they can later be seen in lung tissue.
Allergic pulmonary effects of mold have been well described, ranging from upper airway inflammation (rhinitis with coincident conjunctivitis) to asthma, allergic pulmonary aspergillosis (the latter usually affecting patients with intrinsic lung disease and bronchiectasis), and HP (73, 301, 345, 376). While an in-depth discussion of this topic is beyond the scope of this review, some findings are worth mentioning. Occupational lung disease alone consists of a number of subtypes, including industrial bronchitis, occupational asthma (in which a very small amount of offending agent can cause bronchospasm after airways sensitization, e.g., in animal handlers and crab processors), byssinosis (Monday morning fever), and grain dust-induced bronchitis (73). There is substantial evidence that the last two illnesses are caused by endotoxin exposure (58, 156, 235).
In several cases, HP has been documented after exposure to indoor mold (usually Penicillium species), generally in the setting of faulty ventilation systems (2, 37, 126, 356). Summer-type HP in Japan is probably caused by T. cutaneum (10, 355, 385, 393, 458), although supporting studies are limited by lack of objective data as well as cocontamination (11 case homes had 3,536 strains of fungi [459]). While some forms of HP appear to be due to fungi, the disease can be caused by dozens of agents, ranging from bird proteins to thermophilic actinomycetes (376). Additionally, the differential diagnosis of the condition includes organic dust toxic syndrome, humidifier fever, secondary (from chemotherapy, radiotherapy, inhaled toxins, and pneumoconiosis) and idiopathic pulmonary fibrosis, granulomatous disease (e.g., sarcoid), and congestive heart failure (376). HP is reversible if the patient is removed from exposure to the offending agent, although in some cases prolonged (years) exposure may lead to pulmonary fibrosis and pulmonary hypertension.
Despite claims that building contamination increases asthma symptoms, there is a profound lack of objective data. Notably, one of the few studies to employ objective measures of lung function (PFTs with methacholine challenge) failed to show lower respiratory effects (284). Another paper reported decreased diffusion capacity in individuals with respiratory symptoms who worked a problem building, but this is of unclear significance, given there were no changes in other PFTs and it was not certain if the symptoms were related to the building or preexisting conditions (164). A small Finnish study reported new cases of asthma in occupants of a water-damaged building, confirmed by Sporobolomyces salmonicolor inhalation provocation tests (381) but simultaneously claimed the illnesses were not immunoglobulin E (IgE) mediated. Finally, an experiment which allowed workers to have individual control of their ventilation systems actually led to higher concentrations of airborne dust and fungi while producing fewer symptoms (263).
In some cases it appears there is a correlation between SBS and upper airway allergic symptoms, although the etiology is unclear and is probably varied (176; H. M. Ammann, 2001, Is indoor mold contamination a threat to human health? http://www.doh.wa.gov/ehp/oehas/mold.html). Objective studies have found nasal mucosal hyperreactivity (305), and alterations in tear film stability (285). Jaakkola and Jaakkola (178) found that symptoms were associated with the use of photocopy paper.
Researchers have sometimes found an association between building-related symptoms and mold-specific IgE, although it occurred in a minority of patients. Surprisingly, there was not an association with self-reported hay fever or asthma (222), even though allergic asthma is IgE mediated (345). These authors apparently did not check for IgE to nonmold allergens. Other authors have failed to show links between atopy and symptoms. Higher mold IgE levels have been found in individuals exposed to water damaged structures (242, 368). Several groups have reported links between Alternaria allergen sensitivity (measured by specific IgE), but the relationship was not as strong as that between routine indoor allergens and asthma (325). Of a group of Chinese schoolteachers exposed to moldy sugarcane, 42% developed allergic alveolitis. After the outbreak, patients were found to have elevated IgE levels, and Penicillium and Mucor species were identified on the sugarcane (155). Other studies failed to show associations between IgE and disease (164). Nasal lavage of individuals from contaminated buildings show increased tumor necrosis factor alpha, interleukin-6 (IL-6), and nitric oxide levels in relation to periods of documented exposure (163). However, the study did not document which fungus was responsible or if the results were controlled for confounding factors. Other work found increased concentrations of eosinophils, eosinophil cationic protein, and myeloperoxidase in the nasal lavage fluid of office workers in damp buildings compared to controls (435, 436). Damp buildings had larger amounts of molds and bacteria, as well as evidence of volatiles from degraded polyvinyl chloride floor coatings. Polyvinyl chloride degradation products have been linked to symptoms by using objective physical measures (444). Thus, while evidence suggests a larger amount of allergic markers in individuals exposed to water-damaged structures, the etiology of these changes is far from certain (242). Furthermore, potential fungal antigens are many, and not well identified. While airborne 1-3-β-d-glucan is widely quoted to cause airways inflammation, there is limited evidence to support the assertion (132, 351, 412).
Cleveland infant idiopathic pulmonary hemorrhage outbreak.
In the wake of an outbreak of infant pulmonary hemorrhage (60, 65), Montana et al. (278) described a cluster of 10 infants from the Cleveland area who were diagnosed with IPH after presenting with severe respiratory disturbances requiring intensive care treatment. Of the affected infants, 50% had symptom recurrence after returning home, although these events occurred anywhere from days to many months afterward. Epidemiologic investigation using retrospective questionnaires and home examinations concluded that home water damage prior to the clinical event was the main risk factor (OR = 16). Other factors appeared to include “any relative who coughed blood” (OR = 33), birth weight (normal birth weight OR = 0.12), breastfeeding (OR = 0.16), and home smoking (OR = 7.9); the last two factors became nonsignificant after stratification in a model which included home water damage. Affected infants were found to have significant differences from controls in red blood cell indices, hemolysis, and serum cholinesterase levels. There were several problems with the study. First, case and control infants were significantly different with regard to sex, race, birth weight, breastfeeding, smoking, and the presence of electric fans; nonsignificant differences existed for gestational and maternal age. The definition of close relatives with pulmonary hemorrhage (PH) was erroneous, since there are many causes of hemoptysis in adults other than true PH, especially in smokers (129). Moreover, there was no evidence of increased illness in other family members, despite seemingly common exposure to fungi. Finally, a prior paper had described a cluster of Greek infants with IPH, possibly caused by pesticides in grain stored near children's sleeping areas (56). While the Cleveland study did not find an effect of pesticides, trace amounts of household pesticides were present in case and control homes, and the home of the one child who died had the largest amount of airborne solvents and pesticide residues. The case children in Cleveland had significantly decreased levels of serum cholinesterase compared to controls, though still within the normal range.
The same group examined whether fungal exposure was a risk factor for IPH (123). The hypothesis arose from previous work on the issue of pulmonary disease from wet building and fungal exposure, and because hemolysis on case infants' blood smears might implicate stachybotryotoxicosis (since Stachybotrys toxins may produce hemorrhagic disease and hemolysis in animals) (133, 373, 407). Retrospective questionnaires and home surveys appeared to rule out pesticides, personal care products, or maternal cocaine use as etiologies. In general, case houses had more fungi, but the OR was near 1 for all species but Stachybotrys, which was 9.83 for IPH (risk of disease given a 10-fold increase in fungi). While Aspergillus, Cladosporium, and Penicillium species were abundant in case infant homes, matched analysis failed to demonstrate differences between case and control homes. The authors concluded that infants with IPH were more likely than controls to live in homes with toxigenic S. chartarum and other fungi, although toxigenicity was not in fact demonstrated. As later noted by the Centers for Disease Control and Prevention CDC (61-64), surface samples of mold cannot reliably predict airborne contamination or individual exposure (323), since release of spores is variable and dependent on environmental factors including temperature, relative humidity, light, and air movement (228). Moreover, isolation of organisms may not reflect toxin levels in homes, since toxin production is also variable and depends on many factors, as noted above (157).
In 1999, the same group published an overview of the Cleveland investigations, with 37 case infants (including 12 fatalities) reported from 1993 to 1997; they also mentioned 138 cases in the United States during the preceding 5 years (122). The clinical profile of the larger group of cases was heterogeneous, including 4 with stresses “that do not normally produce respiratory failure” (anesthesia, hypernatremia, water intoxication, and febrile seizure); 4 cases were associated with upper airway obstruction and 1 was associated with probable asphyxiation; 11% had developmental delay or failure to thrive; 22% had seizures; 19% had another infection (including Pneumocystis carinii pneumonia); and there were cases of hemolysis with hemoglobinuria. All 22 patients available for follow-up bronchoscopy had ongoing hemosiderosis (most >6 months), and 39% of infants needed additional therapy for reactive airway disease. The latter finding does not reflect previous reports of this disease. Importantly, gram-negative bacteria and endotoxin levels were not examined. While mold isolates produced mycotoxins, including satratoxins G and H, there was no difference between case and control-homes in this ability (189). Other fungi capable of producing mycotoxins may have confounded the picture, including two strains of Memnoniella echinata (since reclassified as Stachybotrys echinata) (21), which made trichodermol, trichodermin, and griseofulvins (189, 190), as well as Cladosporium species (279). Details regarding over 100 cases reported nationally are not available (405).
Subsequently, the CDC published a retraction of its support of the papers' conclusions (61-64), due to apparent shortcomings of the aforementioned studies. The CDC stated that, because of insufficient evidence, an association between S. chartarum and infant IPH was not proven. They also reported that “while [it is] advisable to remediate homes of mold for a variety of reasons, [it is] not solely because of the purported Stachybotrys/Acute IPH association.” Primary criticisms focused on the calculation of the OR for Stachybotrys exposure, in part due to inclusion of one extreme outlier in the case group. The OR of 9.8 dropped to 1.5 on the CDC reanalysis. Additionally, exclusion of a house with an imputed mold value also brought down the OR from 5.5 to 1.9. Some of the CDC's concerns (e.g., inappropriate age matching, which could in fact affect potential exposure) may be overstated.
Other important CDC concerns were that case home sampling was biased; if true, this could invalidate the studies. Close examination of the data also revealed that Stachybotrys was present in a similar number of water-damaged case and control homes; water damage was not well defined (making the high OR suspect); and sampling was performed weeks to months after exposure. Concerns were strengthened by analysis of another cluster of IPH cases in Chicago, occurring in parallel to the Cleveland cases (59). These patients had a very similar presentation, but no mold-related disease was identified. In fact, a strong negative disease association was noted with Stachybotrys, and other organisms were identified in three of eight cases (Serratia, Staphylococcus aureus, and respiratory syncytial virus). No other family members in the Cleveland cluster were ill, which is inconsistent with the prior Stachybotrys literature.
Most importantly, the reviewers felt that the diagnoses of cases were inadequate and the source of cases was inconsistent (61, 64). There was no consistent definition of lung disease, a problem typical of the indoor-mold literature. Since it is unknown if the illness was a single disease entity, it is unclear if a single etiology could be responsible. Subsequent anecdotal cases demonstrate similar problems. Knapp et al. (209) and Flappan et al. (131) describe a child with pulmonary hemorrhage (as defined by hemosiderin-laden macrophages) and shock. As above, the child was bottle-fed and the parent was a smoker. In addition to anemia, there were many laboratory abnormalities including endocrine, hepatic, and renal. While the authors found Stachybotrys in areas near where the child slept, they in fact found more (both by frequency and amount) Aspergillus, Penicillium, and Cladosporium, as well as Alternaria, Ascospores, Periconia, Chaetomium, and myxomycetes; gram-negative rods and Rhodotorula were also present. While they found mycotoxins (including riordin L-2, roridin E, and satratoxin H) in contaminated wallboard, they did not assay for other compounds. In another case of pulmonary hemorrhage related to fungal exposure, the infant was formula fed and exposed to tobacco smoke (302). Again, there were a host of laboratory abnormalities, particularly renal. Home inspection conducted 4 months after the events revealed water damage and fungal growth. Looking at a variety of in-home sources, the authors found many species of fungi including Penicillium and Trichoderma, but no Stachybotrys. The finding of other species is an important confounder, since Trichoderma can make trichothecenes (157, 422); this genus has been linked to pulmonary disease (STHP), and Penicillium and Alternaria have been associated with animal pulmonary hemorrhage (possibly via rubratoxin) (417). Tripi et al. (418) described an infant with laryngospasm and pulmonary hemorrhage during general anesthesia, who was subsequently found to have prior exposure to Stachybotrys, but detailed laboratory data and home conditions were not reported. Finally, there has been one report of Stachybotrys being isolated from the lungs of a sick child (114). The child was older than the other patients (7 years); while there were hemosiderin-laden macrophages on BAL, and red blood cell microcytosis, there was no hemolysis on blood smears. Other fungal species (including Aspergillis and Penicillium) were recovered from the home. It is not clear what causal link between the lung disease and fungus existed or whether this was more than a variant of atypical farmer's lung or an epiphenomenona (350).
Some concerns raised by the CDC are of limited validity. While there was no evidence documenting actual exposure (i.e., positive serologic tests or organisms on BAL), serologic testing is an ineffective marker for exposure even in heavily exposed animals (92) or humans (164, 196, 197, 419), since toxins and not organisms are the disease-producing agents. Finding organisms on BAL is not a relevant test (Stachybotrys does not germinate in the lungs, nor is there a yeast form; illness is presumably toxin mediated, not via direct infection) (92). Concerns that the clinical syndrome described was not consistent with historic accounts of human and animal illness is of limited relevance: prior descriptions of the disease occurred before modern analytic techniques, and many “new” diseases have been described in the last 20 years that have probably occurred for considerably longer (e.g., human immunodeficiency virus infection). While animal models are open to question, some do include pulmonary hemorrhage.
Potential mechanisms of Stachybotrys-induced lung injury.
Pathogenic mechanisms responsible for Stachybotrys-induced lung injury, if it exists, are unclear. The contention by Dearborn et al. (92) that inhibition of type IV collagen synthesis in rapidly growing young lungs produces capillary fragility, which upon exposure to stressors (e.g., tobacco smoke) results in stress hemorrhage, is not supported by experimental evidence. Work with animal models has shown effects on surfactant production. Early studies showed that surfactant production is up-regulated in type II alveolar cells exposed to pollutants (98, 120, 311). Subsequent work has shown that alveolar type II cells, macrophages, and surfactant production and composition are affected by exposure to fungal spores and their mycotoxins, including Cladosporium and Stachybotrys species (181, 249, 250, 255, 378; W. G. Sorenson, Proc. Int. Conf. Fungi Bacteria Indoor Air Environ., 1994). It is unclear from most studies whether statistical differences observed are physiologically meaningful; moreover it has not been determined whether observed changes are toxic effects or compensatory mechanisms. As discussed below, most studies used transtracheal instillation of a high volume of spores or toxins, which has questionable physiologic relevance. Other toxic mechanisms could be responsible. For example, purified trichothecenes and S. chartarum extracts can cause hemolysis (95, 160, 346). Recently, a new hemolysin, stachylysin, was characterized from S. chartarum (433) from the Cleveland cluster. Hemolysins and siderophores have been isolated from a number of strains, although levels vary markedly across isolates (431), and toxicity does not correlate well with source location or illness (432).
Other animal studies of Stachybotrys pulmonary toxicity suffer similar limitations. Nikulin et al. (296) described experimental murine lung mycotoxicosis, using intranasal installation into the trachea of a high volume of spores of toxin-producing (spirolactones, spirolactams, satratoxins G and H) strains, which caused intra-alveolar, bronchiolar, and interstitial inflammation with hemorrhagic exudates in the alveolar and bronchial lumen. The authors attributed these effects to “toxins.” However in addition to the model limitations, the study lacked proper controls and statistical power. Subsequent work (297) using the same model showed intratracheal instillation of toxigenic spores produced interstitial inflammation with hemorrhagic exudate in the alveolar lumen, severe inflammation, and bronchial obliteration with leukocytes associated with Stachybotrys spores. These findings are similar to those of other researchers (342). As noted below, the presence of spores in pulmonary parenchyma raises concerns about the relevance of the model to actual human and animal disease. Furthermore, no histopathological changes were noted in the other normal target organs of trichothecenes, i.e., thymus, spleen, or intestines. While there were hematological differences between animals exposed to toxic and nontoxic spores, it is questionable if these were of physiologic relevance. Finally, there were marked differences in immune response between animals exposed via the intraperitoneal and the inhalational route; only the former animals developed specific IgG, which is in fact rarely seen in actual illness.
Serious problems with these models exist in regard to routes and levels of exposure, as well as the ability of organisms in “field settings” to actually reach the alveoli. In indoor air settings, spore counts as high as those modeled in the above studies are rarely detected, since Stachybotrys produces spores in a slimy mass that become airborne only when dried (296). While some researchers have thought that the spore burden in models parallels cases of human exposure (92), it is difficult to correlate the number of organisms per cubic meter of room air with concentrations in the nasal passages. Even if there is a sufficient organism burden, spore size in relation to respiratory tract physics must be considered. It has been claimed that Stachybotrys spores can reach the distal airways (92). However, this is based on work involving extensively processed spores, which are of questionable relevance to actual exposure conditions (397). In more appropriate models, even if spores are liberated, it is unlikely they reach the lower airways due to their size (445). Stachybotrys spores are single-celled ellipsoid structures 5 to 7 by 8 to 12 μm in size (144). In mice studied with monodisperse spheres, 4- to 5-μm-median-diameter particles deposited (90%) in the nose and then went on to the gastrointestinal tract; pulmonary deposition was <1% (340). Spores or fragments probably need to be <1 μm in diameter to reach the lower respiratory tract by airborne exposure, and Stachybotrys spores are much larger than this. While intranasal instillation of spores causes them to be flushed into the lungs (296, 297), it does not reflect human exposure conditions (61-64, 445). This may explain why effects seen in murine experimental models have not been seen in humans. Furthermore, the response to toxigenic spores is different from the response to toxins alone (296), and probably only the latter is physiologically relevant.
In one of the only studies using a relevant model, Wilkins et al. (445) grew Stachybotrys in an open dish in a closed chamber (to mimic human exposure) and examined acute murine pulmonary toxicity by a bioassay of respiratory parameters. The estimated exposure level was nearly three times estimated human levels (the satratoxin H level was ≥100 μg/dish). The authors concluded that while there may have been a weak effect of exposure to S. chartarum, there were no major immediate pulmonary effects. They did not examine long-term effects. The substrate was wet, limiting spore release. While this could have been a major limitation of the study, it is more reflective of normal conditions. In response to the low density of spore formation, the authors point out these findings parallel building studies “in which air contained a low number of colony forming units although the ventilation and air conditioning system were extensively colonized by different types of mold (5).” Thus, the degree of liberation of spores and mycelial particles must be considered if model systems are to be useful. Finally, it is worth noting that in this study, control mice had changes of tidal volume of <5%, which, while statistically significant, were neither clinically significant nor due to fungus. This suggests similar changes in physiological parameters reported in some studies discussed in this review may be no more than statistical variation.
Other mycotoxins have been implicated in inhalational toxicity. While aflatoxin is primarily suspected of systemic inhalational toxicity (78, 301, 316), acute inhalation of AFB1 causes tracheobronchial cell destruction in hamsters and guinea pigs (157). Fumonisin may produce pulmonary disease in pigs; animals fed this mycotoxin developed hydrothorax and pulmonary edema (124, 315). These lesions were consistent with outbreaks of porcine pulmonary edema.
In summary, there is clear evidence that exposure to indoor mold may have adverse pulmonary effects, especially by inducing allergic reactions. However, to date there is no sound evidence linking mycotoxin exposure to serious or permanent lung injury. There is also no evidence to support more than mild upper airway allergic effects of SBS and mold exposure. While there are reasonable concerns regarding Stachybotrys exposure, a link to pulmonary disease beyond transient irritative symptoms, and in particular IPH, has not been proven.
Neurologic
Exposure to S. chartarum has been reported to cause human neurotoxicity, although proof is lacking (11, 47, 79, 164, 197, 267, 312, 333, 404, 423). Complicating this has been obvious difficulty in detecting subtle neurological changes, even with sophisticated neuropsychological testing, as well as separating out psychiatric and factitious causes. There is precedent for concern regarding neurotoxicity, both for mycotoxins in general and for stachybotryotoxins in particular. Much of this is based on ergotism, “atypical” equine Stachybotrys disease, and the effects of cyclopeptides and muscarine from toxic mushrooms (4). At the same time, coincident nonfungal compounds (e.g. VOCs) may exert neurologic effects (208, 277, 310).
Neurologic or convulsive ergotism is characterized by symptoms including muscle spasms, seizures, and hallucinations (4), often from compounds produced during the baking of bread using contaminated grain (322). Nonfatal cases may exhibit tabes dorsalis-like features (4). There may be a link to malnutrition. The last major epidemic of ergotism was one of convulsive ergotism in India in 1975 due to C. fusiformis (39). As noted above, the source of the ergot affects the type of alkaloid produced as well as the symptoms, as evidenced by an earlier outbreak in Ethiopia which produced primarily gangrenous rather than neurologic disease (96, 206).
3-Nitropropionic acid is a secondary metabolite of Arthrinium species, which can cause an acute food poisoning known as “mouldy sugarcane poisoning” (230). Affected children suffer dystonia, convulsions, carpopedal spasm, and coma (231), and there may be basal ganglia lesions (270). Fumonisins, of which the most naturally abundant is fumonisin B1, cause equine leukoencephalomalacia (348, 406). This may occur due to their action as competitive inhibitors of sphingosine N-acetyltransferase, which results in the blocking of complex sphingolipid biosynthesis and the accumulation of sphingosine (282). Cyclopiazonic acid produced by Penicillium and Aspergillus may be responsible for Kodua poisoning (from Kodo millet), characterized by somnolence, tremors, and giddiness (221); in chicks it causes convulsions and rigidity (338). Verruculogens and penitrem A may be “tremorgenic mycotoxins,” responsible for tremors, ataxia, weakness, and convulsions in animals (392). Unfortunately, studies of many of these toxins were conducted using intraperitoneal injections (45, 174, 266). Because the route of administration matters greatly and since intraperitoneal injection has no physiologic correlate, the results are of questionable clinical relevance. Postulated disease associations that have not been borne out include the neurologic changes of Reye's syndrome (aflatoxin [97]) and pellagra (365).
An atypical or “shocking” form of stachybotryotoxicosis was first recognized in the 1930s and 1940s in the USSR during the equine stachybotryotoxicosis outbreak. Affected animals developed neurologic problems including areflexia, hyperesthesia, hyperirritability, blindness, and stupor; almost all affected animals died (133). Gastrointestinal disorders and blood dyscrasias of the “typical form” were not seen. However, experimental stachybotryotoxicosis in animals as well as well-documented human cases do not support the existence of significant neurological sequelae. Animal experiments produced pulmonary (361), gastrointestinal (210), and topical (134) toxicity with some systemic toxicity but no specific neurologic signs. Even in cases of heavy exposure, effects described are dermatologic (104, 429), respiratory (104, 361), and possibly gastrointestinal, without neurologic toxicity. Other studies reporting neurologic problems after exposure to Stachybotrys or trichothecenes often do not describe significant specific neurologic symptoms (11, 267, 312, 423) or do so only in vague terms, unsupported by objective neurological findings (47, 79, 164, 197, 333, 404). Notably, in recent cases where S. chartarum has been implicated in pediatric disease, no neurologic effects were reported (122, 278). Finally, some studies have shown a neuroprotective role for certain mycotoxins: stachybotrin C and parvosporin from S. parvispore stimulate neurite outgrowth (303).
In summary, despite many reported subjective complaints, there is no objective evidence for neurological compromise caused by indoor mold exposure, in particular from S. chartarum.
Hematologic and Immunologic
General concerns.
Some mycotoxins have the potential to affect hematopoetic cells, as shown for trichothecenes in equine stachybotryotoxicosis, ATA from Fusarium species, animal experiments showing pancytopenia after ingestion of S. chartarum (210), Akikabi-byo disease (460), and the African idiopathic thrombocytopenic purpura (ITP)-like syndrome Onyalai disease (341). Fungi are the source of immunosuppressive drugs such as cyclosporin, and Stachybotrys species can produce a cyclosporin-like immunosuppressive agent capable of increasing skin graft survival in rats (354). Such findings have become of increasing concern due to reports of “immune suppression” and increased infections in Stachybotrys-exposed individuals (197). It is important to keep in mind that while some mycotoxins have been recognized to affect the immune system, many earlier putative associations (e.g., gliotoxin from Aspergillus as a cause of AIDS [113]) have proven quite erroneous.
Aflatoxin.
Immune effects of mycotoxins have been studied extensively in animals but not in humans beyond observational studies (for an excellent review, see reference 76). Aflatoxin has been the most extensively studied, although immunosuppression is not among its known significant human toxicities. Effects of aflatoxin are probably directly related to impaired protein synthesis (76). Aflatoxin is transformed in vivo into active components that bind DNA and RNA and impair DNA-dependent RNA polymerase, thus inhibiting RNA and protein synthesis (138, 149, 261). In theory, this would impair the proliferation and differentiation of immune cells and perhaps the synthesis of immunomodulating compounds, including chemokines and immunoglobulins. Patulin has similar effects (398).
Aflatoxin effects on immune organs in animals include thymus and bursa atrophy in fowl (140, 328, 411). Cyclopiazonic acid causes bursal lymphoid necrosis (101), as well as similar changes in the spleen and lymph nodes of dogs (304). Trichothecenes, particularly T2 toxin, induce necrosis and lymphoid depletion in the thymus, spleen, and lymph nodes of various laboratory animals, livestock, and fowl (76).
Aflatoxin exerts a variety of cellular and humoral effects. There is suppression of mitogen-induced T- and B-cell blastogenesis in humans (366) and bovines (320). T-cell delayed-type hypersensitivity responses and graft-versus-host responses are suppressed in chickens (140), and there is impaired chemotaxis, phagocytosis, and intracellular killing by heterophils and monocytes in chickens after ingestion (66, 265). High-dose aflatoxin exposure (0.6 to 10 ppm) suppresses IgG or IgA levels in chickens (140), as well as antibody responses to Salmonella (44) and sheep red blood cells (411). There may be clinical veterinary correlates with increased frequency of swine salmonellosis (402), vaccination failure, and fowl cholera, salmonellosis, and coccidiosis (A. C. Pier, J. L. Richard, and J. R. Thurston, Proc. 1978 Symp. Interact. Mycotoxins Anim. Production, 1979). Ochratoxin A (OA) has similar effects, but these vary with dose (only higher doses induce lymphopenia) and species (higher effects in mice, less in cows) (402). Critically, OA effects on immunoglobulin production and the antibody responses vary with the route of administration: intraperitoneal injection suppressed antibody responses, but oral administration did not. Cyclopiazonic acid and, to a lesser extent, zearalenone have similar effects in some animal models. Finally, and perhaps most importantly, the degree of immune suppression and clinical signs is contingent on the nutritional status and general health of the exposed animal (109, 402; G. T. Edds, Proc. 1978 Symp. Interact. Mycotoxins Anim Production, 1979). The presence of diseases and other toxins may affect responses to aflatoxin, and good rations reduce residual tissue levels and improve organ function. Increased dietary protein and vitamins (B12, K, and methionine) are also protective.
Trichothecenes.
Trichothecenes produced by Stachybotrys and Fusarium species have been extensively studied, especially T-2 toxin, due to its toxic and immunosuppressive effects and its potential as a biologic weapon (76, 309). Other potent trichothecenes include DAS, deoxyivalenol (vomitoxin), and fusarenon-X. These compounds are among the most potent small-molecule inhibitors of protein synthesis (243, 258, 372). By blocking RNA and DNA synthesis via inhibition of peptidyltransferase, they can produce characteristic radiomimetic lesions in rapidly dividing tissues (220, 353, 421). This may account for organ and cellular effects noted above and the decreased immunoglobulin synthesis, antibody responses, and complement activity in animal models (240), as well as cell proliferative and activity responses (36, 259). Trichothecenes may affect cytokine production (43, 227, 449).
In theory, there could be a common end point of opportunistic infection, similar to some animal models where aflatoxin has been associated with increased disease susceptibility (329). Trichothecenes have been associated with decreased resistance to infectious organisms, including Salmonella, Mycobacterium tuberculosis, Listeria, herpes simplex virus, Candida, and Cryptococcus (309). One report of an outbreak of stachybotryotoxicosis in sheep reported frequent isolation of a normally commensal organism, Pasteurella haemolytica, from organs; however, it is unknown if this was a result of immunosuppression or breakdown of mucosal barriers (150). Some mycotoxins can enhance immune function, including citrinin, patulin, and even T-2 toxin (119, 343). The latter can increase the number of antibody-producing cells in the spleen, splenocyte IgA production, delayed-type hypersensitivity reactions, and blastogenesis of B and T cells, as well as superinducing IL-1 and IL-2. Often immune stimulation or suppression is related to the amount of toxin; since most studies have been conducted via various noninhalational routes, little is known about the effects of airborne exposure (43).
As Corrier (76) points out, in vivo studies of the immunomodulating effects of mycotoxins may be influenced by contributing factors that are not present or detected during in vitro studies. Almost all studies have been with animals, often using single high-dose purified toxins, by nonnatural routes of administration (e.g., intraperitoneal), and often in animals with different disease susceptibilities from humans (74, 145, 219). Immunological studies of stachybotryotoxin effects in humans are limited to observational studies (82, 197, 198). In contrast to early reports of severe leukopenia and radiomimetic effects in ATA-like syndromes after trichothecene ingestion in Eastern Europe (182, 312, 363, 364), these observational studies have not found the same hematopoietic effects (197). One study (82) claimed that residential fungal contamination affected peripheral lymphocyte counts in children, reporting that contaminated homes were associated with more CD45RO (antigen experienced T-cells) and a reduced CD4/CD8 ratio and thus “that residential fungal contamination leads to chronic stimulation of children's' lymphocytes.” However, close examination of the data leads to questions about these conclusions: in addition to exposure to water damage and apparent mold, affected children were more likely to be in the presence of household smoking, pets, and higher dust mite antigen concentrations, have a bedroom humidifier, be male, on average be 2 years older, and less likely to have a current cold reported by the parent. In addition, environments were not checked for endotoxins or volatile organic compounds, nor was diet examined. Differences of CD45RO, CD29 (B cell), and CD4-to-CD8 ratios were of borderline significance (P = 0.04 to 0.05), and examination of the CD4-to-CD8 ratios for case versus control subjects revealed no significant differences (1.6/0.9 versus 1.5/1.0, with standard deviations of 0.3 to 0.5 for all numbers). The claims of persistent immunological changes over time are not supported statistically. Children from contaminated homes had higher total IgE levels, as well as levels to mite antigens, cat epithelium, and dog dander, suggesting that nonmycotoxin sources may have had a significant impact on the findings.
The study most widely cited as proving immune effects of Stachybotrys exposure in fact does not prove this (197). Fifty-three workers were reportedly exposed to fungal bioaerosols (“particularly S. chartarum,” although multiple other species were isolated). While medical complaints were higher in exposed individuals than in controls, there were no objective measurements of physical complaints, physical examinations, or routine laboratory tests, and there was no documentation of actual infection beyond a retrospective questionnaire. Despite measurement of multiple immune parameters, only white blood cells, CD3 cells, and NK cells were reportedly affected. On closer examination, even these data are questionable, since the statistically significant variations of these numbers (e.g., white blood cell counts of 6,060 versus 6,290 and CD3 counts of 7,566 versus 7,365 cells/ml) lack clinical significance, and low-risk patients had values more different from controls than did high-risk patients. A subsequent study by the same author of 147 children and adults seen in an environmental health specialty clinic failed to show significant differences in immune parameters (198). Despite claims in the latter paper that pediatric cases with preexisting immunodeficiency are at increased risk from “unnecessary” fungal exposure (i.e., indoor fungus) there are no data to support this assertion. The studies are further weakened by the fact that they did not test the levels or effects of other likely contaminants, including volatiles and endotoxins.
In summary, to date there is no good evidence of significant immunological compromise from inhaled fungal toxins. As in the case of neurologic complaints, at most only an association exists. Interested readers are referred to the recent review by Bondy and Pestka (43).
Malignancy
General concerns.
The potential of mycotoxins to act as carcinogens is of concern, due to the probable carcinogenic effects of aflatoxin, as well as recent legal claims that patients exposed to mycotoxins may need long-term cancer monitoring (331). While there are over 100 toxigenic fungi and more than 300 mycotoxins (268, 383), only two (aflatoxin and sterigmatocystin) have been shown to cause tumors in primate experiments (375). Studies with other animals have implicated the following as mutogenic: aflatoxin, sterigmatocystin, ochratoxin, fumonisin, zearalenone, and some Penicillium toxins (citrinin, luteoskyrin, patulin, and penicillic acid). Most are directly genotoxic (e.g., AFB-DNA adducts), except for fumonisin, which disrupts signal transduction (438). Results of animal studies, often cited as proof of carcinogenicity, must be interpreted with extreme caution in regard to humans. From 1961 to 1997, many model carcinogens were found to be tumorogenic in rodents; however, only one (urethane) was found to cause cancer in primates (375). As part of these studies, saccharine, which had been shown to be a mouse urological carcinogen and was subsequently banned as a food additive, was shown to not be a primate carcinogen. The rodent carcinogenicity of saccharine may be due to the high renal concentrating ability of rodents, which is much lower in primates. This wide species variation in susceptibility to mutogenic effects (158) extends to mycotoxins. For example, “a problem in extrapolating animal data to humans is the extremely wide range of species susceptibility to AFB1. For instance, whereas AFB1 appears to be the most hepatocarcinogenic compound known for the rat, the adult mouse is essentially totally resistant to its hepatocarcinogenicity.” (213).
Aflatoxin.
Aflatoxin is the best characterized of the potential human mycotoxin carcinogens (127, 179, 322). While it is acutely lethal in large amounts, chronic low-level exposure produces cancer, particularly hepatocellular carcinoma (HCC) in many animal species (52, 448). It is thought to be the most potent liver carcinogen for a variety of species including humans (102, 172, 406), although Pohland (334) has cautioned that “one must still conclude that only a debatable association, and not a direct causal relationship, has been established to date” for human HCC. Nonetheless, HCC is one of the leading causes of cancer mortality in Asia and Africa, and epidemiological investigations have shown increased aflatoxin ingestion correlates with increased risk (179, 463). AFB1 is produced by Aspergillus flavus and A. parasiticus (12). The primary mode of human exposure is the consumption of contaminated foodstuffs, e.g., peanuts. Aflatoxin can be detected in the blood of pregnant women, cord blood (where levels can be very high), and breast milk; AFM1 and AFM2 (oxidative metabolic products) appear in milk, urine, and feces. That these chemicals are systemically absorbed is supported by data showing a high correlation between exposure and urinary excretion of AFB1-N7-Gua (146, 438).
Mechanisms of aflatoxin carcinogenesis are unclear but probably involve tumor promotion or progression. There is evidence of activation of proto-oncogenes (c-myc, c-Ha-ras, Ki-ras, and N-ras) by aflatoxin, as well as mutations at the p53 suppressor gene target (169, 170, 389, 438). Aflatoxin and p53 mutations have been tightly epidemiologically linked in China and Southern Africa, and a p53 G→T mutation may serve as marker of aflatoxin exposure (165, 223, 384). This mutation may be situationally specific, since AFB1-induced monkey tumors are associated with a different mutation (136). Other data indicate that urine DNA adducts and serum albumen adducts reflect the amount of hepatic genotoxic damage. Levels of these adducts may reflect risk for disease development (438).
As noted, there is great species variability in susceptibility to aflatoxin. Among rodents, rats are very sensitive while mice are resistant, due to efficient conjugation of aflatoxin with glutathione, catalyzed by glutathione S-transferase mYc (158). Similarly, bioactivation of aflatoxin into carcinogenic metabolites shows significant species differences (251). There is strong evidence suggesting cofactors affect carcinogenisis. Coinfection with hepatitis B virus is important, since serum hepatitis B surface antigen (HBsAg) is a key risk marker (439, 456, 462); the relative risk for HCC is as high as 59 if both urinary aflatoxins and positive HBsAg status are present (339, 349, 438). There may be modulation of damage by nutrients, antioxidants, herbs, drugs, and malnutrition (108, 322, 438). Other mycotoxins such as sterigmatocystin may play a role (438). Because of theoretical protective effects of dietary supplements, there have been trials of chemoprotectants like ethoxyquin and oltipraz (204, 464).
There is limited evidence regarding carcinogenicity of inhaled aflatoxins. Aflatoxin can be aerosolized and has been detected in air near farm sources (49, 395, 399). Inhaled aflatoxin causes tracheobronchial cell destruction in hamsters and guinea pigs (157); rats receiving intratracheal doses develop cancer of the liver, intestines, and kidneys (301). The link to human disease is much more tenuous. While epidemiological and anecdotal studies have reported associations between inhaled mycotoxin and cancer, most of these studies were seriously flawed. Hayes et al. (154) found an increased rate of overall cancer and pulmonary cancer mortality in exposed peanut workers but failed to include smoking as a factor and did not find HCC. Olsen et al. (306) showed a two- to threefold increase in the risk of HCC and biliary cancer, but the aflatoxin concentrations were historical and were not measured. Anecdotal studies reported premature cancer in three exposed laboratory workers (106; G. E. Deger, Letter, Ann. Intern. Med. 85:204-205, 1976), but aflatoxin was detected not only in the two patients with lung cancer but also in three with pulmonary fibrosis (107), which has not been postulated to be caused by this compound. The findings may indicate that aflatoxins are secondary findings in chronic lung disease, since lung cancer and pulmonary fibrosis are unrelated illnesses.
Other mycotoxins.
Other mycotoxins have been implicated as carcinogens. Ochratoxins are structurally related metabolites from A. ochraceus and related species, P. viridicatum, and other Penicillium species (216). OA is the only one of major carcinogenic significance (216, 326, 334). In regions where Balkan endemic nephropathy (BEN) is endemic, there are high foodstuff contamination rates, and high levels of OA are found in human blood (428). There have been increasing reports of OA in human tissue samples, including one German study where the toxin was reported in 56% of randomly collected blood samples (33), although such a high prevalence appears suspect. OA can form DNA adducts, and the kidney may be the worst affected organ (438). While OA has been listed as a mammalian carcinogen (426, 451), the data come from a small number of rodent strains, and there is no direct proof in humans.
Patulin, while potentially tumorogenic, illustrates the importance of the route of administration and coincident environmental factors (334). Penicillium species produce patulin and penicillic acid, which can cause tumors when subcutaneously injected into mice. They do not cause murine tumors when administered orally. While patulin frequently contaminates apple juice (P. expansum is a main cause of apple rot), the chemical is unstable in the presence of sulfhydryl groups, which may explain its absence from processed foods.
Zearalenone, produced mainly by Fusarium graminearum, is among the most widely distributed of the fusarial mycotoxins (217) and is found in 6 to 28% of corn samples destined for human consumption (334). Significant concerns have been raised as a result of its estrogenic activity and thus its potential for stimulating estrogen-sensitive tumors (25, 93, 293, 446). It causes increased cancer rates in mice but not rats (173); in fact, in the latter species it may exert protective effects (159). It was once postulated to cause cervical cancer (248), but the assertion has not been borne out due to the clear association of this malignancy with human papillomavirus (125).
There may be a link between mycotoxins and esophageal carcinoma (334). China and areas of southern Africa have the highest rates of esophageal cancer. In these areas, corn is a main staple, and mycotoxins (especially fumonisin B1 from F. moniliforme) are major contaminants found in corn consumed by patients with esophageal cancer (67, 167, 247, 282). Selected fumonisins are hepatocarcinogenic in rats (246) and have been designated group 2b carcinogens, i.e., possibly carcinogenic (172). However, some studies have found many cocontaminants (67) or present only equivocal data (461). Such data must be interpreted with caution, since Fusarium mycotoxins in corn and corn products in areas in China where people are at high risk for gastric cancer do not appear to account for malignancy among those who consume affected foods (148).
Some mycotoxins are antineoplastic agents. Ergosterol peroxide and acetoxyscirpenediol from Paecilomyces tenuipes, a silkworm larva parasite, and Chinese entomopathogenic fungi are more potent than cisplatin against human gastric tumor, hepatoma, colorectal cell lines, and murine sarcoma (288). T-2 toxin inhibits the proliferation of neoplastic cell lines, including mouse melanoma B16 cells, human myeloid leukemia K562 cells, and human cervical carcinoma (HeLa) cells (201). New fungal compounds with similar anti-neoplastic traits are constantly being found (166).
As shown above, some trichothecenes are potentially carcinogenic. To date, there are only conflicting reports in animal studies (402). There is currently no sound evidence linking Stachybotrys-produced mycotoxins to human malignancy or evidence to support claims that individuals exposed to Stachybotrys are at long-term risk of cancer or require cancer surveillance (438).
Hepatic
In addition to HCC, aflatoxin in significant doses can cause acute liver injury. Acute aflatoxicosis generally occurs in outbreaks due to contaminated foodstuffs. The resultant liver injury can be severe, with a 10 to 60% case fatality rate (322). While there have been claims that aflatoxin causes other diseases such as Reye's syndrome (97) and neonatal jaundice (322), they have not been validated. Rubratoxins from T. rubrum can cause liver disease and disseminated hemorrhage in animals (51, 447). In the field, rubratoxins are often mixed with aflatoxin due to cogrowth of A. flavus, and there may be synergistic hepatotoxic effects (447). Hepatobiliary effects have not been reported for Stachybotrys toxins.
Endocrine
Endocrine toxicity has been seen primarily with zearalenone (F-2 toxin) (322). The compound is produced by several Fusarium species under very particular growth conditions (402) and has estrogenic effects in animals. Such effects are well characterized in swine (the most sensitive animals) and include primary effects (e.g., vulval hyperemia) as well as infertility, decreased litter size, and fetal malformations (69). Zearalenone has also been implicated in livestock “scabby grain toxicosis,” characterized by nausea, vomiting, and drowsiness (40, 422), but it was found along with other Fusarium mycotoxins. As pointed out by Stuart and Bedel, “many reports in the literature of ill effects are very questionable due to possible involvement of other mycotoxins…” (402). Similar effects have not been demonstrated in humans, even though zearalenone has been found in the blood of children exposed to contaminated food (352). There have been no reports of Stachybotrys-related endocrine toxicity.
Renal
Diverse renal and urological toxicities have been reported in humans due to OA, although on close inspection the data provide only disease association, not direct proof. Due to abundant circumstantial evidence, OA from Aspergillus and Penicillium has been implicated in porcine nephropathy and BEN (68), as well as urinary tract malignancies noted above. Ochratoxin belongs to a group of secondary metabolites of Aspergillus and Penicillium, found on cereals, coffee, bread, and foods of animal origin (322). Ochratoxin A is the most common and most toxic chemical of its class: it is nephrotoxic, immunosuppressive, carcinogenic, and teratogenic in all animals tested (451). The toxin can cause glomerulonephrosis and may cause cholinergic responses such as bronchial constriction, vasodilation, and mild smooth muscle contraction (290). A nephrotoxic strain of Penicillium was isolated from air samples in areas where BEN occurs, but study of the isolates was limited to animal feeding experiments (280). There has been one case of apparent inhalational nephrotoxicity, and an OA-producing strain of A. ochraceus was subsequently isolated from wheat to which the individual had been exposed (100).
Citrinin is produced by several Penicillium species and is sometimes isolated from contaminated rice (402). Citrinin may exert more renal toxicity than OA, although effects of the latter have been reported more frequently. Citrinin and OA may have a synergistic interaction (55).
Finally, several of the papers postulating a link between infant IPH and Stachybotrys have reported mild renal abnormalities, including non-anion gap acidosis, electrolyte disturbances, and aminoaciduria (209, 302). It is unclear if these findings are part of a mycotoxin syndrome, were secondary to severe illness (e.g., shock), or represent an underlying disease process. To date, there has been no evidence linking Stachybotrys toxins to renal disease.
Pregnancy
A number of mycotoxins affect pregnancy in experimental animals; however, none of the observations extend to humans. Ergotoxins may prevent embryo implantation in mice (244, 245). There are conflicting reports of their ability to induce abortions, and they may cause stillbirths and agalactia (299, 334, 386). Secalonic acid D has been found in dust and aerosols. Produced by P. oxalicum, it is part of the secalonic acid family and is teratogenic in mice (112). Zearalenone has been found in dust and aerosols and may cause infertility and fetal malformations in domestic animals through estrogenic effects discussed above (69, 211, 272). OA may cause fetal death, as well as uterohemorrhagic effects, in rats (211, 400). T-2 toxins and DAS have been reported to cause limb and tail abnormalities (271). While T-2 and DAS in feed do not cause abortion, preconception exposure may cause infertility and reduced litter size (271, 441).
Aflatoxin is of special concern, since it is teratogenic in nearly all animals (451). Various investigators have found uteroplacental hemorrhage and fetal death in rats and mice given intraperitoneal aflatoxin (99, 218), while others have noted only fetal retardation in rats given oral aflatoxin (54). Aflatoxin has been detected in the blood of pregnant women, cord blood, and breast milk (322). The clinical relevance of these findings is unknown.
Finally, there are some animal data suggesting similar effects from Stachybotrys toxins (211). Oral ingestion (of infected grain, liquid growth medium, or partially purified toxin) caused a decrease in the number of pregnant animals; an increased frequency in dead, resorbed, or stunted fetuses; and decreased average litter size. Histopathologic testing showed uteroplacental hemorrhages. There have been some suggestive field data for mare and sow abortions (133, 427). While this evidence has been cited as proof that Stachybotrys can affect fetuses even though no maternal effects may be evident (211), it must be noted that all of the above data are related to oral exposure in animals, which may have no relevance to human inhalational exposure.
Other Toxicities
A host of other mycotoxin effects in animals and to a lesser extent in humans, have been described. These include dermatologic (as described above), gastrointestinal (40, 230, 231), and cardiac (425). These effects are beyond the scope of this review.
CONCLUSIONS
Valid concerns exist regarding the relationship between indoor air, mold exposure, mycotoxins, and human disease. Review of the available literature reveals certain fungus-disease associations, including ergotism, ATA from Fusarium, and liver disease from Aspergillus species (382). However, while many studies suggest a similar relationship between Stachybotrys and human disease, these studies nearly uniformly suffer from significant methodological flaws, making their findings inconclusive. As a result, we have not found supportive evidence for serious illness due to Stachybotrys exposure in the contemporary environment. Our conclusion is supported by several other recent reports (64, 138, 175, 313, 403). To address issues of indoor mold-related illness, there is an urgent need for studies using objective markers of illness, relevant animal models, proper epidemiologic techniques, and careful examination of confounding factors including bacteria, endotoxin, man-made chemicals, and nutritional factors.
Acknowledgments
We thank N. Isham for technical advice, M. Petit and N. McLaughlin of the University Hospitals Core Library for literature searches, and K. Smith for secretarial assistance.
Funding was provided by an unrestricted grant from the CNA Insurance Corp.
REFERENCES
- 1.Abdel-Hafez, S. I., I. A. el-Kady, M. B. Mazen, and O. M. el-Maghraby. 1987. Mycoflora and trichothecene toxins of paddy grains from Egypt. Mycopathologia 100:103-112. [DOI] [PubMed] [Google Scholar]
- 2.Acierno, L. J., J. S. Lytle, and M. S. Sweeney. 1985. Acute hypersensitvity pneumonitis related to forced air systems—a review of selected literature and a commentary on recognition and prevention. J. Environ. Health 48:138-141. [Google Scholar]
- 3.Reference deleted
- 4.Adams, R. D., M. Victor, and A. H. Ropper. 1997. Principles of neurology. McGraw-Hill, New York, N.Y.
- 5.Ahearn, D. G., S. A. Crow, R. B. Simmons, D. L. Price, J. A. Noble, S. K. Mishra, and D. L. Pierson. 1996. Fungal colonization of fiberglass insulation in the air distribution system of a multi-story office building: VOC production and possible relationship to a sick building syndrome. J. Ind. Microbiol. 16:280-285. [DOI] [PubMed] [Google Scholar]
- 6.Ahman, M., A. Lundin, V. Musabasic, and E. Soderman. 2000. Improved health after intervention in a school with moisture problems. Indoor Air 10:57-62. [DOI] [PubMed] [Google Scholar]
- 7.Akkmeteli, M. A. 1977. Epidemiological features of the mycotoxicoses. Ann. Nutr. Aliment. 31:957-975. [PubMed] [Google Scholar]
- 7a.American Academy of Pediatrics Committee on Environmental Health. 1998. Toxic effects of indoor molds. Pediatrics 101:712-714 [PubMed] [Google Scholar]
- 8.Reference deleted
- 9.Andersson, M. A., M. Nikulin, U. Koljalg, M. C. Andersson, F. Rainey, K. Reijula, E. L. Hintikka, and M. Salkinoja-Salonen. 1997. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando, M., K. Yoshida, K. Soda, and S. Araki. 1986. Specific bronchoalveolar lavage IgA antibody in patients with summer-type hypersensitivity pneumonitis induced by Trichosporon cutaneum. Am. Rev. Respir. Dis. 134:177-179. [DOI] [PubMed] [Google Scholar]
- 11.Andrassy, K., I. Horvath, T. Lakos, and Z. Toke. 1980. Mass incidence of mycotoxicoses in Hajdu-Bihar County. Mykosen 23:130-133. [PubMed] [Google Scholar]
- 12.Anonymous. 1992. Aflatoxins from foodborne pathogenic microorganisms and natural toxins handbook. [Online.] U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition, Rockville, Md. http://vm.cf-san.fda.gov/≈mow/chap41.html.
- 13.Anonymous. 2001. Colorado couple files suit alleging defects caused mold. Mealey's Litigation Rep. Mold 1:19-20. [Google Scholar]
- 14.Anonymous. 2000. Employees allege mold caused respiratory, kidney disease; seek $3.2M from landlord. Mealey's Litigation Rep. Mold Preview Issue:22-23.
- 15.Anonymous. 2000. Guidelines on assessment and remediation of fungi in indoor environments. [Online.] New York City Department of Health, New York, N.Y. http://www.ci.nyc.ny.us/html/doh/html/epi/moldrpt1.html.
- 16.Anonymous. 1985. Indoor air quality environmental information task force: combustion sources. Muller Associates, Inc., U.S. Department of Energy, Washington, D.C.
- 17.Anonymous. 2001. Maine employees sue building owner over fungus exposure. Mealey's Litigation Rep. Mold 1:19. [Google Scholar]
- 18.Reference deleted.
- 19.Reference deleted.
- 20.Anonymous. 1985. SF6 tracer gas field sampling procedures. Product Safety Branch, Consumer and Corporate Affairs, Ottawa, Canada.
- 21.Reference deleted.
- 22.Reference deleted.
- 23.Apte, M. G., W. J. Fisk, and J. M. Daisey. 2000. Associations between indoor CO2 concentrations and sick building syndrome symptoms in U.S. office buildings: an analysis of the 1994-1996 BASE study data. Indoor Air 10:246-257. [DOI] [PubMed] [Google Scholar]
- 24.Apter, A., A. Bracker, M. Hodgson, J. Sidman, and W. Y. Leung. 1994. Epidemiology of the sick building syndrome. J. Allergy Clin. Immunol. 94:277-288. [PubMed] [Google Scholar]
- 25.Ardies, C. M., and C. Dees. 1998. Xenoestrogens significantly enhance risk for breast cancer during growth and adolescence. Med. Hypotheses 50:457-464. [DOI] [PubMed] [Google Scholar]
- 26.Arunde, A. V., E. M. Sterling, J. H. Biggin, and T. D. Sterling. 1986. Indirect health effects of relative humidity in indoor environments. Environ Health Perspect. 65:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auger, P. L., P. Gourdeau, and J. D. Miller. 1994. Clinical experience with patients suffering from a chronic fatigue-like syndrome and repeated upper respiratory infections in relation to airborne molds. Am. J. Ind. Med. 25:41-42. [DOI] [PubMed] [Google Scholar]
- 28.Bakai, S. M. 1960. In V. I. Bilai (ed.), Mycotoxicosis of man and agricultural animals, p. 163-167. Izd. Akad. Nauk Ukr. S.S.R., Kiev, USSR.
- 29.Bamburg, J. R., F. M. Strong, and E. B. Smalley. 1969. Toxins from moldy cereals. J. Agric. Food Chem. 17:443-450. [Google Scholar]
- 30.Barletta, M., and C. Ellington. 1998. Foreign suppliers to Iraq's biological weapons program obtain microbial seed stock for standard or novel agent. [Online.] Center for Nonproliferation Studies, Monterey Institute of International Studies, Monterey, Calif. http://cns.miis.edu/research/wmdme/flow/iraq/seed.htm.
- 31.Barsky, A. J., and J. F. Borus. 1999. Functional somatic syndromes. Ann. Intern. Med. 130:910-921. [DOI] [PubMed] [Google Scholar]
- 32.Bata, A., B. Harrach, K. Ujszaszi, A. Kis-Tamas, and R. Lasztity. 1985. Macrocyclic trichothecene toxins produced by Stachybotrys atra strains isolated in Middle Europe. Appl. Environ. Microbiol. 49:678-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer, J., and M. Garies. 1987. Ochratoxin A in the food chain. J Vet. Med. 34:613-627. [DOI] [PubMed] [Google Scholar]
- 34.Belkin, L. 2001. Haunted by mold. The New York Times Sunday Magazine. The New York Times, New York, N.Y.
- 35.Bennett, J. W., J. J. Dunn, and C. I. Goldsman. 1981. Influence of whitelight on production of aflatoxins and anthraquinones in Aspergillus parasiticus. Appl. Environ. Microbiol. 41:488-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berek, L., I. B. Petri, A. Mesterhazy, J. Teren, and J. Molnar. 2001. Effects of mycotoxins on human immune functions in vitro. Toxicol. In Vitro 15:25-30. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein, R. S., W. A. Sorenson, D. Garabrant, C. Reaux, and R. D. Treitman. 1983. Exposure to respirable, airborn Penicillium from a contaminated ventilation system: clinical, environmental and epidemiological aspects. Am. Ind. Hyg. Assoc. J. 44:161-164. [DOI] [PubMed] [Google Scholar]
- 38.Betina, V. E. 1989. Mycotoxins: chemical, biological, and environmental aspects. Elsevier, New York, N.Y.
- 39.Bhat, R. V., and R. N. Rao. 1987. Foodborne diseases in India. Indian J. Pediatr. 54:553-562. [DOI] [PubMed] [Google Scholar]
- 40.Bhat, R. V., P. H. Shetty, R. P. Amruth, and R. V. Sudershan. 1997. A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. J. Toxicol. Clin. Toxicol. 35:249-255. [DOI] [PubMed] [Google Scholar]
- 41.Bissett, J. 1987. Fungi associated with urea-formaldehyde foam insulation in Canada. Mycopathologia 99:47-56. [DOI] [PubMed] [Google Scholar]
- 42.Bjornsson, E., C. Janson, D. Norback, and G. Boman. 1998. Symptoms related to the sick building syndrome in a general population sample: associations with atopy, bronchial hyper-responsiveness and anxiety. Int. J. Tuberc. Lung Dis. 2:1023-1028. [PubMed] [Google Scholar]
- 43.Bondy, G. S., and J. J. Pestka. 2000. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Ser. B 3:109-143. [DOI] [PubMed] [Google Scholar]
- 44.Boonchuvit, B., and P. B. Hamilton. 1975. Interaction of aflatoxin and paratyphoid infections in broiler chickens. Poult Sci. 54:1567-1573. [DOI] [PubMed] [Google Scholar]
- 45.Breton, P., J. C. Bizot, J. Buee, and I. De La Manche. 1998. Brain neurotoxicity of Penitrem A: electrophysiological, behavioral, and histopathological study. Toxicon 36:645-655. [DOI] [PubMed] [Google Scholar]
- 46.Brown, M. P., C. S. Brown-Jenco, and G. A. Payne. 1999. Genetic and molecular analysis of aflatoxin biosynthesis. Fungal Genet. Biol. 26:81-98. [DOI] [PubMed] [Google Scholar]
- 47.Brunekreef, B., D. W. Dockery, F. E. Speizer, J. H. Ware, J. D. Spengler, and B. G. Ferris. 1989. Home dampness and respiratory morbidity in children. Am. Rev. Respir. Dis. 140:1363-1367. [DOI] [PubMed] [Google Scholar]
- 48.Bu'Lock, J. D. 1980. Mycotoxins as secondary metabolites, p. 1-16. In P. S. Steyn (ed.), The biosythesis of mycotoxins. Academic Press, Inc., New York, N.Y.
- 49.Burg, W. R., O. L. Shotwell, and B. E. Saltzman. 1982. Measurement of airborne aflatoxins during the handling of contaminated corn. Am. Ind. Hyg. Assoc. J. 42:580-586. [DOI] [PubMed] [Google Scholar]
- 50.Burge, H., H. J. Su, and J. Spengle. 1984. Moisture, organisms, and health effects, p. 84-90. In H. Trechsel (ed.), Moisture control in buildings. ASTM MNL18. American Society for Testing and Materials, Philadelphia, Pa.
- 51.Burnside, J. E., W. L. Sipple, J. Forgacs, et al. 1957. A disease of swine and cattle caused by eating moldy corn. II. Experimental production with true cultures of molds. Am. J. Vet. Res. 69:817-824. [PubMed] [Google Scholar]
- 52.Busby, W. F., Jr, and G. N. Wogan. 1985. Aflatoxins, p. 487-539. In S. E. Searle (ed.), Chemical carcinogens. American Chemical Society, Washington, D.C.
- 53.Butler, W. H. 1975. Mycotoxins, p. 320-329. In J. E. Smith and D. R. Berry (ed.), The filamentous fungi, vol. 1. Edward Arnold, London, United Kingdom. [Google Scholar]
- 54.Butler, W. H., and J. S. Wigglesworth. 1966. The effects of aflatoxin B1 on the pregnant rat. Br. J. Exp. Pathol. 47:242-247. [Google Scholar]
- 55.Carleton, W. W., and P. Krogh. 1979. Ochratoxins. In W. Shimoda (ed.), Conference on mycotoxins in animal feed and grains related to animal health. FDA/BVM-79/139. U.S. Food and Drug Administration, Washington, D.C.
- 56.Cassimos, C. D., C. Chryssanthoploulos, and C. Panagiotidou. 1983. Epidemiologic observations in idiopathic pulmonary hemosiderosis. J. Pediatr. 102:698-702. [DOI] [PubMed] [Google Scholar]
- 57.CAST. 1989. Mycotoxins: economic and health risks. Task Force report 116. Council for Agricultural Science and Technology, Ames, Iowa.
- 58.Castellan, R. M., S. A. Olenchock, K. B. Kinsley, and J. L. Hankinson. 1987. Inhaled endotoxin and decreased spirometric values. An exposure-response relation for cotton dust. N. Engl. J. Med. 317:605-610. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. 1995. Acute pulmonary hemorrhage among infants—Chicago, April 1992-November 1994. Morb. Mortal. Wkly. Rep. 44:67, 73-74. [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. 1994. Acute pulmonary hemorrhage/hemosiderosis among infants—Cleveland, January 1993-November 1994. Morb. Mortal. Wkly. Rep. 43:881-883. [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. 2000. From the Centers for Disease Control and Prevention. Update: pulmonary hemorrhage/hemosiderosis among infants—Cleveland, Ohio, 1993-1996. JAMA 283:1951-1953. [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention. 1999. Report of the CDC working group on pulmonary hemorrhage/hemosiderosis. [Online.] Centers for Disease Control and Prevention, Atlanta, Ga. http://www.cdc.gov/od/ads.
- 63.Centers for Disease Control and Prevention. 1999. Reports of members of the CDC External Expert Panel on Acute Idiopathic Pulmonary Hemorrhage in Infants: a synthesis. [Online.] Centers for Disease Control and Prevention Atlanta, Ga. http://www.cdc.giv/od/ads.
- 64.Centers for Disease Control and Prevention. 2000. Update: pulmonary hemorrhage/hemosiderosis among infants—Cleveland, Ohio, 1993-1996. Morb. Mortal. Wkly. Rep. 49:180-184. [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. 1997. Update: pulmonary hemorrhage/hemosiderosis among infants—Cleveland, Ohio, 1993-1996. Morb. Mortal. Wkly. Rep. 46:33-35. [PubMed] [Google Scholar]
- 66.Chang, C. F., and P. B. Hamilton. 1979. Impaired phagocytosis by heterophils from chickens during aflatoxicosis. Toxicol. Appl. Pharmacol. 48:459-466. [DOI] [PubMed] [Google Scholar]
- 67.Chu, F. S., and G. Y. Li. 1994. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People's Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 60:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciegler, A., and J. W. Bennett. 1980. Mycotoxins and mycotoxicosis. Bioscience 30:512-515. [Google Scholar]
- 69.Ciegler, A., H. R. Burmeister, R. F. Vesonder, and C. W. Hesseltine. 1981. Mycotoxins: occurrence in the environment, p. 1-50. In R. C. Shank (ed.), Mycotoxins and N-nitroso compounds: environmental risks, vol. 1. CRC Press, Inc., Boca Raton, Fla.
- 70.Ciegler, A., and R. F. Vesonder. 1983. Microbial food and feed toxicants: fungal toxins, p. 57-192. In M. J. Rechcigl (ed.), Handbook of foodborne diseases of biological origin. CRC Press, Inc., Boca Raton, Fla.
- 71.Cole, R. J., and R. H. Cox. 1981. Handbook of toxic fungal metabolites. Academic Press, Inc., New York, N.Y.
- 72.Cooley, J. D., W. C. Wong, C. A. Jumper, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper, J. A. J. 1998. Occupational asthma, byssinosis, and industrial bronchitis, p. 915-924. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw-Hill, New York, N.Y.
- 74.Cooray, R., and P. Jonsson. 1990. Modulation of resistance to mastitis pathogens by pretreatment of mice with T-2 toxin. Food Chem. Toxicol. 28:687-692. [DOI] [PubMed] [Google Scholar]
- 75.Corda, A. C. 1837. Icones fungorum hucusque cognitorum I. Prague, Czechoslovakia.
- 76.Corrier, D. E. 1991. Mycotoxicosis: mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 30:73-87. [DOI] [PubMed] [Google Scholar]
- 77.Costa, M. F., and L. S. Brickus. 2000. Effect of ventilation systems on prevalence of symptoms associated with “sick buildings” in Brazilian commercial establishments. Arch. Environ. Health 55:279-83. [DOI] [PubMed] [Google Scholar]
- 78.Creasia, D. A., and R. J. Lambert. 1989. Acute respiratory toxicity of the trichothecene mycotoxin, T-2 toxin, p. 162-170. In V. R. Beasley (ed.), Trichothecene mycotoxicosis: pathophysiologic effects, vol. 1. CRC Press, Inc., Boca Raton, Fla.
- 79.Croft, W. A., B. B. Jarvis, and C. S. Yatawara. 1986. Airborne outbreak of trichothecane toxicosis. Atmos. Environ. 20:549-552. [Google Scholar]
- 80.Cruz-Perez, P., M. P. Buttner, and L. D. Stetzenbach. 2001. Specific detection of Stachybotrys chartarum in pure culture using quantitative polymerase chain reaction. Mol. Cell. Probes 15:129-138. [DOI] [PubMed] [Google Scholar]
- 81.Cuijpers, C. E., G. M. Swaen, G. Wesseling, F. Sturmans, and E. F. Wouters. 1995. Adverse effects of the indoor environment on respiratory health in primary school children. Environ. Res. 68:11-23. [DOI] [PubMed] [Google Scholar]
- 82.Dales, R., D. Miller, J. White, C. Dulberg, and A. I. Lazarovitz. 1998. Influence of residential fungal contamination on peripheral blood lymphocyte population in children. Arch. Environ. Health 53:190-195. [DOI] [PubMed] [Google Scholar]
- 83.Dales, R., S. Schweitzer, S. Bartlett, M. Raizienne, and R. Burnett. 1994. Indoor air and health: reproducibility of symptoms and reported home dampness and molds using a self-administered questionaire. Indoor Air 4:2-7. [Google Scholar]
- 84.Dales, R. E., R. Burnett, and H. Zwanenburg. 1991. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 143:505-509. [DOI] [PubMed] [Google Scholar]
- 85.Dales, R. E., and D. Miller. 1999. Residential fungal contamination and health: microbial cohabitants as covariants. Environ. Health Perspect. 107:A63-A64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dales, R. E., D. Miller, and E. McMullen. 1997. Indoor air quality and health: validity and determinants of reported home dampness and moulds. Int. J. Epidemiol. 26:120-125. [DOI] [PubMed] [Google Scholar]
- 87.Dales, R. E., D. Miller, and J. White. 1999. Testing the association between residential fungus and health using ergosterol measures and cough recordings. Mycopathologia 147:21-27. [DOI] [PubMed] [Google Scholar]
- 88.Dales, R. E., J. White, C. Bhumgara, and E. McMullen. 1997. Parental reporting of children's coughing is biased. Eur. J. Epidemiol. 13:541-545. [DOI] [PubMed] [Google Scholar]
- 89.Dalton, P. 1999. Cognitive influences on health symptoms from acute chemical exposure. Health Psychol. 18:579-590. [DOI] [PubMed] [Google Scholar]
- 90.Day, J. H. 1986. UFFI-fungal interaction. Working paper provided to the Health and Welfare Canada Working Group on Fungi and Indoor Air. Environmental Health Directorate, Health and Welfare Canada, Ottawa.
- 91.Dearborn, D. G. 1997. Pulmonary hemorrhage in infants and children. Curr. Opin. Pediatr. 9:219-224. [DOI] [PubMed] [Google Scholar]
- 92.Dearborn, D. G., I. Yike, W. G. Sorenson, M. J. Miller, and R. A. Etzel. 1999. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. 107(Suppl. 3):495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dees, C., J. S. Foster, S. Ahamed, and J. Wimalasena. 1997. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environ Health Perspect 105(Suppl. 3):633-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reference deleted.
- 95.DeLoach, J. R., M. I. C. Gyongyossy-Issa, and G. G. Khachatourians. 1989. Species-specific hemolysis of erythrocytes by T-2 toxin. Toxicol. Appl. Pharmacol. 97:107-112. [DOI] [PubMed] [Google Scholar]
- 96.Demeke, T., Y. Kidane, and E. Wuhib. 1979. Ergotism—a report on an epidemic, 1977-78. Ethiop. Med. J. 17:107-113. [PubMed] [Google Scholar]
- 97.Denning, D. W. 1987. Aflatoxin and human diseases, p. 175-209. In D. M. Davies and H. deGlanville (ed.), Adverse drug reactions and acute poisoning reviews, vol. 4. Oxford University Press, Oxford, United Kingdom. [PubMed]
- 98.Dethloff, L. A., L. B. Gilmore, and G. E. R. Hook. 1986. The relationship between intra- and inter-cellular surfactant phospholoids in the lung of rabbits and the effects of silica induced lung injury. Biochem. J. 239:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DiPaolo, J. A., J. Elis, and H. Erwin. 1967. Teratogenic response by hamsters, rats, and mice to aflatoxin B1. Nature 215:638-639. [DOI] [PubMed] [Google Scholar]
- 100.DiPaolo, N., A. Guarnieri, F. Loi, G. Sacchi, A. M. Mangiarotti, and M. Dipaulo. 1993. Acute renal failure from inhalation of mycotoxins. Nephron 4:621-625. [DOI] [PubMed] [Google Scholar]
- 101.Dorner, J. W., R. J. Cole, and L. G. Lomax. 1983. Cyclopiazonic acid production by Aspergillus flavus and its effects on broiler chickens. Appl. Environ. Microbiol. 46:698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dragan, Y. P., and H. C. Pitot. 1994. Aflatoxin carcinogenesis in the context of the multistage nature of cancer, p. 179-206. In D. L. Eaton and J. D. Groopman (ed.), The toxicology of aflatoxins. Academic Press, Ltd., London, United Kingdom.
- 103.Drobotko, V. G. 1945. Stachybotryotoxicosis, a new disease of horses and humans. Am. Rev. Soviet Med. 2:238-242. [Google Scholar]
- 104.Drobotko, V. G., P. E. Marushenko, B. E. Aizeman, N. G. Kolesnik, P. D. Iatel, and V. D. Meknichenko. 1946. Stachybotryotoxicosis, a new disease of horses and humans (relation to agranulocytic angina). Vrachebnoe Delo 26:125-128. [Google Scholar]
- 105.Dungy, C. I., P. P. Kozak, J. Gallup, and S. P. Galant. 1986. Aeroallergen exposure in the elementary school setting. Ann. Allergy 56:218-221. [PubMed] [Google Scholar]
- 106.Dvorackova, I. 1976. Aflatoxin inhalation and alveolar cell carcinoma. Br. Med. J. 1:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dvorackova, I. 1990. Potential professional risk due to aflatoxin exposure via the respiratory route, p. 135-150. In A. J. Gilman et al. (ed.), Aflatoxins and human health. CRC Press, Inc., Boca Raton, Fla.
- 108.Eaton, D. L., and J. D. Groopman (ed.). 1994. The toxicology of aflatoxins: human health, veterinary, and agricultural significance. Academic Press, Inc., San Diego, Calif.
- 109.Edds, G. T. 1979. Aflatoxins. In W. Shimoda (ed.), Conference on mycotoxins in animal feeds and grains related to animal health. FDA/BVM-79/139. U.S. Food and Drug Administration, Washington, D.C.
- 110.Reference deleted.
- 111.Ehrlich, K. C., and L. S. Lee. 1984. Mycotoxins in grain dust: method for analysis of mycotoxins, ochratoxin A, zearalenone, vomitoxin, and secalonic acid D. J. Assoc. Off. Anal. Chem. 67:963-967. [PubMed] [Google Scholar]
- 112.Ehrlich, K. C., L. S. Lee, A. Ciegler, and M. S. Palmgren. 1982. Secalonic acid D: natural contaminant of corn dust. Appl. Environ. Microbiol. 44:1007-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eichner, R., and A. Mullbacher. 1984. Hypothesis: fungal toxins are involved in aspergillis and AIDS. Aust. J. Exp. Med. Sci. 62:479-484. [DOI] [PubMed] [Google Scholar]
- 114.Elidemir, O., G. N. Colasurdo, S. N. Rossmann, and L. L. Fan. 1999. Isolation of Stachybotrys from the lung of a child with pulmonary hemosiderosis. Pediatrics 104:964-966. [DOI] [PubMed] [Google Scholar]
- 115.El-Maghraby, O. M., and M. A. Abdel-Sater. 1993. Mycoflora and natural occurrence of mycotoxins in tobacco from cigarettes in Egypt. Zentbl. Mikrobiol. 148:253-264. [PubMed] [Google Scholar]
- 116.Emanuel, D. A., F. J. Wenzel, and B. R. Lawton. 1975. Pulmonary mycotoxicosis. Chest 67:293-297. [DOI] [PubMed] [Google Scholar]
- 117.Engvall, K., C. Norrby, and D. Norback. 2001. Asthma symptoms in relation to building dampness and odour in older multifamily houses in Stockholm. Int. J. Tuberc. Lung Dis. 5:468-477. [PubMed] [Google Scholar]
- 118.Engvall, K., C. Norrby, and D. Norback. 2001. Sick building syndrome in relation to building dampness in multi-family residential buildings in Stockholm. Int. Arch. Occup. Environ. Health 74:270-278. [DOI] [PubMed] [Google Scholar]
- 119.Escoula, L., D. Bourdiol, M. D. Limas, P. Recco, and J. P. Sequela. 1988. Enhancing resistance and modulation of humoral immune response to experimental Candida albicans infection by patulin. Mycopathologia 103:153-156. [DOI] [PubMed] [Google Scholar]
- 120.Eskelson, C. D., M. Chvapil, K. A. Strom, and J. J. Vostal. 1987. Pulmonary phospholipases in rats respiring air containing deisel particulates. Environ. Res. 44:260-271. [DOI] [PubMed] [Google Scholar]
- 121.Etzel, R., and R. Rylander. 1999. Indoor mold and children's health. Environ. Health Perspect. 107(Suppl. 3):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Etzel, R. A., and D. G. Dearborn. 1999. Pulmonary hemorrhage among infants with exposure to toxigenic molds: an update, p. 79-83. In E. Johanning (ed.), Bioaerosols, fungi, and mycotoxins: health effects, assessment, prevention, and control. Eastern New York Occupational and Environmental Health Center, Albany, N.Y.
- 123.Etzel, R. A., E. Montana, W. G. Sorenson, G. J. Kullman, T. M. Allan, D. G. Dearborn, D. R. Olson, B. B. Jarvis, and J. D. Miller. 1998. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch. Pediatr. Adolesc. Med. 152:757-762. [DOI] [PubMed] [Google Scholar]
- 124.Fazekas, B., E. Bajmocy, R. Glavits, A. Fenyvesi, and J. Tanyi. 1998. Fumonisin B1 contamination of maize and experimental acute fumonisin toxicosis in pigs. Zentralbl. Veterinaermed. Ser. B 45:171-181. [DOI] [PubMed] [Google Scholar]
- 125.Ferenczy, A., and E. Franco. 2002. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 3:11-16. [DOI] [PubMed] [Google Scholar]
- 126.Fergusson, R. J., L. J. R. Milne, and B. K. Crompton. 1984. Penicillium allergic alveolitis: faulty installation of central heating. Thorax 39:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fink-Gremmels, J. 1999. Mycotoxins: their implications for human and animal health. Vet. Q. 21:115-120. [DOI] [PubMed] [Google Scholar]
- 128.Fish, J. E., and S. P. Peters. 1998. Asthma: clinical presentation and management, p. 757-775. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw Hill, New York, N.Y.
- 129.Fishman, A. P. 1998. Approach to the patient with respiratory symptoms, p. 361-393. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw Hill, New York, N.Y.
- 130.Flannigan, B., E. M. McCabe, and F. McGarry. 1991. Allergenic and toxigenic micro-organisms in houses. J. Appl. Bacteriol. Symp. Suppl. 70:61S-73S. [PubMed] [Google Scholar]
- 131.Flappan, S. M., J. Portnoy, P. Jones, and C. Barnes. 1999. Infant pulmonary hemorrhage in a suburban home with water damage and mold (Stachybotrys atra). Environ. Health Perspect. 107:927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fogelmark, B., H. Goto, K. Yuasa, B. Marchai, and R. Rylander. 1992. Acute pulmonary toxicity of inhaled beta-1,3-glucan and endotoxin. Agents Actions 35:50-56. [DOI] [PubMed] [Google Scholar]
- 133.Forgacs, J. 1972. Stachybotrytoxicosis, p. 95-128. In S. Kadis, A. Ciegler, and S. J. Ajl (ed.), Microbial toxins, vol. 8. Academic Press, Inc., New York, N.Y.
- 134.Forgacs, J., W. T. Carll, A. S. Herring, and W. R. Hinshaw. 1958. Toxicity of Stachybotrys atra for animals. Trans. N. Y. Acad. Sci. 20:787. [DOI] [PubMed] [Google Scholar]
- 135.Fradkin, A. 1987. Sampling of micorbiological contaminants in indoor air. ASTM Spec. Tech. Publ. 957:66-77. [Google Scholar]
- 136.Fujimoto, Y., L. L. Hampton, L. Luo, P. J. Wirth, and S. S. Thorgeirsson. 1992. Low frequency of p53 gene mutations in tumors induced by aflatoxin B1 in nonhuman primates. Cancer Res. 52:1044-1046. [PubMed] [Google Scholar]
- 137.Fung, F., R. Clark, and S. Williams. 1998. Stachybotrys, a mycotoxin-producing fungus of increasing toxicologic importance. J. Toxicol. Clin. Toxicol. 36:79-86. [DOI] [PubMed] [Google Scholar]
- 138.Fu-Yi, Y. 1977. Mechanism of aflatoxin B1 inhibition of rat hepatic nuclear RNA synthesis. J. Biol. Chem. 252:3245-3251. [PubMed] [Google Scholar]
- 139.Gao, P., and J. R. Martin. 2002. Volatile metabolites produced by three strains of Stachybotrys chartarum cultivated on rice and gypsum board. Appl. Environ. Microbiol. 17:430-436. [DOI] [PubMed] [Google Scholar]
- 140.Giambrone, J. J., D. L. Ewert, R. D. Wyatt, and C. S. Eidson. 1978. Effect of aflatoxin on the humoral and cell-mediated immune systems of the chicken. Am. J. Vet. Res. 39:305-308. [PubMed] [Google Scholar]
- 141.Giddings, M. J. 1986. Occupational health problems due to air exposue to fungal contamination. In Health and Welfare Canada Working Group on Fungi and Indoor Air (ed.), Significance of fungi in indoor air: report of a working group. Health and Welfare Canada, Ottawa.
- 142.Grant, C., C. A. Hunter, B. Flannigan, and A. F. Bravery. 1989. The moisture requirements of moulds isolated from domestic buildings. Int. Biodeterior. 25:259-284. [Google Scholar]
- 143.Gravesen, S., L. Larsen, F. Gyntelberg, and P. Skov. 1986. Demonstration of microorganisms and dust in schools and offices. An observational study of non- industrial buildings. Allergy 41:520-525. [DOI] [PubMed] [Google Scholar]
- 144.Gravesen, S., P. A. Nielsen, R. Iversen, and K. F. Nielsen. 1999. Microfungal contamination of damp buildings—examples of risk constructions and risk materials. Environ. Health Perspect. 107(Suppl. 3):505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greene, D. M., J. I. Azcona-Olivera, and J. J. Pestka. 1994. Vomitoxin (deoxynivalenol)-induced IgA nephropathy in the B6C3F1 mouse: dose response and male predilection. Toxicology 92:245-260. [DOI] [PubMed] [Google Scholar]
- 146.Groopman, J. D., J. Q. Zhu, P. R. Donahue, A. Pikul, L. S. Zhang, J. S. Chen, and G. N. Wogan. 1992. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Res. 52:45-52. [PubMed] [Google Scholar]
- 147.Grove, J. F. 1988. Non-macrocylic trichithecenes. Nat. Prod. Rep. 5:187-209. [DOI] [PubMed] [Google Scholar]
- 148.Groves, F. D., L. Zhang, Y. S. Chang, P. F. Ross, H. Casper, W. P. Norred, W. C. You, and J. F. Fraumeni, Jr. 1999. Fusarium mycotoxins in corn and corn products in a high-risk area for gastric cancer in Shandong Province, China. J. AOAC Int. 82:657-662. [PubMed] [Google Scholar]
- 149.Gurtoo, H. L., and V. D. Chondrakant. 1975. In vitro metabolic conversion of aflatoxin and benzopyrene to nucleic acid-binding metabolites. Cancer Res. 35:382-389. [PubMed] [Google Scholar]
- 150.Harrach, B., A. Bata, E. Bajmocy, and M. Benko. 1983. Isolation of satratoxins from the bedding straw of a sheep flock with fatal stachybotryotoxicosis. Appl. Environ. Microbiol. 45:1419-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harrach, B., A. Bata, G. Sandor, and A. Vanyi. 1987. Isolation of macrocyclic and non-macrocyclic trichothecenes (Stachybotrys and Fusarium toxins) from the environment of 200 ill sport horses). Mycot. Res. 3:65-68. [DOI] [PubMed] [Google Scholar]
- 152.Harrach, B., C. J. Mirocha, S. V. Pathre, and M. Palyusik. 1981. Macrocyclic trichothecene toxins produced by a strain of Stachybotrys atra from Hungary. Appl. Environ. Microbiol. 41:1428-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harrach, B., M. Nummi, M. L. Niku-Paavola, C. J. Mirocha, and M. Palyusik. 1982. Identification of “water-soluble” toxins produced by a Stachybotrys atra strain from Finland. Appl. Environ. Microbiol. 44:494-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayes, R. B., J. P. van Nieuwenhuize, J. W. Raatgever, and F. J. W. Ten Kate. 1984. Aflatoxin exposure in the industrial setting: an epidemiologic study of mortality. Food Chem. Toxicol. 22:39-43. [DOI] [PubMed] [Google Scholar]
- 155.He, Z. Y. 1989. Investigation on an outbreak of allergic pulmonary alveolitis. Zhonhua Liu Xing Bing Xue Za Zhi 10:129-132. (In Chinese.) [PubMed] [Google Scholar]
- 156.Held, H. D., and S. Uhlig. 2000. Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice. Am. J. Respir. Crit. Care Med. 162:1547-1552. [DOI] [PubMed] [Google Scholar]
- 157.Hendry, K. M., and E. C. Cole. 1993. A review of mycotoxins in indoor air. J. Toxicol. Environ. Health 38:183-198. [DOI] [PubMed] [Google Scholar]
- 158.Hengstler, J. G., B. Van der Burg, P. Steinberg, and F. Oesch. 1999. Interspecies differences in cancer susceptibility and toxicity. Drug Metab. Rev. 31:917-970. [DOI] [PubMed] [Google Scholar]
- 159.Hilakivi-Clarke, L., I. Onojafe, M. Raygada, E. Cho, T. Skaar, I. Russo, and R. Clarke. 1999. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br. J. Cancer 80:1682-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hintikka, E.-L. 1977. The genus Stachybotrys, p. 87-89. In T. D. Wyllie and L. G. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicosis, vol. 3. Marcel Dekker, Inc., New York, N.Y.
- 161.Hintikka, E.-L., and M. Nikulin. 1998. Airborne mycotoxins in agricultural and indoor environments. Indoor Air Suppl. 4:66-70. [Google Scholar]
- 162.Hirsch, S. R., and J. A. Sosman. 1976. A one-year survey of mould growth inside twelve homes. Ann. Allergy 36:30-38. [PubMed] [Google Scholar]
- 163.Hirvonen, M. R., M. Ruotsalainen, M. Roponen, A. Hyvarinen, T. Husman, V. M. Kosma, H. Komulainen, K. Savolainen, and A. Nevalainen. 1999. Nitric oxide and proinflammatory cytokines in nasal lavage fluid associated with symptoms and exposure to moldy building microbes. Am. J. Respir. Crit. Care Med. 160:1943-1946. [DOI] [PubMed] [Google Scholar]
- 164.Hodgson, M. J., P. Morey, W. Y. Leung, L. Morrow, D. Miller, B. B. Jarvis, H. Robbins, J. F. Halsey, and E. Storey. 1998. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Occup. Environ. Med. 40:241-249. [DOI] [PubMed] [Google Scholar]
- 165.Hollstein, M. C., C. P. Wild, F. Bleicher, S. Chutimataewin, C. C. Harris, P. Srivatanakul, and R. Montesano. 1993. p53 mutations and aflatoxin B1 exposure in hepatocellular carcinoma patients from Thailand. Int. J. Cancer 53:51-55. [DOI] [PubMed] [Google Scholar]
- 166.Honda, Y., M. Ueki, G. Okada, R. Onose, R. Usami, K. Horikoshi, and H. Osada. 2001. Isolation, and biological properties of a new cell cycle inhibitor, curvularol, isolated from Curvularia sp. RK97-F166. J. Antibiot. (Tokyo) 54:10-16. [DOI] [PubMed] [Google Scholar]
- 167.Hsia, C., J. Wu, X. Qui Lu, and Y. Li. 1988. Natural occurrence and clastogenic effects of nivalenol, deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol and zearalenone in corn from a high risk area of esophageal cancer. Cancer Detect. Prevent. 13:79-86. [PubMed] [Google Scholar]
- 168.Hunter, C. A., C. Grant, B. Flannigan, and A. F. Bravery. 1988. Mould in buildings: the air spora of domestic dwellings. Int. Biodeterior. 24:81-101. [Google Scholar]
- 169.Hussain, S. P., and C. C. Harris. 2000. Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens. Mutat. Res. 462:311-322. [DOI] [PubMed] [Google Scholar]
- 170.Hussain, S. P., and C. C. Harris. 1998. Molecular epidemiology of human cancer. Recent Results Cancer Res. 154:22-36. [DOI] [PubMed] [Google Scholar]
- 171.Hyvarinen, A., T. Reponen, T. Husman, and A. Nevalainen. 2001. Comparison of the indoor air quality in mould damaged and reference buildings in a subarctic climate. Cent. Eur. J. Public Health 9:133-139. [PubMed] [Google Scholar]
- 172.IARC. 1987. Aflatoxins, p. 83-87. IARC monograph on the evaluation of carcinogenic risk of chemicals to humans, suppl 7. International Agency for Research on Cancer, Lyon, France.
- 173.IARC. 1993. Toxins derived from Fusarium graminearum, F. culmorum and F. crookwellense: zearalenone, deoxynivalenol, nivalenol and fusarenone X. IARC Monogr. Eval. Carcinog. Risks Hum. 56:397-444. [PMC free article] [PubMed] [Google Scholar]
- 174.Ikegwuonu, F. I. 1983. The neurotoxicity of aflatoxin B1 in the rat. Toxicology 28:247-259. [DOI] [PubMed] [Google Scholar]
- 175.Institute of Medicine (ed.). 2000. Clearing the air: asthma and indoor air exposures. Committee on Health Effects and Indoor Allergens, Division of Health Promotion and Disease Prevention, Institute of Medicine. National Academy Press, Washington, D.C.
- 176.Institute of Medicine (ed.). 1993. Indoor allergens. Assessing and controlling adverse health effects. Committee on Health Effects and Indoor Allergens, Division of Health Promotion and Disease Prevention, Institute of Medicine. National Academy Press, Washington, D.C. [PubMed]
- 177.Jaakkola, J. J., N. Jaakkola, and R. Ruotsalain. 1998. Home dampness and molds as determinants of respiratory symptoms and asthma in pre-school children. J. Exp. Anal. Environ. Epidemiol. 3(Suppl. 1):129-142. [PubMed] [Google Scholar]
- 178.Jaakkola, M. S., and J. J. Jaakkola. 1999. Office equipment and supplies: a modern occupational health concern? Am. J. Epidemiol. 150:1223-1228. [DOI] [PubMed] [Google Scholar]
- 179.Jackson, P. E., and J. D. Groopman. 1999. Aflatoxin and liver cancer. Baillieres Best Pract. Res. Clin. Gastroenterol. 13:545-555. [DOI] [PubMed] [Google Scholar]
- 180.Jacobsen, B. J., K. L. Bowen, R. A. Shelby, U. L. Diener, B. W. Kemppainen, and J. Floyd. 1993. Circular ANR 767: mycotoxins and mycotoxicosis. [Online.] Alabama Cooperative Extension System. Alabama A&M and Auburn Universities. http://www.aces.edu/department/grain/ANR767.htm.
- 181.Jakab, G. J., R. R. Hmieleki, A. Zarba, D. R. Hemenway, and J. D. Groopman. 1994. Respiratory aflatoxicosis: suppression of pulmonary and systemic host defenses in rats and mice. Toxicol. Appl. Pharmacol. 125:198-205. [DOI] [PubMed] [Google Scholar]
- 182.Jarmai, K. 1929. Viskosusseptikamien bei alteren Fohlen und erwachsenen Pferden. Dtsch. Tierarztl. Wochenschr. 33:517-519. [Google Scholar]
- 183.Jarvis, B. B. 1991. Macrocyclic trichothecenes, p. 361-421. In R. P. Sharma and D. K. Salunkhe (ed.), Mycotoxins and phytoalexins. CRC Press, Inc., Boca Raton, Fla.
- 184.Jarvis, B. B. 1990. Mycotoxins and indoor air quality. Biological contaminants in indoor environments, vol. ASTM STP 107. American Society for Testing and Materials, Philadelphia, Pa.
- 185.Jarvis, B. B. 1986. Potential indoor air pollution problems associated with macrocyclic trichothecene producing fungi. In Health and Welfare Canada Working Group on Fungi and Indoor Air (ed.), Significance of fungi in indoor air: report of a Working Group. Health and Welfare Canada, Ottawa.
- 186.Jarvis, B. B., R. M. Eppley, and E. P. Mazzolla. 1983. Chemistry and bioproduction of macrocyclic trichothecenes, p. 20-38. In Y. Ueno (ed.), Trichothecenes—chemical, biological, and toxicological aspects, vol. 4. Elsevier, New York, N.Y.
- 187.Jarvis, B. B., and E. P. Mazzola. 1982. Macrocyclic and other novel trichothecenes: their structure, synthesis, and biological significance. Acc. Chem. Res. 15:388-395. [Google Scholar]
- 188.Jarvis, B. B., J. Salemme, and A. Morais. 1995. Stachybotrys toxins. 1. Nat. Toxins 3:10-16. [DOI] [PubMed] [Google Scholar]
- 189.Jarvis, B. B., W. G. Sorenson, E. L. Hintikka, M. Nikulin, Y. Zhou, J. Jiang, S. Wang, S. Hinkley, R. A. Etzel, and D. Dearborn. 1998. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 64:3620-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jarvis, B. B., Y. Zhou, J. Jiang, S. Wang, W. G. Sorenson, E. L. Hintikka, M. Nikulin, P. Parikka, R. A. Etzel, and D. G. Dearborn. 1996. Toxigenic molds in water-damaged buildings: dechlorogriseofulvins from Memnoniella echinata. J. Nat. Prod. 59:553-554. [DOI] [PubMed] [Google Scholar]
- 191.Jarvis, J. Q., and P. R. Morey. 2001. Allergic respiratory disease and fungal remediation in a building in a subtropical climate. Appl. Occup. Environ. Hyg. 16:380-388. [DOI] [PubMed] [Google Scholar]
- 192.Jelinek, C., A. Pohland, and G. Wood. 1989. Worldwide occurrence of mycotoxins in foods and feeds. J. Assoc. Offic. Anal. Chem. 72:223-230. [PubMed] [Google Scholar]
- 193.Joffe, A. Z. 1970. Alimentary toxic aleukia, p. 139-189. In S. Kadis, A. Ciegler, and S. J. Ajl (ed.), Microbial toxins. Academic Press, Inc., New York, N.Y.
- 194.Joffe, A. Z. 1978. Fusarium poae and F. sporotrichoides as principle causal agents of alimentary toxic aleukia, p. 27-41. In T. Willie and L. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicosis. Marcel Dekker, Inc., New York, N.Y.
- 195.Joffe, A. Z. 1986. Fusarium species: their biology and toxicology, p. 225-292. John Wiley & Sons, Inc., New York, N.Y.
- 196.Johanning, E. 1995. Health problems related to fungal exposure: the example of toxigenic Stachybotrys chartarum (atra), p. 201-208. In E. Johanning and C. S. Yang (ed.), Fungi and bacteria in indoor air environments. Eastern New York Occupational Health Program, Latham, N.Y.
- 197.Johanning, E., R. Biagini, D. Hull, P. Morey, B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 68:207-218. [DOI] [PubMed] [Google Scholar]
- 198.Johanning, E., P. Landsbergis, M. Gareis, C. S. Yang, and E. Olmsted. 1999. Clinical experience and results of a Sentinel Health Investigation related to indoor fungal exposure. Environ. Health Perspect. 107(Suppl. 3):489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reference deleted.
- 200.Jong, S. C., and E. E. Davis. 1976. Contribution to the knowlege of Stachybotrys and Memnoniella in culture. Mycotaxon 3:409-485. [Google Scholar]
- 201.Juranic, Z., M. P. Stojiljkovic, A. Bocarov-Stancic, V. Kilibarda, S. R. Milovanovic, I. Juranic, S. Bijelogrlic, N. Vuletic, and S. Radulovic. 1998. T-2 toxin affects proliferation of three different neoplastic cell lines. J. Exp. Clin. Cancer Res. 17:33-40. [PubMed] [Google Scholar]
- 202.Karunasena, E., N. Markham, T. Brasel, J. D. Cooley, and D. C. Straus. 2001. Evaluation of fungal growth on cellulose-containing and inorganic ceiling tile. Mycopathologia 150:91-95. [DOI] [PubMed] [Google Scholar]
- 203.Kendrick, B. 1985. The fifth kingdom. Mycologue, Waterloo, Ontario, Canada.
- 204.Kensler, T. W., P. A. Egner, N. E. Davidson, B. D. Roebuck, A. Pikul, and J. D. Groopman. 1986. Modulation of aflatoxin metabolism, aflatoxin-N7-guanine formation, and hepatic tumorigenesis in rats fed ethoxyquin: role of induction of glutathione S-transferases. Cancer Res. 46:3924-3931. [PubMed] [Google Scholar]
- 205.Kilburn, K. H. 2000. Indoor air effects after building renovation and in manufactured homes. Am. J. Med. Sci. 320:249-254. [DOI] [PubMed] [Google Scholar]
- 206.King, B. 1979. Outbreak of ergotism in Wollo, Ethiopia. Lancet i:1411. [DOI] [PubMed] [Google Scholar]
- 207.Reference deleted.
- 208.Kjaergaard, S., L. Molhave, and O. F. Pedersen. 1990. Human reactions to a mixture of indoor air volatile organic compounds. Atmos. Environ. 25A:1417-1426. [Google Scholar]
- 209.Knapp, J. F., J. G. Michael, M. A. Hegenbarth, P. E. Jones, and P. G. Black. 1999. Case records of the Children's Mercy Hospital, case 02-1999: a 1-month- old infant with respiratory distress and shock. Pediatr. Emerg. Care 15:288-293. [PubMed] [Google Scholar]
- 210.Korneev, N. E. 1948. Veterinarya 25:36. [Google Scholar]
- 211.Korpinen, E. L. 1974. Studies on Stachybotrys alternans. IV. Effect of low doses of stachbotrys toxins on pregnancy of mice. Acta Pathol. Microbiol. Scand. Ser. B 82:457-464. [PubMed] [Google Scholar]
- 212.Korpinen, E. L., and A. Ylimaki. 1972. Discovery of toxicogenic Stachybotrys chartarum strains in Finland. Experientia 28:108-109. [DOI] [PubMed] [Google Scholar]
- 213.Kotsonis, F. N., G. A. Burdock, and W. G. Flamm. 1996. Food toxicology, p. 936-939. In C. D. Klassen (ed.), Casarett and Doull's toxicology: the basic science of poisons, 5th ed. McGraw-Hill, New York, N.Y.
- 214.Kozak, P. P., J. Gallup, L. H. Cummins, and S. A. Gillman. 1985. Endogenous mold exposure: environmental risk to atopic and nonatopic patients, p. 149-170. In R. B. Gammage and S. V. Kay (ed.), Indoor air and human health. Lewis Publishers, Chelsea, Mich.
- 215.Kozak, P. P., J. Gallup, L. H. Cummins, and S. A. Gillman. 1979. Factors of importance in determining the prevelence of indoor moulds. Ann. Allergy 43:88-94. [PubMed] [Google Scholar]
- 216.Kuiper-Goodman, T., and P. M. Scott. 1989. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 2:179-248. [PubMed] [Google Scholar]
- 217.Kuiper-Goodman, T., P. M. Scott, and H. Watanabe. 1987. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 7:253-306. [DOI] [PubMed] [Google Scholar]
- 218.La Breton, E., C. Frayssinet, C. Lafarge, and A. M. de Recondo. 1964. Aflatoxine—mechanisme de l'action. Food Cosmet. Toxicol. 2:675-676. [PubMed] [Google Scholar]
- 219.LaFarge-Frayssinet, C., K. Chakor, P. Lafont, and C. Frayssinet. 1990. Transplacental transfer of T2-toxin: pathological effect. J. Environ. Pathol. Toxicol. Oncol. 10:64-68. [PubMed] [Google Scholar]
- 220.LaFarge-Frayssinet, C., F. DeCloitre, S. Mousset, M. Martin, and C. Frayssinet. 1981. Induction of DNA single strand breaks by T-2 toxin, a trichothecene metabolite of Fusarium. Effects on lymphoid organs and liver. Mutat. Res. 88:115-123. [DOI] [PubMed] [Google Scholar]
- 221.Lalitha Rao, B., and A. Husain. 1985. Presence of cyclopiazonic acid in kodo millet (Paspalum scrobiculatum) causing ‘kodua poisoning' in man and its production by associated fungi. Mycopathologia 89:177-180. [DOI] [PubMed] [Google Scholar]
- 222.Lander, F., H. W. Meyer, and S. Norn. 2001. Serum IgE specific to indoor moulds, measured by basophil histamine release, is associated with building-related symptoms in damp buildings. Inflamm. Res. 50:227-231. [DOI] [PubMed] [Google Scholar]
- 223.Lasky, T., and L. Magder. 1997. Hepatocellular carcinoma p53 G → T transversions at codon 249: the fingerprint of aflatoxin exposure? Environ. Health Perspect. 105:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le Bars, J. 1977. La Stachybotryotoxicose: une mycotoxicose fatale due a Stachybotrys atra Cda. Rev. Med. Vet. 128:51-69. [Google Scholar]
- 225.Le Bars, J., J. P. Gerard, and C. Michel. 1977. Demonstration of stachybotryotoxicosis in France: a case of acute intoxication in deer. Ann. Nutr. Aliment. 31:509-517. (In French.) [PubMed] [Google Scholar]
- 226.Reference deleted.
- 227.Lee, M. G., S. Li, B. B. Jarvis, and J. J. Pestka. 1999. Effects of satratoxins and other macrocyclic trichothecenes on IL-2 production and viability of EL-4 thymoma cells. J. Toxicol. Environ. Health Ser. A 57:459-474. [DOI] [PubMed] [Google Scholar]
- 228.Levetin, E. 1995. Fungi, p. 87-120. In H. A. Burge (ed.), Bioaerosols. Lewis Publishers, Boca Raton, Fla.
- 229.Linnik, F. A. 1949. New fungal diseases of horses and people, p. 27-33. Kiev, USSR.
- 230.Liu, X., X. Luo, and W. Hu. 1992. Studies on epidemiology and etiology of moldy sugarcane poisoning in China. Biomed. Environ. Sci. 5:161-177. [PubMed] [Google Scholar]
- 231.Ludorf, A. C., F. He, P. S. Spencer, J. Hammerstad, and M. Sabri. 1991. 3-Nitropropionic acid—exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 18:492-498. [DOI] [PubMed] [Google Scholar]
- 232.Lundin, L. 1999. Allergic and non-allergic students' perception of the same high school environment. Indoor Air 9:92-102. [DOI] [PubMed] [Google Scholar]
- 233.Lutsky, I. I., and N. Mor. 1981. Alimentary toxic aleukia (septic angina, endemic panmyelotoxicosis, alimentary hemorrhagic aleukia): T-2 toxin-induced intoxication of cats. Am. J. Pathol. 104:189-191. [PMC free article] [PubMed] [Google Scholar]
- 234.Lutsky, I. I., and N. Mor. 1981. Experimental alimentary toxic aleukia in cats. Lab. Anim. Sci. 31:43-47. [PubMed] [Google Scholar]
- 235.Macek, C. 1982. Bacterial endotoxin may be culprit in ‘Monday fever'. JAMA 247:2765-2766. [PubMed] [Google Scholar]
- 236.Reference deleted.
- 237.Machado, L. C., and C. Kemmelmeier. 2001. Identification of deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone in the galactose oxidase-producing fungus Dactylium dendroides. Mycopathologia 149:79-85. [DOI] [PubMed] [Google Scholar]
- 238.Macher, J. M. 1986. Air sampling methods for biological contaminants. Working paper provided to the Health and Welfare Canada Working Group on Fungi and Indoor Air. Environmental Health Directorate, Health and Welfare Canada, Ottawa.
- 239.Madsen, J. M. 2001. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin. Lab. Med. 21:593-605. [PubMed] [Google Scholar]
- 240.Mahmoudi, M., and M. E. Gershwin. 2000. Sick building syndrome. III. Stachybotrys chartarum. J. Asthma 37:191-198. [DOI] [PubMed] [Google Scholar]
- 241.Mainville, C., P. L. Auger, W. Smoaogiewica, N. D., N. J., and L. M. 1988. Mycotoxins and chronic fatigue syndrome in a hospital, p. 1-10. In K. Andersson (ed.), Healthy Building Conference. Swedish Council of Building Research, Stockholm.
- 242.Malkin, R., K. Martinez, V. Marinkovich, T. Wilcox, D. Wall, and R. Biagini. 1998. The relationship between symptoms and IgG and IgE antibodies in an office environment. Environ. Res. 76:85-93. [DOI] [PubMed] [Google Scholar]
- 243.Mann, D. D., G. M. Buening, B. Hook, and G. D. Osweiler. 1983. Effects of T- 2 on bovine serum proteins. Am. J. Vet. Res. 44:1757-1759. [PubMed] [Google Scholar]
- 244.Mantle, P. G. 1969. Interruption of early pregnancy by oral administration of acroclavine and sclerotica of Claviceps fusiformis (loveless). J. Reprod. Fertil. 18:81-88. [DOI] [PubMed] [Google Scholar]
- 245.Mantle, P. G., and C. A. Finn. 1971. Investigations on the mode of action of d-methyl-8-cyanomethylergoline in suppressing pregnancy in mice. J. Reprod. Fertil. 24:441-444. [DOI] [PubMed] [Google Scholar]
- 246.Marasas, W. F., N. P. Kriek, J. E. Fincham, and S. J. van Rensburg. 1984. Primary liver cancer and oesophageal basal cell hyperplasia in rats caused by Fusarium moniliforme. Int. J. Cancer 34:383-387. [DOI] [PubMed] [Google Scholar]
- 247.Marasas, W. F. O., K. Jaskiewicz, F. S. Venter, and D. J. van Schalkwyk. 1988. Fusarium moniliforme contamination of maize in oesophogeal cancer areas in Transkei. S. Afr. Med. J. 74:110-114. [PubMed] [Google Scholar]
- 248.Martin, P. M. D., and P. Keen. 1978. The occurence of zearalenone in raw and fermented products from Swaziland and Lesotho. Sabouraudia 16:15-22. [DOI] [PubMed] [Google Scholar]
- 249.Mason, C. D., T. G. Rand, M. Oulton, J. MacDonald, and M. Anthes. 2001. Effects of Stachybotrys chartarum on surfactant convertase activity in juvenile mice. Toxicol. Appl. Pharmacol. 172:21-28. [DOI] [PubMed] [Google Scholar]
- 250.Mason, C. D., T. G. Rand, M. Oulton, J. M. MacDonald, and J. E. Scott. 1998. Effects of Stachybotrys chartarum (atra) conidia and isolated toxin on lung surfactant production and homeostasis. Nat. Toxins 6:27-33. [PubMed] [Google Scholar]
- 251.Massey, T. E., G. B. Smith, and A. S. Tam. 2000. Mechanisms of aflatoxin B1 lung tumorigenesis. Exp. Lung Res. 26:673-683. [DOI] [PubMed] [Google Scholar]
- 252.Mayer, C. F. 1953. Endemic panmyelotoxicosis in the Russian grain belt. I. The clinical aspects of alimentary toxic aleukia (ATA): a comprehensive review. Mil. Surg. 113:173-189. [PubMed] [Google Scholar]
- 253.Mayer, C. F. 1953. Endemic panmyelotoxicosis in the Russian grain belt. II. The botany, phytopathology, and toxicology of Russian cereal food. Mil. Surg. 113:295-315. [PubMed] [Google Scholar]
- 254.Reference deleted.
- 255.McCrae, K. C., T. Rand, R. A. Shaw, C. Mason, M. R. Oulton, C. Hastings, T. Cherlet, J. A. Thliveris, H. H. Mantsch, J. MacDonald, and J. E. Scott. 2001. Analysis of pulmonary surfactant by Fourier-transform infrared spectroscopy following exposure to Stachybotrys chartarum (atra) spores. Chem. Phys. Lipids 110:1-10. [DOI] [PubMed] [Google Scholar]
- 256.McGregor, R. G., W. B. Walker, and E. V. Letourneau. 1985. Radon and radon daughter levels in energy efficient housing. Sci. Total Environ. 45:271-278. [DOI] [PubMed] [Google Scholar]
- 257.Reference deleted.
- 258.McLaughlin, C. S., M. H. Vaughan, I. M. Campbell, C. M. Wei, M. E. Stafford, and B. S. Hansen. 1977. Inhibition of protein synthesis by trichothecenes, p. 263-274. In J. V. Rodricks, C. W. Hesseltine, and M. A. Mehlmang (ed.), Mycotoxins in human and animal health, vol. 19. Pathotox Publishers, Park Forest South, Ill.
- 259.Meky, F. A., L. J. Hardie, S. W. Evans, and C. P. Wild. 2001. Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem. Toxicol. 39:827-836. [DOI] [PubMed] [Google Scholar]
- 260.Mendell, M. J. 1993. Non-specific symptoms in office workers: a review and summary of the literature. Indoor Air 4:227-236. [Google Scholar]
- 261.Meneghini, R., and R. I. Schumacher. 1977. Aflatoxin B1, a selective inhibitor of DNA synthesis in mammalian cells. Chem. Biol. Interact. 18:267-276. [DOI] [PubMed] [Google Scholar]
- 262.Menzies, D., P. Comtois, J. Pasztor, F. Nunes, and J. A. Hanley. 1998. Aeroallergens and work-related respiratory symptoms among office workers. J. Allergy Clin. Immunol. 101:38-44. [DOI] [PubMed] [Google Scholar]
- 263.Menzies, D., J. Pasztor, F. Nunes, J. Leduc, and C. H. Chan. 1997. Effect of a new ventilation system on health and well-being of office workers. Arch. Environ. Health 52:360-367. [DOI] [PubMed] [Google Scholar]
- 264.Menzies, D., R. M. Tamblyn, F. Nunes, J. Hanley, and R. T. Tamblyn. 1996. Exposure to varying levels of contaminants and symptoms among workers in two office buildings. Am. J. Public Health 86:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Michael, G. Y., P. Thaxton, and P. B. Hamilton. 1973. Impairment of the reticuloendothelial system of chickens during aflatoxicosis. Poult. Sci. 52:1206-1207. [DOI] [PubMed] [Google Scholar]
- 266.Miki, T., Y. Fukui, N. Uemura, and Y. Takeuchi. 1994. Regional difference in the neurotoxicity of ochratoxin A on the developing cerebral cortex in mice. Brain Res. Dev. Brain Res. 82:259-264. [DOI] [PubMed] [Google Scholar]
- 267.Miller, J. D., A. M. Laflamme, Y. Sobol, P. Lafontaine, and R. Greenalgh. 1988. Fungi and fungal products in some Canadian houses. Int. Biodeterior. 24:103-120. [Google Scholar]
- 268.Miller, J. D., and H. L. Trenholm (ed.). 1994. Mycotoxins in grain: compounds other than aflatoxins. Eagan Press, St. Paul, Minn.
- 269.Minagawa, K., S. Kouzuki, H. Tani, K. Ishii, T. Tanimoto, Y. Terui, and T. Kamigauchi. 2002. Novel stachyflin derivatives from Stachybotrys sp. RF-7260. Fermentation, structure elucidation and biological activities. J. Antibiot. (Tokyo) 55:239-248. [DOI] [PubMed] [Google Scholar]
- 270.Ming, L. 1995. Moldy sugarcane poisoning—a case report with a brief review. Clin. Toxicol. 33:363-367. [DOI] [PubMed] [Google Scholar]
- 271.Mirocha, C. H. 1979. Trichothecene toxins produced by Fusaria, p. 345-364. In W. Shimoda (ed.), Conference on Mycotoxins in Animals Feeds and Grains Related to Animal Health. FDA/BVM-79/139. Food and Drug Administration, Washington, D.C.
- 272.Mirocha, C. J., C. M. Christensen, and G. H. Nelson. 1971. F-2 (zearalenone) estrogenic mycotoxin from Fusarium, p. 107-138. In S. Kadis, A. Ciegler, and S. J. Ajl (ed.), Microbial toxins, vol. VII. Academic Press, Inc., New York, N.Y.
- 273.Mirocha, C. J., and S. Pathre. 1973. Identification of the toxic principle in a sample of poaefusarin. Appl. Microbiol. 26:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mironov, S. G., and N. K. Baklardina. 1947. To the problem of etiology of alimentary toxic aleukia. Sci. Pap. Ckalov Opblast Svechnikov's Inst. Epidemiol. Microbiol. 2:1-10. (In Russian.) [Google Scholar]
- 275.Miyazaki, W., H. Tamaoka, M. Shinohara, H. Kaise, T. Izawa, Y. Nakano, T. Kinoshita, K. Hong, and K. Inoue. 1980. A complement inhibitor produced by Stachybotrys complementi, nov. sp. K-76, a new species of fungi imperfecti. Microbiol. Immunol. 24:1091-108. [DOI] [PubMed] [Google Scholar]
- 276.Mizoue, T., K. Reijula, and K. Andersson. 2001. Environmental tobacco smoke exposure and overtime work as risk factors for sick building syndrome in Japan. Am. J. Epidemiol. 154:803-808. [DOI] [PubMed] [Google Scholar]
- 277.Molhave, L. B., O. F. Bach, and D. F. Pederson. 1986. Human reaction to low concentrations of volatile organic compounds. Environ. Int. 12:167-175. [Google Scholar]
- 278.Montana, E., R. A. Etzel, T. Allan, T. E. Horgan, and D. G. Dearborn. 1997. Environmental risk factors associated with pediatric idiopathic pulmonary hemorrhage and hemosiderosis in a Cleveland community. Pediatrics 99:E1-E8. [DOI] [PubMed] [Google Scholar]
- 279.Moreau, C. 1979. Other mycotoxicoses, p. 252-272. In C. Moreau (ed.), Molds, toxins, and food. John Wiley & Sons, Inc., New York, N.Y.
- 280.Morey, P. R., M. J. Hodgson, W. G. Sorenson, G. K. Kullman, W. W. Rhodes, and G. S. Visvesvara. 1984. Environmental studies in moldy office buildings: biological agents, sources, and preventive measures. Ann. Am. Conf. Gov. Ind. Hyg. 10:21-35. [Google Scholar]
- 281.Reference deleted.
- 282.Moss, M. O. 1998. Recent studies of mycotoxins. J. Appl. Microbiol. Symp. Suppl. 84:62S-76S. [DOI] [PubMed] [Google Scholar]
- 283.Murtoniemi, T., A. Nevalainen, M. Suutari, M. Toivola, H. Komulainen, and M. R. Hirvonen. 2001. Induction of cytotoxicity and production of inflammatory mediators in raw264.7 macrophages by spores grown on six different plasterboards. Inhal. Toxicol. 13:233-247. [DOI] [PubMed] [Google Scholar]
- 284.Muzi, G., G. Abbritti, M. P. Accattoli, and M. dell'Omo. 1998. Prevalence of irritative symptoms in a nonproblem air-conditioned office building. Int. Arch. Occup. Environ. Health 71:372-378. [DOI] [PubMed] [Google Scholar]
- 285.Muzi, G., M. dell'Omo, G. Abbritti, P. Accattoli, M. C. Fiore, and A. R. Gabrielli. 1998. Objective assessment of ocular and respiratory alterations in employees in a sick building. Am. J. Ind. Med. 34:79-88. [DOI] [PubMed] [Google Scholar]
- 286.Nagai, K., N. Sukoh, H. Yamamoto, A. Suzuki, M. Inuoe, N. Watanabe, R. Kuroda, and E. Yamaguchi. 1998. Pulmonary disease after massive inhalation of Aspergillis niger. Nihon Kokyuki Gakkai Zasshi 36:551-555. (In Japanese.) [PubMed] [Google Scholar]
- 287.Nakamura, M., Y. Ito, K. Ogawa, Y. Michisuji, S. Sato, M. Takada, M. Hayashi, S. Yaginuma, and S. Yamamoto. 1995. Stachybocins, novel endothelin receptor antagonists, produced by Stachybotrys sp. M6222. I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. (Tokyo) 48:1389-1395. [DOI] [PubMed] [Google Scholar]
- 288.Nam, K. S., Y. S. Jo, Y. H. Kim, J. W. Hyun, and H. W. Kim. 2001. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 69:229-237. [DOI] [PubMed] [Google Scholar]
- 289.Reference deleted.
- 290.Newberne, P. M. 1974. Mycotoxins: toxicity, carcinogenicity, and the influence of various nutritional conditions. Environ. Health Perspect. 9:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nielson, K. F., S. Gravesen, P. A. Nielsen, B. Anderson, U. Thrane, and J. C. Frisvad. 1999. Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia 145:43-56. [DOI] [PubMed] [Google Scholar]
- 292.Nikodemusz, I., L. Szam, and I. L. Vedres. 1978. Analysis of the germ content of the air in university lecture rooms. Zentbl. Bakteriol. Mikrobiol. Hyg. 166:353-360. (In German.) [PubMed] [Google Scholar]
- 293.Nikov, G. N., N. E. Hopkins, S. Boue, and W. L. Alworth. 2000. Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environ. Health Perspect. 108:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nikulin, M., A.-L. Pasanen, S. Berg, and E.-L. Hintikka. 1994. Stachybotrys atra growth and toxin production in some building materials and fodder under different relative humidities. Appl. Environ. Microbiol. 60:3421-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nikulin, M., S. Lappalainen, A. L. Pasanen, I. Laamanen, P. Veijalainen, S. Berg, and E. L. Hintikka. 1996. Comparison of detection methods for trichothecenes produced by Fusarium sporotrichioides on fodder and grains at different air humidities. Nat. Toxins 4:117-121. [DOI] [PubMed] [Google Scholar]
- 296.Nikulin, M., K. Reijula, B. B. Jarvis, and E. L. Hintikka. 1996. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int. J. Exp. Pathol. 77:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nikulin, M., K. Reijula, B. B. Jarvis, P. Veijalainen, and E. L. Hintikka. 1997. Effects of intranasal exposure to spores of Stachybotrys atra in mice. Fundam. Appl. Toxicol. 35:182-188. [PubMed] [Google Scholar]
- 298.Niven, R. M., A. M. Fletcher, C. A. Pickering, E. B. Faragher, I. N. Potter, W. B. Booth, T. J. Jones, and P. D. Potter. 2000. Building sickness syndrome in healthy and unhealthy buildings: an epidemiological and environmental assessment with cluster analysis. Occup. Environ. Med. 57:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nordskog, A. W., and R. T. Clark. 1945. Ergotism in pregnant sows, female rats, and guinea pigs. Am. J. Vet. Res. 6:107. [Google Scholar]
- 300.Reference deleted.
- 301.Northrup, S. C., and K. H. Kilburn. 1978. The role of mycotoxins in human pulmonary disease, p. 91-108. In T. Wylie and L. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicosis, and encyclopedic handbook, vol. 3. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 302.Novotny, W. E., and A. Dixit. 2000. Pulmonary hemorrhage in an infant following 2 weeks of fungal exposure. Arch. Pediatr. Adolesc. Med. 154:271-275. [DOI] [PubMed] [Google Scholar]
- 303.Nozawa, Y., K. Yamamoto, M. Ito, N. Sakai, K. Mizoue, F. Mizobe, and K. Hanada. 1997. Stachybotrin C and parvisporin, novel neuritogenic compounds. I. Taxonomy, isolation, physico-chemical and biological properties. J. Antibiot. (Tokyo) 50:635-640. [DOI] [PubMed] [Google Scholar]
- 304.Nuehring, L. P., G. N. Rowland, L. R. Harrison, R. J. Cole, and J. W. Dorner. 1985. Cyclopiazonic acid mycotoxicosis in the dog. Am. J. Vet. Res. 46:1670-1676. [PubMed] [Google Scholar]
- 305.Ohm, M., J. E. Juto, K. Andersson, and L. Bodin. 1997. Nasal histamine provocation of tenants in a sick-building residential area. Am. J. Rhinol. 11:167-175. [DOI] [PubMed] [Google Scholar]
- 306.Olsen, J. H., L. Dragsted, and H. Autrup. 1988. Cancer risk and occupational exposure to aflatoxins in Denmark. Br. J. Cancer 58:392-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ooi, P. L., and K. T. Goh. 1997. Sick building syndrome: an emerging stress- related disorder? Int. J. Epidemiol. 26:1243-1249. [DOI] [PubMed] [Google Scholar]
- 308.Ooi, P. L., K. T. Goh, M. H. Phoon, S. C. Foo, and H. M. Yap. 1998. Epidemiology of sick building syndrome and its associated risk factors in Singapore. Occup. Environ. Med. 55:188-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Otokawa, M. 1983. Immunological disorders, p. 163-170. In Y. Ueno (ed.), Trichothecenes—chemical, biological, and toxicological aspects, vol. 4. Elsevier, New York, N.Y.
- 310.Otto, D., L. Molhave, G. Rose, H. K. Hudnell, and D. House. 1990. Neurobehavioral and sensory effects of controlled exposure to a complex mixture of volatile organic compounds. Neurotoxicol. Teratol. 12:649-652. [DOI] [PubMed] [Google Scholar]
- 311.Oulton, M. R., D. T. Janigan, J. MacDonald, G. T. Faulkner, and J. E. Scott. 1994. Effects of smoke inhalation on alveolar surfactant subtype in mice. Am. J. Pathol. 145:941-950. [PMC free article] [PubMed] [Google Scholar]
- 312.Ozegovic, L., R. Pavlovic, and B. Milosev. 1971. Toxic dermatitis, conjunctivitis, rhinitis, pharyngitis, and laryngitis in fattening cattle and farm workers caused by molds from contaminated straw (stachybotryotoxicosis?). Veterinaria (Sarajevo) 20:263-267. [Google Scholar]
- 313.Page, E. H., and D. B. Trout. 2001. The role of Stachybotrys mycotoxins in building related illness. AIHAJ 62:644-648. [PubMed] [Google Scholar]
- 314.Palmgren, M. S., and L. S. Lee. 1986. Separation of mycotoxin-containing sources in grain dust and determination of their mycotoxin potential. Environ. Health Perspect. 66:105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Palyusik, M., and E. M. Moran. 1994. Porcine pulmonary edema with hydrothorax; a review. J. Environ. Pathol. Toxicol. Oncol. 13:63-66. [PubMed] [Google Scholar]
- 316.Pang, V. F., R. J. Lambert, P. J. Felsburg, V. R. Beasley, W. B. Buck, and W. M. Haschek. 1988. Experimental T-2 toxicosis in swine following inhalation exposure: clinical signs and effects on hematology, serum biochemistry, and immune response. Fundam. Appl. Toxicol. 11:100-109. [DOI] [PubMed] [Google Scholar]
- 317.Panigrahi, S. 1993. Biossay of mycotoxins using terrestrial and aquatic, animal and plant species. Food Chem. Toxicol. 31:767-790. [DOI] [PubMed] [Google Scholar]
- 318.Pasanen, A. L., J. Keinanen, P. Kalliokoski, P. I. Martikainen, and J. Ruuskanen. 1993. Microbial growth on respirator filters from improper storage. Scand. J. Work Environ. Health 19:421-425. [DOI] [PubMed] [Google Scholar]
- 319.Patterson, R. 1998. Allergic bronchopulmonary aspergillosis and hypersensitivity to fungi, p. 777-782. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw Hill, New York, N.Y.
- 320.Paul, P. S., D. W. Johnson, C. J. Mirocha, F. F. Soper, C. O. Thoen, C. C. Muscoplat, and A. F. Weber. 1977. In vitro stimulation of bovine peripheral blood lymphocytes: suppression of phytomitogen and specific antigen lymphocyte responses by aflatoxin. Am. J. Vet. Res. 38:2033-2035. [PubMed] [Google Scholar]
- 321.Pejtersen, J., H. Brohus, C. E. Hyldgaard, J. B. Nielsen, O. Valbjorn, P. Hauschildt, S. K. Kjaergaard, and P. Wolkoff. 2001. Effect of renovating an office building on occupants' comfort and health. Indoor Air 11:10-25. [DOI] [PubMed] [Google Scholar]
- 322.Peraica, M., B. Radic, A. Lucic, and M. Pavlovic. 1999. Toxic effects of mycotoxins in humans. Bull. W. H. O. 77:754-766. [PMC free article] [PubMed] [Google Scholar]
- 323.Perkins, J. L. 1997. Modern industrial hygiene. Van Nostrand Reinhold, New York, N.Y.
- 324.Perry, L. P., M. Iwata, H. D. Tazelhaar, T. V. Colby, and S. A. Yousem. 1998. Pulmonary mycotoxicosis: a clinicopathologic study of three cases. Mod. Pathol. 11:432-436. [PubMed] [Google Scholar]
- 325.Perzanowski, M. S., R. Sporik, S. P. Squillace, L. E. Gelber, R. Call, M. Carter, and T. A. Platts-Mills. 1998. Association of sensitization to Alternaria allergens with asthma among school-age children. J. Allergy Clin. Immunol. 101:626-632. [DOI] [PubMed] [Google Scholar]
- 326.Petkova-Bocharova, T., I. N. Chernozensky, and M. Castegnaro. 1988. Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary system tumors in Bulgaria. Food Additives Contam. 5:299-301. [DOI] [PubMed] [Google Scholar]
- 327.Pieckova, E., and Z. Jesenska. 1999. Microscopic fungi in dwellings and their implications in humans. Ann. Agric. Environ. Med. 6:1-11. [PubMed] [Google Scholar]
- 328.Pier, A. C., K. L. Heddleston, S. J. Cysewski, and J. M. Patterson. 1972. Effect of aflatoxin on immunity in turkeys. II. Reversal of impaired resistance to bacterial infection by passive transfer of plasma. Avian Dis. 16:381-387. [PubMed] [Google Scholar]
- 329.Pier, A. C., J. L. Richard, and S. J. Cysewski. 1980. Implications of mycotoxins in animal disease. J. Am. Vet. Med. Assoc. 176:719-724. [PubMed] [Google Scholar]
- 330.Reference deleted.
- 331.Pietrykowski, M., W. Peters, L. Geist, M. McKay, B. Stewart, and H. Norton. 2001. Can residential mold trigger a prop 65 violation? Gordon & Reese, LLP Vol. 1, No. 2. [Online.] http://www.gordonrees.com/pubs/pdf/moldmat0801.pdf.
- 332.Pitten, F. A., J. Bremer, and A. Kramer. 2000. Air pollution by volatile organic compounds (VOC) and health complaints. Dtsch. Med. Wochenschr. 125:545-550. (In German.) [DOI] [PubMed] [Google Scholar]
- 333.Platt, S. D., C. J. Martin, S. M. Hunt, and C. W. Lewis. 1989. Damp housing, mould growth, and symptomatic health state. Br. Med. J. 298:1673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Pohland, A. E. 1993. Mycotoxins in review. Food Additives Contam. 10:17-28. [DOI] [PubMed] [Google Scholar]
- 335.Pohland, A. E., and G. E. Wood. 1991. Natural occurrence of mycotoxins, p. 32-52. In G. Bray and D. Ryan (ed.), Pennington Center nutrition series: mycotoxins, cancer, and health. Louisiana State University Press, Baton Rouge.
- 336.Poliakov, A. A. 1948. Rukovodstvo po Veterinarnoi Dezinfeksii, p. 154-158. Ogiz-Selskhozgiz, Moscow, USSR.
- 337.Prelusky, D. B., B. A. Rotter, and B. G. Rotter. 1994. Toxicology of mycotoxins, p. 359-403. In J. D. Miller and H. L. Trenholm (ed.), Mycotoxins in grain compounds other than aflatoxins. Egan Press, St. Paul, Minn.
- 338.Purchase, I. F. 1971. The acute toxicity of the mycotoxin cyclopiazonic acid to rats. Toxicol. Appl. Pharmacol. 18:114-123. [DOI] [PubMed] [Google Scholar]
- 339.Qian, G. S., M. C. Yu, R. Ross, J. M. Yuan, Y. T. Gao, B. E. Henderson, G. N. Wogan, and J. D. Groopman. 1994. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, PRC. Cancer Epidemiol. Biomarkers Prev. 3:3-11. [PubMed] [Google Scholar]
- 340.Raabe, O. G., M. A. Al-Bayati, S. V. Teague, and A. Rasolt. 1988. Regional deposition of inhaled monodisperse course and fine aerosol particles in small laboratory animals. Ann. Occup. Hyg. 32(Suppl. 1):53-63. [Google Scholar]
- 341.Rabie, C. J., S. J. Van Rensburg, J. J. Van Der Watt, and A. Lubben. 1975. Onyalai—the possible involvement of a mycotoxin produced by Phoma sorghina in the aetiology. S. Afr. Med. J. 49:1647-1650. [PubMed] [Google Scholar]
- 342.Rao, C. Y., H. A. Burge, and J. D. Brain. 2000. The time course of responses to intratracheally instilled toxic Stachybotrys chartarum spores in rats. Mycopathologia 149:27-34. [DOI] [PubMed] [Google Scholar]
- 343.Reddy, R. V., M. J. Taylor, and R. P. Sharma. 1988. Evaluation of citrinin toxicity on the immune functions of mice. J. Food Prot. 51:32-36. [DOI] [PubMed] [Google Scholar]
- 344.Reinikainen, L. M., and J. J. Jaakkola. 2001. Effects of temperature and humidification in the office environment. Arch. Environ. Health 56:365-368. [DOI] [PubMed] [Google Scholar]
- 345.Richerson, H. B. 1990. Unifying concepts underlying the effects of organic dust exposures. Am. J. Ind. Med. 17:139-142. [DOI] [PubMed] [Google Scholar]
- 346.Rizzo, A. F., F. Atroshi, T. Hirvi, and H. Saloniemi. 1992. The hemolytic activity of deoxynivalenol and T-2 toxin. Nat. Toxins 1:106-110. [DOI] [PubMed] [Google Scholar]
- 347.Roe, J. D., R. A. Haugland, S. J. Vesper, and L. J. Wymer. 2001. Quantification of Stachybotrys chartarum conidia in indoor dust using real time, fluorescent probe-based detection of PCR products. J. Exp. Anal. Environ. Epidemiol. 11:12-20. [DOI] [PubMed] [Google Scholar]
- 348.Rosiles, M. R., J. Bautista, V. O. Fuentes, and F. Ross. 1998. An outbreak of equine leukoencephalomalacia at Oaxaca, Mexico, associated with fumonisin B1. Zentbl Veterinaermed. Ser. A 45:299-302. [DOI] [PubMed] [Google Scholar]
- 349.Ross, R., J.-M. Yuan, M. C. Yu, G. N. Wogan, G. S. Qian, J. C. Tu, J. D. Groopman, Y. T. Gao, and B. E. Henderson. 1992. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 339:943-946. [DOI] [PubMed] [Google Scholar]
- 350.Rybakova, S. G. 1955. Mikrobiologiya 24. [PubMed]
- 351.Rylander, R., M. Norrhall, U. Engdahl, A. Tunsater, and P. G. Holt. 1998. Airways inflammation, atopy, and (1→3)-beta-d-glucan exposures in two schools. Am. J. Respir. Crit. Care Med. 158:1685-1687. [DOI] [PubMed] [Google Scholar]
- 352.Saenz de Rodriguez, C. 1984. Environmental hormone contamination in Puerto Rico. N. Engl. J. Med. 310:1741-1742. [DOI] [PubMed] [Google Scholar]
- 353.Saito, M., and T. Tatsuno. 1971. Toxins of Fusarium nivale., p. 293-315. In S. Kadis, A. Ciegler, and S. J. Ajl (ed.), Microbial toxins. Algal and fungal toxins, vol. 7. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 354.Sakamoto, K., E. Tsujii, M. Miyauchi, T. Nakanishi, M. Yamashita, N. Shigematsu, T. Tada, S. Izumi, and M. Okuhara. 1993. FR901459, a novel immunosuppressant isolated from Stachybotrys chartarum no. 19392. Taxonomy of the producing organism, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. (Tokyo) 46:1788-1798. [DOI] [PubMed] [Google Scholar]
- 355.Sakata, T., K. Soda, M. Ando, K. Shimazu, and S. Araki. 1988. Experimental hypersensitivity pneumonitis in rabbits induced by Trichosporon cutaneum: role of local cellular and humoral immune responses. J. Clin. Lab. Immunol. 25:191-199. [PubMed] [Google Scholar]
- 356.Saltos, N., N. A. Saunders, S. B. Bhagwandeen, and B. Jarvie. 1982. Hypersensitivity pneumonitis in a mouldy house. Med. J. Aust. 2:244-246. [PubMed] [Google Scholar]
- 357.Samet, J. M., and M. J. Utell. 1998. Indoor and outdoor air pollution, p. 941-963. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw Hill, New York, N.Y.
- 358.Sampson, R. A. 1985. Occurrence of molds in modern living and working environments. Eur. J. Epidemiol. 1:54-61. [DOI] [PubMed] [Google Scholar]
- 359.Samson, R. A. 1986. Fungi in indoor air associated with adverse health effects. Working paper provided to the Health and Welfare Canada Working Group on Fungi and Indoor Air. Environmental Health Directorate, Health and Welfare Canada, Ottawa.
- 360.Samsonov, P. F. 1960. P. 131-140. In V. I. Bilai (ed.), Mycotoxicoses of man and agricultural animals. Izd. Akad. Nauk. Ukr. S.S.R., Kiev, USSR.
- 361.Samsonov, P. F., and A. P. Samsonov. 1960. P. 140-151. In V. I. Bilai (ed.), Mycotoxicosis of man and agricultural animals. Izd. Akad. Nauk. Ukr. S.S.R., Kiev, USSR.
- 362.Sanders, C. H., and J. P. Cornish. 1982. Dampness: one week's complaints in five local authorities in England and Wales. BRE Report. Her Majesty's Stationery Office, London, United Kingdom.
- 363.Sarkisov, A. K. 1960. P. 155-163. In V. I. Bilai (ed.), Mycotoxicoses of man and agricultural animals. Izd. Akad. Nauk Ukr. S.S.R., Kiev, USSR.
- 364.Sarkisov, A. K. 1950. Etiology of “septic angina” in man. J. Microbiol. Epidemiol. Immunol. 1:43-47. [Google Scholar]
- 365.Sashidhar, R. B., Y. Ramakrishna, T. Ramnath, and R. V. Bhat. 1991. Lack of relationship between sorghum consumption, mycotoxin contamination and pellagra in a traditionally sorghum eating population. Trop. Geogr. Med. 43:165-170. [PubMed] [Google Scholar]
- 366.Savel, H., B. Forsyth, W. Schaeffer, and T. Cordella. 1970. Effect of aflatoxin B1 upon phytohemagglutinin-transformed human lymphocytes. Proc. Soc. Exp. Biol. Med. 134:1112-1115. [DOI] [PubMed] [Google Scholar]
- 367.Savilahti, R., J. Uitti, P. Laippala, T. Husman, and P. Roto. 2000. Respiratory morbidity among children following renovation of a water-damaged school. Arch. Environ. Health 55:405-410. [DOI] [PubMed] [Google Scholar]
- 368.Savilahti, R., J. Uitti, P. Roto, P. Laippala, and T. Husman. 2001. Increased prevalence of atopy among children exposed to mold in a school building. Allergy 56:175-179. [DOI] [PubMed] [Google Scholar]
- 369.Schalman, L. B. 1945. Clinical and epidemiological observations on the “septic sore throat”, p. 61-66. In Z. I. Malkin (ed.), Alimentarno-toxicesrai aleucia. Tatgosizabat, Kasan, USSR. (In Russian.)
- 370.Schiefer, H. B. 1986. Health effects from mycotoxins (volatile or absorbed to particulates): a review of the relevant data in animal experiments. In Health and Welfare Canada Working Group on Fungi and Indoor Air (ed.), Significance of fungi in indoor air: report of a Working Group. Health and Welfare Canada, Ottawa.
- 371.Reference deleted.
- 372.Schindler, D. 1974. Two classes of inhibitors of peptidyl transferase activity in eukaryotes. Nature 249:38-41. [DOI] [PubMed] [Google Scholar]
- 373.Schneider, D. J., W. F. Marasas, J. C. Dale Kuys, N. P. Kriek, and G. C. Van Schalkwyk. 1979. A field outbreak of suspected stachybotryotoxicosis in sheep. J. S. Afr. Vet. Assoc. 50:73-81. [PubMed] [Google Scholar]
- 374.Schneider, D. J., C. O. Miles, I. Garthwaite, A. Van Halderen, J. C. Wessels, and H. J. Lategan. 1996. First report of field outbreaks of ergot-alkaloid toxicity in South Africa. Onderstepoort J. Vet. Res. 63:97-108. [PubMed] [Google Scholar]
- 375.Schoeffner, D. J., and U. P. Thorgeirsson. 2000. Susceptibility of nonhuman primates to carcinogens of human relevance. In Vivo 14:149-156. [PubMed] [Google Scholar]
- 376.Schuyler, M. 1998. Hypersensitivity pneumonitis, p. 1086-1097. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw Hill, New York, N.Y.
- 377.Schwartz, D. A., and C. A. Blaski. 1998. Toxic inhalations, p. 925-940. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders. McGraw-Hill, New York, N.Y.
- 378.Scott, J. E. 1992. Phosphatidylcholine synthesis, secretion, and reutilization during differentiation of the surfactant-producing type II alveolar cell from fetal rabbit lungs. Exp. Lung Res. 18:563-580. [DOI] [PubMed] [Google Scholar]
- 379.Scott, P. M. 1986. Potential indoor air pollution problems with macrocyclic trichothecene by producing fungi. Working paper provided to the Health and Welfare Canada Working Group on Fungi and Indoor Air. Environmental Health Directorate, Health and Welfare Canada, Ottawa.
- 380.Sergiev, P. G. 1945. Epidemiology of alimentary-toxic aleukia, p. 7-11. In H. I. Nesterov, H. N. Sisin, and L. N. Karlin (ed.), Alimentary-toxic aleukia. (In Russian.)
- 381.Seuri, M., K. Husman, H. Kinnunen, M. Reiman, R. Kreus, P. Kuronen, K. Lehtomaki, and M. Paananen. 2000. An outbreak of respiratory diseases among workers at a water-damaged building—a case report. Indoor Air 10:138-145. [DOI] [PubMed] [Google Scholar]
- 382.Shank, R. C. 1978. Mycotoxicosis of man: dietary and epidemiologic considerations, p. 1-19. In T. Wyllie and L. G. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicosis: an encyclopedic handbook, vol. 3. Marcel Dekker, Inc., New York, N.Y.
- 383.Sharma, R. P., and D. K. Salunkhe (ed.). 1991. Mycotoxins and phytotoxins. CRC Press, Inc., Boca Raton, Fla.
- 384.Shen, H. M., and C. N. Ong. 1996. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat. Res. 366:23-44. [DOI] [PubMed] [Google Scholar]
- 385.Shimazu, K., M. Ando, T. Sakata, K. Yoshida, and S. Araki. 1984. Hypersensitivity pneumonitis induced by Trichosporon cutaneum. Am. Rev. Respir. Dis. 130:407-411. [DOI] [PubMed] [Google Scholar]
- 386.Shone, D. K., J. R. Philip, and G. J. Christie. 1959. Agalactia of cows caused by feeding the ergot of the bulrush millet, Pennisetum typhoides. Vet. Rec. 71:129-132. [Google Scholar]
- 387.Shulyumov, Y. S., A. F. Kuzmin, and P. A. Fedko. 1960. P. 167-180. In V. I. Bilai (ed.), Mycotoxicoses of man and agricultural animals. Izd. Akad. Nauk Ukr. S.S.R., Kiev, USSR.
- 388.Silas, J. C., M. A. Harrison, J. A. Carpenter, and I. L. Roth. 1987. Airborne aflatoxin in corn processing facilities in Georgia. Am. Ind. Hyg. Assoc. J. 48:198-201. [DOI] [PubMed] [Google Scholar]
- 389.Smela, M. E., S. S. Currier, E. A. Bailey, and J. M. Essigmann. 2001. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis 22:535-545. [DOI] [PubMed] [Google Scholar]
- 390.Smoragiewicz, W., B. Cossette, A. Boutard, and K. Krzystyniak. 1993. Trichothecene mycotoxins in the dust of ventilation systems in office buildings. Int. Arch. Occup. Environ. Health 65:113-117. [DOI] [PubMed] [Google Scholar]
- 391.Sneller, M. R., and R. R. Roby. 1979. Incidence of fungal spores at the homes of allergic patients in an agricultural community. I. A 12 month study in and out of doors. Ann. Allergy 43:225-228. [PubMed] [Google Scholar]
- 392.Sobotka, T. J., R. E. Brodie, and S. L. Spaid. 1978. Neurobehavioral studies of the tremorgenic mycotoxins verruculogen and penitrem A. Pharmacology 16:287-294. [DOI] [PubMed] [Google Scholar]
- 393.Soda, K., M. Ando, K. Shimazu, T. Sakata, K. Yoshida, and S. Araki. 1986. Different classes of antibody activities to Trichosporon cutaneum antigen in summer-type hypersensitivity pneumonitis by enzyme-linked immunosorbent assay. Am. Rev. Respir. Dis. 133:83-87. [DOI] [PubMed] [Google Scholar]
- 394.Solomon, W. R. 1976. A volumetric study of winter fungus prevelence in the air of midwestern homes. J. Allergy Clin. Immunol. 57:46-55. [DOI] [PubMed] [Google Scholar]
- 395.Reference deleted.
- 396.Sorenson, W. G. 1999. Fungal spores: hazardous to health? Environ. Health Perspect. 107:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Sorenson, W. G., D. G. Frazer, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sorenson, W. G., G. F. Gerberick, D. M. Lewis, and V. Castranova. 1986. Toxicity of mycotoxins for the rat pulmonary macrophage in vitro. Environ. Health Perspect. 66:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sorenson, W. G., J. P. Simpson, M. J. I. Peach, T. D. Thedell, and S. A. Olenchock. 1981. Aflatoxin in respirable corn dust particles. J. Toxicol. Environ. Health 7:669-672. [DOI] [PubMed] [Google Scholar]
- 400.Still, P. E., A. W. Macklin, W. E. Ribelin, and E. B. Smalley. 1971. Relationship of ochratoxin A to foetal death in the laboratory and domestic animals. Nature 234:563-564. [DOI] [PubMed] [Google Scholar]
- 401.Strachan, D. P. 1988. Damp housing and childhood asthma: validation of reporting of symptoms. Br. Med. J. 297:1223-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Stuart, B. P., and D. M. Bedell. 1982. Mycotoxicosis in swine. Vet. Clin. North Am. Large Anim. Pract. 4:377-388. [DOI] [PubMed] [Google Scholar]
- 403.Sudakin, D. L. 2000. Stachybotrys chartarum: current knowledge of its role in disease. MedGenMed. 2000:E11. [PubMed] [Google Scholar]
- 404.Sudakin, D. L. 1998. Toxigenic fungi in a water-damaged building: an intervention study. Am. J. Ind. Med. 34:183-190. [DOI] [PubMed] [Google Scholar]
- 405.Sudakin, D. L., R. A. Etzel, E. G. Sorenson, D. M. Miller, B. B. Jarvis, T. Allan, and D. G. Dearborn. 1999. Mycotoxins and pulmonary hemorrhage. Arch. Pediatr. Adolesc. Med. 153:205-206. [DOI] [PubMed] [Google Scholar]
- 406.Sweeney, M. J., and A. D. Dobson. 1999. Molecular biology of mycotoxin biosynthesis. FEMS Microbiol. Lett. 175:149-163. [DOI] [PubMed] [Google Scholar]
- 407.Szathmary, C. I. 1983. Trichothecene toxicosis and natural occurence in Hungary, p. 229-250. In Y. Ueno (ed.), Trichothecenes: developments in food science, vol. 4. Elsevier, Amsterdam, The Netherlands.
- 408.Tantaoui-Elaraki, A., S. L. Mekouar, M. el Hamidi, and M. Senhaji. 1994. Toxigenic strains of Stachybotrys atra associated with poisonous straw in Morocco. Vet. Hum. Toxicol. 36:93-96. [PubMed] [Google Scholar]
- 409.Taskinen, T., A. Hyvarinen, and T. Meklin. 1999. Asthma and repiratory infections in school children with special reference to moisture and mold problems in the school. Acta Paediatr. 88:1373-1379. [DOI] [PubMed] [Google Scholar]
- 410.Taskinen, T., T. Meklin, M. Nousiainen, T. Husman, A. Nevalainen, and M. Korppi. 1997. Moisture and mold problems in schools and respiratory manifestations in school children: clinical and skin test findings. Acta Paediatr. 86:1153-1154. [DOI] [PubMed] [Google Scholar]
- 411.Thaxton, J. P., H. T. Tung, and P. B. Hamilton. 1974. Immunosuppression in chickens by aflatoxin. Poult. Sci. 53:721-725. [DOI] [PubMed] [Google Scholar]
- 412.Thor, J., and R. Rylander. 1998. Airways inflammation and glucan in a rowhouse area. Am. J. Respir. Crit. Care Med. 157:1798-1803. [DOI] [PubMed] [Google Scholar]
- 413.Thorn, A. 2000. Emergence and preservation of a chronically sick building. J. Epidemiol. Community Health 54:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Thorn, A., M. Lewne, and L. Belin. 1996. Allergic alveolitis in a school environment. Scand. J. Work Environ. Health 22:311-314. [DOI] [PubMed] [Google Scholar]
- 415.Tobin, R. S. 1986. Health effects of airborne bacteria. Environmental Health Directorate, Health and Welfare Canada, Ottawa.
- 416.Tobin, R. S., E. Baranowski, A. P. Gilman, T. Kuiper-Goodman, J. D. Miller, and M. Giddings. 1987. Significance of fungi in indoor air: report of a working group. Can. J. Public Health 78:S1-S32. [PubMed] [Google Scholar]
- 417.Townsend, R. J., M. O. Moss, and H. M. Peck. 1966. Isolation and characterization of hepatoxins from Penicillium rubrum. J. Pharm. Pharmacol. 18:471-473. [DOI] [PubMed] [Google Scholar]
- 418.Tripi, P. A., S. Modlin, W. G. Sorenson, and D. G. Dearborn. 2000. Acute pulmonary haemorrhage in an infant during induction of general anaesthesia. Paediatr. Anaesth. 10:92-94. [DOI] [PubMed] [Google Scholar]
- 419.Trout, D., J. Bernstein, K. Martinez, R. Biagini, and K. Wallingford. 2001. Bioaerosol lung damage in a worker with repeated exposure to fungi in a water- damaged building. Environ. Health Perspect. 109:641-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Tuomi, T., K. Reijula, T. Johnsson, K. Hemminki, E. L. Hintikka, O. Lindroos, S. Kalso, P. Koukila-Kahkola, H. Mussalo-Rauhamaa, and T. Haahtela. 2000. Mycotoxins in crude building materials from water damaged buildings. Appl. Environ. Microbiol. 66:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Ueno, Y. 1971. Toxicolgical and biological properties of fusarenon-x, a cytoxic mycotoxin of Fusarium nivale Fn-2B, p. 163-178. In I. F. H. Purchase (ed.), Mycotoxins in human health. Proceedings of a symposium. Macmillan, Edinburgh, United Kingdom.
- 422.Ueno, Y. 1984. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 4(Suppl.):S124-S132. [DOI] [PubMed] [Google Scholar]
- 423.Ueno, Y. 1980. Trichothecene mycotoxins—mycology, chemistry, and toxicology. Adv. Nutr. Sci. 3:301-353. [Google Scholar]
- 424.Ueno, Y. 1977. Trichothecenes: overview address, p. 189-208. In J. V. Rodericks, C. W. Hesseltine, and M. A. Mehlman (ed.), Mycotoxins in human and animal health. Pathtox, Park Forest South, Ill.
- 425.Uraguchi, K., and M. Yamazaki. 1978. In Y. Yeno (ed.), Toxicology, biochemistry, and pathology of mycotoxins, p. 6-11. John Wiley & Sons, New York, N.Y.
- 426.U.S. Dept. of Health and Human Services. 1989. NTP technical report on the toxicology and carcinogenesis studies of ochratoxin A in F344/N rats. NTP TR 358. NIH publication 89-2813.
- 427.Vachev, V., L. Dyakov, P. Peichev, and B. Tabakov. 1970. Abortion caused by Stachybotrys toxin in swine. Veterinar. Sbir. 67:7-10. [Google Scholar]
- 428.Vainio, H., E. Heseltine, and J. Wilbourn. 1993. Report on an IARC working group meeting on some naturally occurring substances. Int. J. Cancer 53:535-537. [DOI] [PubMed] [Google Scholar]
- 429.Vertinskii, K. L. 1940. Stachybotryotoxicosis in horses. Veterinariya 17:61-68. [Google Scholar]
- 430.Vesper, S., D. G. Dearborn, I. Yike, T. Allan, J. Sobolewski, S. F. Hinkley, B. B. Jarvis, and R. A. Haugland. 2000b. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: quantitative assessment before, during, and after remediation. J. Urban Health 77:68-85. [DOI] [PMC free article] [PubMed]
- 431.Vesper, S. J., D. G. Dearborn, O. Elidemir, and R. A. Haugland. 2000. Quantification of siderophore and hemolysin from Stachybotrys chartarum strains, including a strain isolated from the lung of a child with pulmonary hemorrhage and hemosiderosis. Appl. Environ. Microbiol. 66:2678-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Vesper, S. J., D. G. Dearborn, I. Yike, W. G. Sorenson, and R. A. Haugland. 1999. Hemolysis, toxicity, and randomly amplified polymorphic DNA analysis of Stachybotrys chartarum strains. Appl. Environ. Microbiol. 65:3175-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Vesper, S. J., M. L. Magnuson, D. G. Dearborn, I. Yike, and R. A. Haugland. 2001. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect. Immun. 69:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Vyshelesski, S. N. 1948. Special epizootiology. Ozig-Selskozgiz, Moscow, USSR.
- 435.Walinder, R., D. Norback, B. Wessen, and P. Venge. 2001. Nasal lavage biomarkers: effect of water damage and microbial growth in an office building. Arch. Environ. Health 56:30-36. [DOI] [PubMed] [Google Scholar]
- 436.Walinder, R., D. Norback, G. Wieslander, G. Smedje, C. Erwall, and P. Venge. 2001. Acoustic rhinometry and lavage biomarkers in relation to some building characteristics in Swedish schools. Indoor Air 11:2-9. [DOI] [PubMed] [Google Scholar]
- 437.Wan, G. H., and C. S. Li. 1999. Dampness and airway inflammation and systemic symptoms in office building workers. Arch. Environ. Health 54:58-63. [DOI] [PubMed] [Google Scholar]
- 438.Wang, J. S., and J. D. Groopman. 1999. DNA damage by mycotoxins. Mutat. Res. 424:167-181. [DOI] [PubMed] [Google Scholar]
- 439.Wang, L. Y., M. Hatch, C. J. Chen, B. Levin, S. L. You, S. N. Lu, M. H. Wu, W. P. Wu, L. W. Wang, Q. Wang, G. T. Huang, P. M. Yang, H. S. Lee, and R. M. Santella. 1996. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int. J. Cancer 67:620-625. [DOI] [PubMed] [Google Scholar]
- 440.Wargocki, P., D. P. Wyon, J. Sundell, G. Clausen, and P. O. Fanger. 2000. The effects of outdoor air supply rate in an office on perceived air quality, sick building syndrome (SBS) symptoms and productivity. Indoor Air 10:222-236. [DOI] [PubMed] [Google Scholar]
- 441.Weaver, G. A., H. J. Kurtz, F. Y. Bates, M. S. Chi, C. J. Mirocha, J. C. Behrens, and T. S. Robison. 1978. Acute and chronic toxicity of T-2 mycotoxin in swine. Vet. Rec. 103:531-535. [DOI] [PubMed] [Google Scholar]
- 442.White, W. L., C. C. Yeager, and H. Shotts. 1949. History, distribution, and economic significance of cellulose-destroying fungus Memnoniella echinata. Farlowia 3:399. [Google Scholar]
- 443.Wicklow, D. T., and O. L. Shotwell. 1983. Intrafungal distribution of aflatoxins among conidia and sclerotia of Aspergillus flavus and Aspergillus parasiticus. Can. J. Microbiol. 29:1-5. [DOI] [PubMed] [Google Scholar]
- 444.Wieslander, G., D. Norback, K. Nordstrom, R. Walinder, and P. Venge. 1999. Nasal and ocular symptoms, tear film stability and biomarkers in nasal lavage, in relation to building-dampness and building design in hospitals. Int. Arch. Occup. Environ. Health 72:451-461. [DOI] [PubMed] [Google Scholar]
- 445.Wilkins, C. K., S. T. Larsen, M. Hammer, O. M. Poulsen, P. Wolkoff, and G. D. Nielsen. 1998. Respiratory effects in mice exposed to airborne emissions from Stachybotrys chartarum and implications for risk assessment. Pharmacol. Toxicol. 83:112-119. [DOI] [PubMed] [Google Scholar]
- 446.Withanage, G. S., H. Murata, T. Koyama, and I. Ishiwata. 2001. Agonistic and antagonistic effects of zearalenone, an etrogenic mycotoxin, on SKN, HHUA, and HepG2 human cancer cell lines. Vet. Hum. Toxicol. 43:6-10. [PubMed] [Google Scholar]
- 447.Wogan, G. N., G. S. Edwards, and P. M. Newberne. 1971. Acute and chronic toxicity of rubratoxin B. Toxicol. Appl. Pharmacol. 19:712-720. [DOI] [PubMed] [Google Scholar]
- 448.Wogan, G. N., and P. M. Newberne. 1967. Dose-response characteristics of aflatoxin B1 carcinogenesis in rats. Cancer Res. 27:2370-2376. [PubMed] [Google Scholar]
- 449.Wong, S., R. C. Schwartz, and J. J. Pestka. 2001. Superinduction of TNF-alpha and IL-6 in macrophages by vomitoxin (deoxynivalenol) modulated by mRNA stabilization. Toxicology 161:139-149. [DOI] [PubMed] [Google Scholar]
- 450.World Health Organization. 1982. Indoor air pollutants, exposure and health effect assessments. Nordlinger, Copenhagen, Denmark.
- 451.World Health Organization. 1990. Selected mycotoxins: ochratoxins, trichothecenes, ergot. Report of an expert committee. Environmental health criteria no. 105. World Health Organization. Geneva, Switzerland.
- 452.Wyatt, R. D., W. M. Colwell, P. B. Hamilton, and H. R. Burmeister. 1973. Neural disturbances in chickens caused by dietary T-2 toxin. Appl. Microbiol. 26:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wyatt, R. D., B. A. Weeks, P. B. Hamilton, and H. R. Burmeister. 1972. Severe oral lesions in chickens caused by ingestion of dietary fusariotoxin. Appl. Microbiol. 24:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Wyllie, T. D., and L. G. Morehouse. 1978. Introduction, p. vii-xviii. In T. D. Wyllie and L. G. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicosis, vol. 3. Marcel Dekker, Inc., New York, NY.
- 455.Ye, T. T., J. X. Huang, Y. E. Shen, P. L. Lu, and D. C. Christiani. 1998. Respiratory symptoms and pulmonary function among Chinese rice-granary workers. Int. J. Occup. Environ. Health 4:155-159. [DOI] [PubMed] [Google Scholar]
- 456.Yeh, F.-S., and M. Yu. 1986. Epidemiology and early diagnosis of primary liver cancer in China. Adv. Cancer Res. 47:297-329. [PubMed] [Google Scholar]
- 457.Yike, I., T. Allan, W. G. Sorenson, and D. G. Dearborn. 1999. Highly sensitive protein translation assay for trichothecene toxicity in airborne particulates: comparison with cytotoxicity assays. Appl. Environ. Microbiol. 65:88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yoshida, K., M. Ando, T. Sakata, and S. Araki. 1988. Environmental mycological studies on the causative agent of summer-type hypersensitivity pneumonitis. J. Allergy Clin. Immunol. 81:475-483. [DOI] [PubMed] [Google Scholar]
- 459.Yoshida, K., M. Ando, T. Sakata, and S. Araki. 1989. Prevention of summer- type hypersensitivity pneumonitis: effect of elimination of Trichosporum cutaneum from the patients' homes. Arch. Environ. Health 44:317-322. [DOI] [PubMed] [Google Scholar]
- 460.Yoshizawa, T. 1983. Red-mold diseases and natural occurrence in Japan, p. 195-209. In Y. Ueno (ed.), Trichothecenes: chemical, biological, and toxicological aspects. Elsevier, New York, N.Y.
- 461.Yoshizawa, T., A. Yamashita, and Y. Luo. 1994. Fumonisin occurrence in corn from high- and low-risk areas fro human esophageal cancer in China. Appl. Environ. Microbiol. 60:1626-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Yu, M.-W., J.-P. Lien, Y. H. Chiu, R. M. Santella, Y. F. Liaw, and C. J. Chen. 1997. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J. Hepatol. 27:320-330. [DOI] [PubMed] [Google Scholar]
- 463.Yu, M. C., J. M. Yuan, S. Govindarajan, and R. K. Ross. 2000. Epidemiology of hepatocellular carcinoma. Can. J. Gastroenterol. 14:703-709. [DOI] [PubMed] [Google Scholar]
- 464.Zhang, B. C., Y. R. Zhu, J. B. Wang, Y. Wu, Q. N. Zhang, G. S. Qian, S. Y. Kuang, Y. F. Li, X. Fang, L. Y. Yu, L. P. De Flora, L. P. Jacobson, A. Zarba, P. A. Egner, X. He, J. S. Wang, B. Chen, C. L. Enger, N. E. Davidson, G. B. Gordon, M. B. Gorman, H. J. Prochaska, J. D. Groopman, A. Munoz, and T. W. Kensler. 1997. Olipraz chemoprevention trial in Qidong, Jiangsu Province, People's Republic of China. J. Cell. Biochem. Suppl. 28-29:166-173. [PubMed] [Google Scholar]
- 465.Zilinskas, R. A. 1997. Iraq's biological weapons. The past as future? JAMA 278:418-424. [PubMed] [Google Scholar]