Abstract
In a proteasome-lacking mutant of Streptomyces coelicolor A3(2), an intracellular enzyme with chymotrypsin-like activity, absent from the wild type, was detected. Complementation that restored proteasome function did not suppress expression of the endopeptidase. Since the enzyme was not found in two other S. coelicolor proteasome mutants, its expression probably resulted from a secondary mutation arisen in the proteasome mutant. Purification of the endopeptidase revealed its identity to SCO7095, a putative hydrolase encoded by the S. coelicolor A3(2) genome with no known homologue. Based on the prediction of a Ser-Asp-His catalytic triad and an α/β hydrolase fold, SCO7095 was assigned to peptidase clan SC. N-terminally His-tagged SCO7095 was efficiently expressed in Escherichia coli cells and purified for further characterization. Although SCO7095 is distantly related to several proline iminopeptidases, including Thermoplasma acidophilum tricorn-interacting F1, no aminopeptidase activity was detected. On synthetic substrates, the monomeric enzyme exhibited not only chymotrypsin-like activity but also thrombin-like activity.
Proteases are key players in various processes occurring in eukaryotic and prokaryotic cells. For instance, proteolysis plays a global role in the bacterial cell cycle (13). Since the cellular environment is crowded with proteins, tight control of intracellular protein breakdown is vital. This may be the result of a strict substrate specificity of the intracellular proteases degrading only few target proteins. Another control mechanism is provided by the architecture of self-compartmentalizing proteases. These multisubunit assemblies dispose of internal active sites that are not accessible to folded proteins. However, substrates can be targeted to the proteolytic chamber following interaction with protease-associated ATP-dependent “unfoldases.” Well-characterized prokaryotic proteases operating according to this principle include ClpP (34), HslV (38, 50), and the 20S proteasome (4, 49). Evolution has given rise to various combinations of these self-compartmentalizing proteases in the proteolytic complements of individual prokaryotes, with HslV and 20S proteasome apparently being mutually exclusive (8). Although ubiquitous in Archaea (27), in Bacteria the 20S proteasome is confined to the actinomycetes, a lineage of high-GC gram-positive bacteria (23, 30, 35, 42).
The actinomycete genus Streptomyces has proven a valuable resource for a wide variety of peptidases and proteases as well as potent inhibitors of such enzymes (19, 48). The complete genome sequence of Streptomyces coelicolor A3(2) has provided a comprehensive picture of the protease complement of this bacterium (5). As might be anticipated from the large size of the genome, encoding almost 8,000 proteins, this analysis has revealed a highly complex proteolytic network. In addition to 60 predicted secreted proteases and peptidases, the intracellular proteolytic system comprises the 20S proteasome (30), five ClpP-like proteins (47), several other ATP-dependent proteases (including two FtsH proteins and Lon protease [14]), two tricorn proteases (41), and more than 60 putative protein/peptide-hydrolyzing enzymes.
In this paper we report on the characterization of a novel enzyme that is part of the intracellular proteolytic machinery of S. coelicolor A3(2). This new member of the α/β hydrolase family, not annotated as a protein- or peptide-hydrolyzing enzyme but classified as a putative hydrolase (SCO7095), was identified in a S. coelicolor mutant lacking the 20S proteasome and characterized as a serine peptidase with unusual specificity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Strains and plasmids used in this study are described in Table 1. Escherichia coli strains were routinely cultured in Luria-Bertani medium at 37°C. Where appropriate, antibiotics were added to the medium as follows: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml. S. coelicolor strains were grown in CYG medium without NaCl (Casitone peptone, 10 g/liter; yeast extract, 5 g/liter; glucose, 5 g/liter) at 30°C. Antibiotics were added as follows: thiostrepton, 10 μg/ml (in liquid medium) or 50 μg/ml (in solid medium); kanamycin, 50 μg/ml. To generate spores, Streptomyces cells were sprayed on the CYG plates and incubated for 7 days at 30°C. The CYG medium was supplemented with 5 mM MgCl2 and 0.5% glycine to grow cells for the preparation of protoplasts.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 37 |
| GM119 | F−dam-3 dcm-6 metB1 lacY1 galK2 galT22 tonA31 tsx-78 supE44 mtl-1 (thi-1) | 2 |
| BL21 (DE3) | F−ompT hsdSB(rB−mB−) gal dcm (DE3) | 37 |
| Streptomyces coelicolor | ||
| A3(2) | Wild-type strain | 18 |
| FAJ2037 | Proteasome mutant of strain A3(2) with thiostrepton resistance gene tsr replacing part of the prcBA operon | This work |
| FAJ2038 | Proteasome mutant of strain A3(2) with tsr replacing part of the prcBA operon | This work |
| FAJ2053 | Proteasome mutant of strain A3(2) with tsr replacing part of the prcBA operon | This work |
| Plasmids | ||
| pET-28a | E. coli expression vector | Novagen |
| pUC18 | E. coli general cloning vector | 37 |
| pIJ486 | Streptomyces promoter-probe vector | 18 |
| pFD666 | Streptomyces shuttle cosmid vector | 18 |
| pFAJ2594 | pUC18 with the 5,128-bp KpnI fragment of S. coelicolor A3(2) carrying the prcBA operon | 30 |
| pFAJ2608 | pUC18 with the 1,055-bp BclI fragment of pIJ486 containing tsr cloned into the BamHI site | This work |
| pFAJ2727 | pFAJ2594 with the internal 1.26-kb ScaI-MluI fragment of the S. coelicolor A3(2) prcBA operon replaced with the 1.09-kb EcoRI-PstI fragment (tsr gene) of FAJ2608 | This work |
| pFAJ2761 | pFD666 with the 2,946-bp HindIII-SrfI fragment of pFAJ2594 (carrying the entire proteasome operon) inserted in the HindIII-ScaI sites | This work |
| pFAJ2773 | pUC18 with the PCR-amplified gene encoding SCO7095 cloned into the SmaI site | This work |
| pFAJ2775 | pET-28a containing the NdeI-XhoI fragment of pFAJ2773 cloned into the corresponding sites of the expression vector creating an N-terminal His6 tag | This work |
Construction of an S. coelicolor proteasome mutant.
A pUC18-based DNA replacement vector was constructed by replacing an internal fragment of the previously cloned prcBA operon (30) with a thiostrepton resistance gene. The internal 1,261-bp ScaI-MluI fragment of the prcBA operon was removed from the insert of pFAJ2594 by digestion with the corresponding restriction endonucleases (partial digestion with ScaI required due to an internal site of pUC18), and the overhanging MluI end of the plasmid remnant was filled up with the Klenow fragment of DNA polymerase. The thiostrepton resistance gene, tsr, originating from pIJ486 was isolated from pFAJ2608 by digestion with EcoRI and PstI. The purified DNA fragment (1,090 bp) was blunted and ligated to the remnant of pFAJ2594. This resulted in a plasmid, pFAJ2727, not replicating in Streptomyces cells and carrying a marker-disrupted proteasome operon. Preparation and transformation of S. coelicolor protoplasts were carried out by the method of Oh and Chater (32). Thiostrepton-resistant colonies were checked by colony PCR using primers corresponding to the 5′ end of prcB (5′-TGGAAGCCAACACTCGTAGCACCG-3′) and the 3′ end of prcA (5′-CTACTCCTCGTCCGACCCGGAGTC-3′). Putative double homologous recombinants showing only the PCR product of the size predicted for the marker-disrupted prcBA operon were selected (3 colonies of 100 screened). For these strains, the absence of the delivery vector pUC18 and the replacement of the ScaI-MluI DNA fragment of the proteasome operon with the thiostrepton resistance gene were verified by Southern hybridization on total DNA digested with StuI and EcoRI, using the digoxigenin-labeled DNAs (Roche Applied Science) of the target fragments (pUC18, ScaI-MluI fragment, or tsr gene) as probes. The absence of the 20S proteasome in the mutants was confirmed by Western blotting, using a cross-reactive polyclonal antibody raised against the Rhodococcus erythropolis 20S proteasome.
Phenotypic characterization of the S. coelicolor proteasome mutant.
The ability of cells of S. coelicolor A3(2) and the proteasome-deficient mutants to grow under different kinds of environmental stress were analyzed. Growth was assessed qualitatively on solid media since the mycelial growth characteristics and clumping did not allow a reliable quantitative analysis based on culture turbidity measurements. A 20-μl volume of spore suspension was spread on the surface of solid CYG medium, and plates were incubated at 30°C for 5 days. The conditions used were essentially those described by Knipfer and Shrader (23). The CYG medium was supplemented with ethanol (7%, vol/vol), cadmium chloride (0.1 mM), puromycin (0.1 or 0.01 mg/ml), or sodium chloride (6 or 7%, wt/vol).
To visualize the lack of proteasome activity and detect possible other changes in the intracellular protease-peptidase profile of the mutant, zymograms using different synthetic substrates were prepared for wild-type and mutant cells. Cellular extracts were obtained by a mild extraction method. Cells were cultured for 48 h in the growth medium used to prepare protoplasts, collected by centrifugation, washed twice, and resuspended in 5 ml of 25 mM Tris-HCl (pH 7.5) supplemented with 1 mg of lyzozyme per ml. The cell suspension was incubated on ice for 2 h, and 60 U of endonuclease (Sigma) was added to degrade DNA and RNA. Cell debris was removed by centrifugation (27,000 × g for 30 min) at 4°C. Cell extracts were analyzed by loading aliquots containing 30 μg of protein onto a native polyacrylamide gradient gel (4 to 20%) run as recommended by the supplier (Novex). After completion of the run, the gel was overlaid with 5 ml of 25 mM Tris-HCl (pH 7.5) supplemented with 0.1 mM of fluorogenic substrate (Bachem): Z-GGL-AMC (benzyloxycarbonyl-Gly-Gly-Leu-7-amido-4-methylcoumarin), Suc-LLVY-AMC (succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin), or Suc-AAF-AMC (succinyl-Ala-Ala-Phe-7-amido-4-methylcoumarin). After incubation for 15 min at 37°C, the peptidase activity was visualized under UV illumination.
To complement the proteasome deficiency of mutant FAJ2038, protoplasts were transformed with pFAJ2761 and then subjected to regeneration. Kanamycin-resistant transformants were selected, and expression of the proteasome was verified by zymography using Suc-LLVY-AMC as a substrate.
Partial purification of the enzyme hydrolyzing Z-GGL-AMC.
The enzyme hydrolyzing the synthetic substrate Z-GGL-AMC was partially purified by fast protein liquid chromatography. All purification steps were performed at 4°C. During the different purification steps, enzymatic activity was monitored by Z-GGL-AMC zymogram analysis. Crude cell extract was prepared from 12 g of S. coelicolor FAJ2038 cells as described above. A 20-ml volume of supernatant containing 200 mg of protein was loaded onto a Q-Sepharose column (2 by 10 cm; Amersham Biosciences) and equilibrated with 25 mM Tris-HCl (pH 7). Bound proteins were eluted using a 0 to 600 mM NaCl linear gradient in the same buffer. Active fractions eluting at approximately 200 mM NaCl were pooled and dialyzed against 50 mM Tris-HCl (pH 7.5). The dialyzed sample was loaded onto a DEAE-Sephacel column (2 by 8 cm [Amersham Biosciences]) equilibrated with the same buffer, and bound proteins were eluted with a 0 to 600 mM NaCl linear gradient. Active fractions, eluting at approximately 350 mM NaCl, were pooled and dialyzed against 10 mM potassium phosphate buffer (pH 7). The dialyzed sample was loaded onto a hydroxyapatite colum (1.4 by 6 cm [Bio-Rad]) equilibrated with the same buffer, and bound proteins were eluted with 0 to 300 mM potassium phosphate buffer (pH 7). The active fractions, eluting at approximately 180 mM phosphate, were pooled, dialyzed against 25 mM Tris-HCl (pH 7.0), and loaded onto a MonoQ column (0.5 by 5 cm [Amersham Biosciences]). Bound proteins were eluted with a gradient of 0 to 600 mM NaCl in the same buffer. The enzyme, emerging at approximately 220 mM NaCl, was concentrated by centrifugation in a Centricon-10 apparatus (Amicon) and then loaded onto a Superose 6 HR column (1 by 30 cm [Amersham Biosciences]) equilibrated with 25 mM Tris-HCl (pH 7.0). The active fractions were combined, and this sample was subjected to native gradient gel electrophoresis to locate the band associated with Z-GGL-AMC-hydrolyzing activity. SDS-polyacrylamide gel electrophoresis (PAGE) analysis of the excised band confirmed that this activity corresponded to the major protein in the active Superose 6 fractions. Following electroblotting to a polyvinylidene difluoride membrane, the major band was subjected to Edman degradation, but the protein appeared to be N-terminally blocked. Therefore, the active band from native gradient gel was subjected to in-situ digestion with trypsin. The resulting peptides were separated by high-performance liquid chromatography before being subjected to Edman degradation (42).
Cloning of the S. coelicolor gene encoding the Z-GGL-AMC-hydrolyzing enzyme SCO7095.
The amino acid sequences of five internal fragments resulting from trypsin digestion of the Z-GGL-AMC-hydrolyzing enzyme were determined: (a) DHVQDHPEVADYAATLTTR, (b) DYTVATYAHFLHAVVQHL, (c) WAALLHEGLFK, (d) AELTVLEEAGHLAHLEQPAR, and (e) TPEDPSAPLSPGTHTITVP. Since the relative positions of the internal amino acid sequences was not known, degenerate forward (F) and reverse (R) primers corresponding to the sequences (a) through (d) were designed: a.F (5′-GACCACGTKCAGGACCACCCKGAGGTKGCKGACTACGC-3′), a.R (5′-GCGTAGTCMGCMACCTCMGGGTGGTCCTGMACGTGGTC-3′), b.F (5′-GACTACACKGTKGCKACKTACGCKCACTT-3′), b.R (5′-AAGTGMGCGTAMGTMGCMACMGTGTAGTC-3′), c.F (5′-TGGGCKGCKCTKCTKCACGAGGGKCTKTTCAA-3′), c.R (5′-TTGAAMAGMCCCTCGTGMAGMAGMGCMGCCCA-3′), d.F (5′-GAGGAGGCKGGKCACCTKGCKCACCTKGAGCAGCC-3′), and d.R (5′-GGCTGCTCMAGGTGMGCMAGGTGMCCMGCCTCCTC-3′). From the primer combinations that produced DNA bands in PCR reactions using Pfu polymerase, the PCR product (604 bp) produced by primer combination b.F and d.R was selected, phosphorylated with T4 polynucleotide kinase (New England Biolabs), cloned into the SmaI site of pUC18, and verified by sequence analysis. A digoxigenin-labeled probe was synthesized by PCR on this DNA fragment and used in a Southern hybridization on total DNA of S. coelicolor digested with EcoRI, SmaI, PstI, or BglII. Based on the size estimates of the hybridizing bands, it was decided to construct a minilibrary in pUC18 containing fragments of about 5 kb from PstI-digested total DNA. The construct carrying the gene encoding the Z-GGL-AMC-hydrolyzing peptidase was isolated from this minilibrary as described previously (29), except that the library was screened by colony PCR. The DNA sequence of the PstI fragment (5,128 bp) was determined. This sequence perfectly matched a DNA sequence located on S. coelicolor A3(2) cosmid SC3A4 that was released in the course of this work through the genomic sequencing project (5). Therefore, the Z-GGL-AMC-hydrolyzing activity could be assigned to the putative hydrolase SCO7095 (= SC3A4.21c). In silico analyses of DNA and protein sequences was carried out using the Vector NTI 7 software package (InforMax). Multiple alignments were edited using GeneDoc (http://www.psc.edu/biomed/genedoc/).
Heterologous expression of SCO7095.
The gene encoding SCO7095 was synthesized by PCR with the primers 5′-GCATATGAAGCACACGCATCGGCCGGGGACTC-3′ (forward primer with added NdeI site in bold) and GCTCGAGCTATGGGTTCCGGGGTCCGCGGACGACT-3′ (reverse primer with added XhoI site in bold). The amplified fragment was cloned into the SmaI site of pUC18, and the insert of this plasmid (pFAJ2773) was verified by sequence analysis. Subsequently, the gene was cut out with NdeI and XhoI and cloned into the corresponding sites of the pET-28a vector, generating plasmid pFAJ2773. The N-terminally His6-tagged recombinant protein was expressed in E. coli BL21(DE3) and purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography (Qiagen). The enzyme preparation was checked for purity by SDS-PAGE and stored in 50 mM Tris-HCl buffer (pH 7.5) containing 10% (vol/vol) glycerol. The N-terminal amino acid sequence was determined by automated Edman degradation on a Procise 491 cLC protein sequencer (Applied Biosystems).
Biochemical characterization of SCO7095.
For the characterization of the substrate specificity of SCO7095, 0.2 or 1 μg of purified recombinant protein (depending on the level of activity) was incubated with 10 nmol of synthetic substrate in 100 μl of 50 mM Tris-HCl (pH 7.5) at 37°C for 30 min. Previously, enzyme titration experiments were conducted to assess the linear range of concentration-dependent enzyme activity. The reaction was terminated with 100 μl of 10% sodium dodecyl sulfate (SDS), and the volume was increased to 1 ml with water. Cleavage of the peptide substrates was monitored fluorometrically by release of AMC (7-amino-4 methylcoumarin).
To determine thrombin activity, a chromogenic assay with the synthetic substrate h-d-phenylalanyl-l-pipecolyl-l-arginine-p-nitroaniline hydrochloride (S-2238; Chromogenix) was used. SCO7095 at 40 μg/ml was serially diluted twofold using 50 mM Tris-HCl (pH 8.3). Then 50-μl volumes of these dilutions were added to a 96-well plate. Subsequently, 50 μl of 0.4 mM S-2238 was added, and the mixtures were incubated at 37°C. As a positive control, thrombin (from bovine plasma at 1,700 NIH U/mg [Sigma]) at 0.096 μg/ml was serially diluted twofold using 50 mM Tris-HCl (pH 8.3). Then 50-μl volumes of these dilutions were added to a 96-well plate. Subsequently, 50 μl of 0.4 mM S-2238 was added and the mixtures were incubated at 37°C. After a 30-min incubation, the absorbance at 405 nm was measured using an EL 808 Ultra microplate reader (Bio-Tek Instruments Inc).
Carboxypeptidase activity was determined spectrophotometrically using the 3-(2-furyl)acryloyl-dipeptides FA-AR, FA-GL, and FA-RL as substrates (51). Purified recombinant enzyme (2 or 8 μg/ml) was incubated at 37°C with the respective substrates (100 μM final concentration) in 0.1 mM Tris-HCl (pH 7.5). The reaction was stopped after 30 or 60 min with an equal volume of 10% SDS. Activity was measured from the decrease in absorption at 340 nm. Esterase activity was assayed by the method of Jackman et al. (17). The peptide degradation assay using oxidized insulin B-chain as a substrate was described previously (40). The protease database MEROPS (3) was used as a resource for information about the activities of proteases on synthetic substrates.
The inhibitory effect of phenylmethylsulfonyl fluoride (PMSF) and h-d-Phe-Pro-Arg-chloromethylketone (PPACK) on the activity of SCO7095 was analyzed by determining the residual activity after preincubation of the enzyme in the presence of inhibitor (40). Purified enzyme (20 ng) was incubated with different concentrations of PMSF or PPACK in 50 μl of Tris-HCl (pH 7.5) at 37°C for 15 min. Then the reaction was initiated by addition of 50 μl of 50 mM Tris-HCl containing 10 nmol of Z-PR-AMC, and the mixture was incubated at 37°C for 30 min. Reactions were terminated by addition of 100 μl of 10% SDS, and the amount of AMC released was measured.
The plasminogen activator inhibitor type 1 (PAI-1) inhibition assay was carried out as described previously (11). PAI-1 was diluted with phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]) to 6.7 μM. Both thrombin (purified from bovine plasma at 1,700 NIH U/mg [Sigma]) and SCO7095 were diluted to 13.4 μM with phosphate-buffered saline. Equal volumes of PAI-1 and either thrombin or SCO7095 were mixed and incubated at 37°C for 25 min. The reaction was terminated by adding SDS (final concentration, 1%) and heating for 30s at 100°C. Reaction products were analyzed by SDS-polyacrylamide gel electrophoresis.
The Bio-Rad protein assay, based on the method of Bradford, was used for determination of protein concentrations with bovine serum albumin as a standard. The apparent molecular weight of purified SCO7095 was determined by gel filtration on a calibrated Superdex 200 HR column (Amersham Biosciences) equilibrated with 50 mM sodium phosphate buffer (pH 7) containing 0.15 M NaCl. The average molecular mass was determined by electrospray ion trap mass spectrometry (Esquire-LC-MS; Bruker Daltonics) and calculated from the results of four experiments with an average of at least 2,000 scans per experiment.
RESULTS AND DISCUSSION
Construction and phenotypic characterization of proteasome-lacking S. coelicolor mutants.
Proteasome knockout mutants were isolated by exchanging an internal fragment of the prcBA operon, encoding the β and α subunits of the 20S proteasome, with a thiostrepton resistance marker through double homologous recombination. Three such mutants (FAJ2037, FAJ2038, and FAJ2053) with the predicted gene disruption were obtained. The absence of the 20S proteasome was confirmed by zymography (using Suc-LLVY-AMC as a substrate) and by Western analysis. Growth of the mutants on rich or minimal medium at 37 and 42°C was not impaired. In addition, the mutants could not be distinguished from the wild-type strain based on sensitivity to ethanol (organic solvent stress), cadmium ions (heavy metal stress), puromycin (generating misfolded puromycyl-containing peptides), or high salt concentration (osmotic stress). The lack of an obvious growth- or stress-related phenotype was previously reported for a proteasome mutant of Mycobacterium smegmatis (23). In the archaeon Thermoplasma acidophilum, specific inhibition of proteasome activity impaired cell viability following heat shock but not under normal growth conditions (36). In E. coli, several proteases, including the self-compartmentalizing protease ClpP, are involved in the response to conditions that cause damage to cellular proteins (52) or when foreign proteins are overexpressed (9). The ClpP-encoding operons clpP1 clpP2 and clpP3 clpP4 of Streptomyces lividans, however, are not inducible by heat shock (47). Likewise, the expression of proteasome genes in R. erythropolis is constitutive and not enhanced by heat shock (I. Nagy and R. De Mot, unpublished data). At present, no specific function has been attributed to these self-compartmentalizing ATP-dependent proteases in actinomycetes.
The observation that the 20S proteasome is dispensable under a variety of growth conditions indicates that this system is dedicated to some, as yet unidentified, specialized function(s). It has been suggested that the actinomycetes may have expanded their proteolytic machinery, after separation from other gram-positive bacteria, by lateral gene transfer of proteasome genes from a eukaryotic organism (7, 25). Proteasomes have been identified in diverse representatives of the order Actinomycetales (8): Frankia (suborder Frankineae), Mycobacterium and Rhodococcus (suborder Corynebacterineae), and Streptomyces (suborder Streptomycineae). Through analysis of data generated by (ongoing) genomic sequencing efforts, we can add Thermobifida (suborder Streptosporangineae) to this list (draft genome analysis of Thermobifida fusca by the DOE Joint Genome Institute: http://www.jgi.doe.gov/JGI_microbial/html/thermobifida/thermob_homepage.html). However, this survey also revealed two notable exceptions. No homologues of the proteasome genes are found in the genomes of two Corynebacterium species, namely, C. diphtheriae (complete genome sequence by the Sanger Institute: http://www.sanger.ac.uk/Projects/C_diphtheriae/) and C. glutamicum (33), although they belong to a family (Corynebacteriaceae) that is phylogenetically close to the proteasome-containing Mycobacteriaceae and Nocardiaceae families (39). This suggests that the proteasome genes have been lost from Corynebacterium, which is in line with the observation that the actinomycete 20S proteasome fulfills no essential cellular function(s).
Identification of the putative hydrolase SCO7095 from S. coelicolor as a novel endopeptidase of the α/β hydrolase family.
For one of the proteasome-minus mutants, FAJ2038, an additional band was detected in the zymogram using Z-GGL-AMC. This substrate for chymotrypsin-like activity is a poor substrate for the S. coelicolor 20S proteasome (only 5% of the activity on Suc-LLVY-AMC, another chymotrypsin-like substrate [30]). When plasmid pFD666 carrying the proteasome operon (pFAJ2761) was introduced in FAJ2038, proteasome expression was restored but expression of the peptidase hydrolyzing Z-GGL-AMC was not repressed. This lack of phenotypic complementation and the fact that the peptidase was absent from the other mutants indicate that its appearance in mutant FAJ2038 was probably not due to the lack of proteasome but may have been caused by secondary mutation.
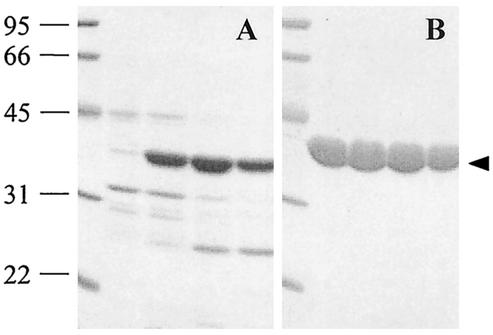
The enzyme with Z-GGL-AMC-hydrolyzing activity was partially purified from mutant FAJ2038 cell extracts by a combination of chromatographic steps (Fig. 1A). The major band in this sample displayed the Z-GGL-AMC hydrolytic activity. Several internal amino acid sequences were determined following tryptic digestion of enzyme further purified by native gradient gel electrophoresis. Based on these sequences, degenerate primers were designed that allowed amplification of the corresponding gene and, using this as a probe, isolation of a genomic clone containing the entire gene and flanking sequences. The DNA sequence was confirmed on release of the entire sequence of S. coelicolor A3(2) cosmid SC3A4 (GenBank accession number AL354616). The enzyme could thus unequivocally be identified as SCO7095 (= SC3A4.21c), annotated as a putative hydrolase (5).
FIG. 1.
SDS-PAGE analysis of active fractions of the partially purified native enzyme after Superose 6 HR column chromatography (A) and recombinant His-tagged SCO7095 after Ni-NTA affinity chromatography (B). The solid arrowhead indicates the position of the protein displaying Z-GGL-AMC-hydrolyzing activity in the native gradient gel. The molecular mass of the marker proteins is indicated in kilodaltons.
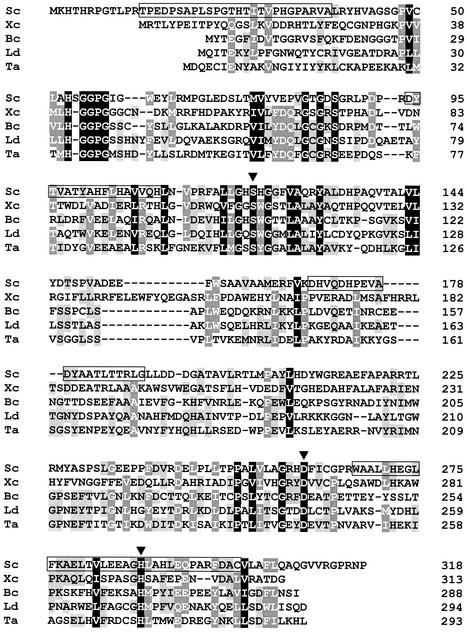
Database searches (including the National Center for Biotechnology Information unfinished microbial genomes; version 30 August 2002; http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi) revealed no homologue for SCO7095. The absence of such enzyme from proteasome-containing actinomycetes makes it rather unlikely that SCO7095 would have a function directly related to the proteasome. The current picture of the distribution pattern of SCO7095 among actinomycetes, however, matches the one of tricorn protease, both presently being reported only for S. coelicolor (41). Between residues 73 and 306, the protein contains the Pfam motif PF00561, characteristic for members of the α/β hydrolase family (15, 31). Borderline homology (<30% amino acid sequence identity) was detected with several (putative) members of this family. The best matches were with putative α/β hydrolases revealed by genomic sequencing of Sinorhizobium meliloti (SMc04033; 28% identity in a 276-residue overlap) and of Mycobacterium tuberculosis (Rv0840c; 26% identity in a 295-residue overlap). Both deduced gene products are related to several bacterial proline iminopeptidases (about 35% identity). Proline iminopeptidase hydrolyses peptide substrates containing proline at the amino terminus, releasing free proline. In Fig. 2, the SCO7095 sequence is aligned with those of some functionally characterized α/β hydrolase family members with peptidase activity. These enzymes, originating from low-GC gram-positive bacteria (Bacillus and Lactobacillus), gram-negative bacteria (Xanthomonas), and Archaea (Thermoplasma), are also proline iminopeptidases (EC 3.4.11.5). Despite the low overall homology of these proteins (∼30% amino acid similarity), the regions containing the residues of the catalytic triad Ser-Asp-His aligned well (Fig. 2). Considerable sequence divergence was apparent in the central part of SCO7095 (residues 150 to 230).
FIG. 2.
Multiple alignment of SCO7095 from S. coelicolor (Sc) with proline iminopeptidases from Xanthomonas campestris pv. citri (Xc) (1), Bacillus coagulans (Bc) (20), Lactobacillus delbrueckii subsp. lactis (Lc) (22), and tricorn-interacting factor 1 (F1) from Thermoplasma acidophilum (Ta) (44). The level of sequence conservation is indicated by differential shading. Percent sequence identity and similarity for SCO7095 were 20 and 33% (X. campestris), 17 and 30% (B. coagulans), 15 and 33% (L. delbrueckii), and 13 and 27% (T. acidophilum). The residues (Ser122-Asp260-His288) of the predicted catalytic triad in SCO7095 are labeled with solid arrowheads. Residues confirmed by amino acid sequencing are boxed.
In the aminopeptidase from Xanthomonas campestris pv. citri (28) and the tricorn-interacting aminopeptidase F1 from Thermoplasma acidophilum (12), the corresponding segment constitutes a separate “upper” domain, capping the “lower” α/β hydrolase domain. The respective active sites of this enzyme, containing the catalytic triad, are located at the interface between the two domains. The upper domain plays a major role in determining enzymatic activity and specificity of the hydrolase (12, 28). Mutational analysis has confirmed the requirement of a conserved serine residue, acting as the active-site nucleophile, in the aminopeptidases from Bacillus coagulans (21) and T. acidophilum (44). The latter enzyme displayed broad substrate specificity as an aminopeptidase (44). Based on the conserved catalytic triad Ser-Asp-His (Fig. 2) and predicted α/β hydrolase fold in SCO7095, this S. coelicolor enzyme can be assigned to clan SC of serine peptidases (24).
Biochemical characterization of SCO7095.
To facilitate the further characterization of purified SCO7095, the recombinant His-tagged protein was overexpressed in E. coli and purified by Ni-NTA affinity chromatography (Fig. 1B). The mass of the protein was determined by electrospray ion trap mass spectrometry to be 36,733.55 Da, corresponding to the His-tagged protein lacking the N-terminal methionine (calculated molecular mass of 36,733.60 Da). This was confirmed by N-terminal sequencing, yielding the sequence of the histidine tag with methionine cleaved off (GSSHHHHHH). On SDS-PAGE, the protein migrated as a single band with Mr = 35,300. The size estimated by gel filtration yielded a Mr of 34,500. This indicates that the enzyme was purified as a monomer, as reported for the Thermoplasma F1 enzyme (44) and the Xanthomonas aminopeptidase (28).
The activity of SCO7095 on several peptidyl-AMC substrates was analyzed (Table 2). Although the enzyme is distantly related to several proline iminopeptidases, no such activity (with H-P-AMC) or other aminopeptidase activity (with H-X-AMC, where X is a glycine, leucine, valine, arginine, glutamate, phenylalanine, or tyrosine) was detected for SCO7095. Lack of hydrolysis of several N-protected substrates with a P1 proline (Z-GP-AMC, Suc-GP-AMC, or Suc-GPLGP-AMC) indicated that SCO7095 has no prolyl-endopeptidase activity. The divergent central part (Fig. 2), which probably folds into a separate “cap” domain (12, 28), most probably plays a major role in determining the catalytic activity, which is quite different from that of the distantly related proline iminopeptidases.
TABLE 2.
Hydrolysis of synthetic substrates by purified recombinant SCO7095
| Substratea | Sp act (nmol/min/mg of protein)b |
|---|---|
| H-R-AMC | NDc |
| H-AR-AMC | 0.3 |
| H-PR-AMC | 1.1 |
| Z-R-AMC | 1.6 |
| Z-PR-AMC | 24.0 |
| Boc-d(Obzl)PR-AMC | ND |
| Z-RR-AMC | 0.4 |
| Z-ARR-AMC | ND |
| Z-GGR-AMC | ND |
| Z-GGL-AMC | 10.6 |
| Z-GPR-AMC | 3.2 |
| Boc-VPR-AMC | 8.4 |
| Boc-LRR-AMC | 1.4 |
| Boc-VGR-AMC | ND |
| Bz-VGR-AMC | ND |
| H-F-AMC | ND |
| H-AAF-AMC | 5.9 |
| Suc-AAF-AMC | ND |
| Suc-AAV-AMC | ND |
| Suc-AAA-AMC | ND |
| H-Y-AMC | ND |
| Suc-LY-AMC | 4.6 |
| Suc-LLVY-AMC | 0.7 |
Boc, t-butyloxycarbonyl; Bz, benzoyl; Suc, succinyl; Obzl, benzyl ester; Z, benzyloxycarbonyl. For each of the substrates, the concentration was 100 μM.
The values represent the mean of three independent determinations.
ND, no activity was detected. This was also the case for the substrates H-E-AMC, H-G-AMC, H-L-AMC, H-V-AMC, H-P-AMC, Z-GP-AMC, Suc-GP-AMC, and Suc-GPLGP-AMC.
On Z-GGL-AMC, the enzyme had the highest activity at pH 7.9, retaining 90% of this activity at pH 8.5 and pH 7.2. Only 10% of this activity remained at pH 5.5 (data not shown). Chymotrypsin-like activity, inferred from cleavage of Z-GGL-AMC (100% relative activity), was confirmed by the ability to hydrolyze H-AAF-AMC (56%), Suc-LY-AMC (43%), and Suc-LLVY-AMC (7%), albeit at a reduced rate. Changing the hydrophobic P1 residue into a positively charged amino acid (Z-GGL → Z-GGR) or introducing a negatively charged N-terminal blocking group (H-AAF → Suc-AAF) abolished activity. Hydrolysis of the chymotrypsin-like tripeptide substrates Z-GGL-AMC and H-AAF-AMC has also been reported for the distantly related nonspecific aminopeptidase F1 from T. acidophilum (43, 44). This enzyme also showed little or no activity on Z-GGR-AMC and Suc-AAF-AMC.
The highest endopeptidase activity was found with Z-PR-AMC (226%). Several other N-protected substrates with a P1 arginine (Z-R-AMC, Z-RR-AMC, and Boc-LRR) were hydrolyzed at a low rate (15% or less compared to Z-GGL-AMC). Conversely, no activity was measured with Z-ARR-AMC, Z-GGR-AMC, and Boc-VGR-AMC. The presence of a free amino terminus in the Pro-Arg dipeptide strongly affected the activity of the enzyme (>20-fold reduction). Apparently, the presence of an amino-terminally substituted proline at position P2 promotes the endopeptidase activity of SCO7095. The importance of the nature of the P3 residue is apparent from the relatively high activities with Boc-VPR-AMC (79%), and Z-GPR-AMC (30%) and the lack of detectable activity with Boc-D(OBzl)PR-AMC (Table 2).
The postarginine cleavage of the X-P-R-Y motif is reminiscent of the activity of thrombin on polypeptides in the coagulation cascade (46). According to the MEROPS database, post-VPR cleavage is known for thrombin (acting on coagulation factor XIII) and for activated coagulation factor C from the horseshoe crab but is not documented for a prokaryotic enzyme. Unlike SCO7095, these coagulation-active serine endopeptidases have a His-Asp-Ser triad and belong to a different clan (24). Toward S-2238 (h-d-phenylalanyl-l-pipecolyl-l-arginine-p-nitroaniline hydrochloride), a thrombin-specific chromogenic substrate (26), SCO7095 revealed only low activity (0.01% of that of thrombin). The enzyme was unable to degrade oxidized insulin B-chain that is a substrate for tricorn protease from S. coelicolor (41).
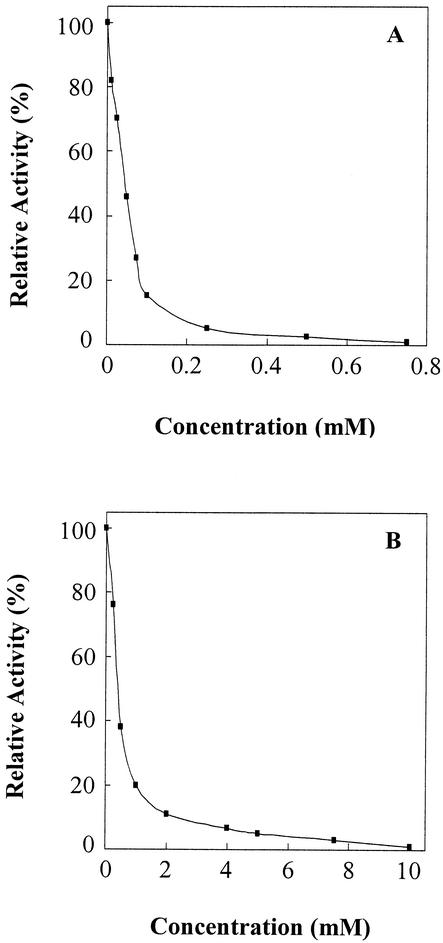
The activity of SCO7095 on Z-PR-AMC was inhibited by PMSF (Fig. 3A), consistent with our prediction that SCO7095 is most probably a serine hydrolase. Such inhibition was also observed with Z-GGL-AMC as a substrate (data not shown). Inhibition by PMSF was also reported for aminopeptidase F1 from Thermoplasma (12). Z-PR-AMC hydrolysis was also inhibited in a dose-dependent fashion by the irreversible thrombin inhibitor PPACK (6), but the concentration required to attain a comparable level of inactivation was about 10-fold higher than for PMSF (Fig. 3B). A similar inhibition curve was obtained with Boc-VPR-AMC as a substrate (data not shown). The Streptomyces enzyme was not able to form a complex with PAI-1. As a member of the serpin (serine protease inhibitor) family, PAI-1 inhibits certain serine proteases, such as thrombin, by forming a covalent 1:1 complex, resulting from the formation of an ester bond between the P1 residue (i.e., Arg346 in PAI-1) and the active-site serine of the protease (10).
FIG. 3.
Inhibition of SCO7095 activity on Z-PR-AMC by the serine hydrolase inhibitor PMSF (A) and by the thrombin inhibitor PPACK (B). Residual activity was determined after a 15-min preincubation of the enzyme with different concentrations of inhibitor.
Using benzoyl-tyrosine ethyl ester as a substrate, esterase activity was demonstrated for SCO7095 (data not shown). Ester hydrolysis by an endopeptidase is not an unusual feature, since the catalytic triad in α/β hydrolases, comprising a nucleophile, an acidic residue, and a conserved histidine, enables different types of reactions, including carboxyl ester hydrolysis and peptide hydrolysis (16, 45). No carboxypeptidase activity was detectable with three different N-blocked furylacryloyl dipeptides that have been used as substrates for carboxypeptidases B, U, N, or Y.
Although SCO7095 displays thrombin-like activity on some synthetic substrates, the poor inhibition by PPACK and the lack of inhibition by PAI-1 indicate that the catalytic centers of the two enzymes are quite different. At present, the physiological function(s) of SCO7095 in S. coelicolor remains obscure. Conditions inducing its expression and natural substrate(s) need to be identified. Valuable information may be obtained by comparing genome-wide expression profiles of the wild-type strain and a SCO7095-lacking mutant under various conditions.
Acknowledgments
I.N. and T.B. contributed equally to this work.
This work was supported by a grant (Onderzoeksproject G.0369.98N to R.D.M.) from the Fund for Scientific Research (F.W.O-Vlaanderen). T.B. acknowledges a Visiting Postdoctoral Fellowship from this organization. A.G. and P.P. are postdoctoral fellows of the F.W.O.-Vlaanderen.
M.-N. Pouch (Department of Molecular Structural Biology, Max-Planck-Institute of Biochemistry, Martinsried, Germany) kindly provided proteasome antibodies.
REFERENCES
- 1.Alonso, J., and J. L. Garcia. 1996. Proline iminopeptidase gene from Xanthomonas campestris pv. citri. Microbiology 142:2951-2957. [DOI] [PubMed] [Google Scholar]
- 2.Arraj, J. A., and M. G. Marinus. 1983. Phenotypic reversal in dam mutants of Escherichia coli K-12 by a recombinant plasmid containing the dam+ gene. J. Bacteriol. 153:562-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, A. J., N. D. Rawlings, and E. A. O'Brien. 2001. The MEROPS database as a protease information system. J. Struct. Biol. 134:95-102. [DOI] [PubMed] [Google Scholar]
- 4.Baumeister, W., J. Walz, F. Zühl, and E. Seemüller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367-380. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, S.D., K. F. Chater, A.-M. Cerdeño-Tárraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C.-H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M.-A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 6.Bode, W., D. Turk, and A. Karshikov. 1992. The refined 1.9-Å X-ray crystal structure of d-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1:426-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouzat, J. L., L. K. McNeil, H. M. Robertson, L. F. Solter, J. E. Nixon, J. E. Beever, H. R. Gaskins, G. Olsen, S. Subramaniam, M. L. Sogin, and H. A. Lewin. 2000. Phylogenomic analysis of the α proteasome gene family from early-diverging eukaryotes. J. Mol. Evol. 51:532-543. [DOI] [PubMed] [Google Scholar]
- 8.De Mot, R., I. Nagy, J. Walz, and W. Baumeister. 1999. Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends Microbiol. 7:88-92. [DOI] [PubMed] [Google Scholar]
- 9.Gill, R. T., J. J. Valdes, and W. E. Bentley. 2000. A comparative study of global stress gene regulation in response to overexpression of recombinant proteins in Escherichia coli. Metab. Eng. 2:178-189. [DOI] [PubMed] [Google Scholar]
- 10.Gils, A., and P. J. Declerck. 1998. Structure-function relationships in serpins: current concepts and controversies. Thromb. Haemostasis 80:531-541. [PubMed] [Google Scholar]
- 11.Gils, A., I. Knockaert, and P. J. Declerck. 1996. Substrate behavior of plasminogen activator inhibitor-1 is not associated with a lack of insertion of the reactive site loop. Biochemistry 35:7474-7481. [DOI] [PubMed] [Google Scholar]
- 12.Goettig, P., M. Groll, J.-S. Kim, R. Huber, and H. Brandstetter. 2002. Structures of the tricorn-interacting aminopeptidase F1 with different ligands explain its catalytic mechanism. EMBO J. 21:5343-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grünenfelder, B., G. Rummel, J. Vohradsky, D. Röder, H. Langen, and U. Jenal. 2001. Proteomic analysis of the bacterial cell cycle. Proc. Natl. Acad. Sci. USA 98:4681-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guglielmi, G., P. Mazodier, C. J. Thompson, and J. Davies. 1991. A survey of the heat shock response in four Streptomyces species reveals two groEL-like genes and three groEL-like proteins in Streptomyces albus. J. Bacteriol. 173:7374-7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heikinheimo, P., A. Goldman, C. Jeffries, and D. L. Ollis. 1999. Of barn owls and bankers: a lush variety of α/β hydrolases. Struct. Fold. Des. 7:R141-R146. [DOI] [PubMed] [Google Scholar]
- 16.Huang, Y.-T., Y.-C. Liaw, V. Y. Gorbatyuk, and T.-H. Huang. 2001. Backbone dynamics of Escherichia coli thioesterase/protease I: evidence of a flexible active-site environment for a serine protease. J. Mol. Biol. 307:1075-1090. [DOI] [PubMed] [Google Scholar]
- 17.Jackman, H. L., F. Tan, H. Tamei, C. Beurling-Harbury, X.-Y. Li, R. A. Skidgel, and E. G. Erdos. 1990. A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal “protective protein”. J. Biol. Chem. 265:11265-11272. [PubMed] [Google Scholar]
- 18.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, England.
- 19.Kisselev, A. F., and A. L. Goldberg. 2001. Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8:739-758. [DOI] [PubMed] [Google Scholar]
- 20.Kitazono, A., T. Yoshimoto, and D. Tsuru. 1992. Cloning, sequencing, and high expression of the proline iminopeptidase gene from Bacillus coagulans. J. Bacteriol. 174:7919-7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitazono, A., K. Ito, and T. Yoshimoto. 1994. Prolyl aminopeptidase is not a sulfhydryl enzyme: identification of the active serine residue by site-directed mutagenesis. J. Biochem. 116:943-945. [DOI] [PubMed] [Google Scholar]
- 22.Klein, J. R., U. Schmidt, and R. Plapp. 1994. Cloning, heterologous expression, and sequencing of a novel proline iminopeptidase gene, pepI, from Lactobacillus delbrueckii subsp. lactis DSM 7290. Microbiology 140:1133-1139. [DOI] [PubMed] [Google Scholar]
- 23.Knipfer, N., and T. E. Shrader. 1997. Inactivation of the 20S proteasome in Mycobacterium smegmatis. Mol. Microbiol. 25:375-383. [DOI] [PubMed] [Google Scholar]
- 24.Krem, M. M., and E. Di Cera. 2001. Molecular markers of serine protease evolution. EMBO J. 20:3036-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupas, A., F. Zühl, T. Tamura, S. Wolf, I. Nagy, R. De Mot, and W. Baumeister. 1997. Eubacterial proteasomes. Mol. Biol. Rep. 24:125-131. [DOI] [PubMed] [Google Scholar]
- 26.Mattler, L. E., and N. U. Bang. 1977. Serine protease specificity for peptide chromogenic substrates. Thromb. Haemostasis 38:776-792. [PubMed] [Google Scholar]
- 27.Maupin-Furlow, J. A., S. J. Kaczowka, M. S. Ou, and H. L. Wilson. 2001. Archaeal proteasomes: proteolytic nanocompartments of the cell. Adv. Appl. Microbiol. 50:279-338. [DOI] [PubMed] [Google Scholar]
- 28.Medrano, F. J., J. Alonso, J. L. Garcia, A. Romero, W. Bode, and F. X. Gomis-Ruth. 1998. Structure of proline iminopeptidase from Xanthomonas campestris pv. citri: a prototype for the prolyl oligopeptidase family. EMBO J. 17:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy, I., G. Schoofs, F. Compernolle, P. Proost, J. Vanderleyden, and R. De Mot. 1995. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J. Bacteriol. 177:676-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy, I., T. Tamura, J. Vanderleyden, W. Baumeister, and R. De Mot. 1998. The 20S proteasome of Streptomyces coelicolor. J. Bacteriol. 180:5448-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardini, M., and B. Dijkstra. 1999. α/β hydrolase fold enzymes: the family keeps growing. Curr. Opin. Struct. Biol. 9:732-737. [DOI] [PubMed] [Google Scholar]
- 32.Oh, S. H., and K. F. Chater. 1997. Denaturation of circular or linear DNA facilitates targeted integrative transformation of Streptomyces coelicolor A3(2): possible relevance to other organisms. J. Bacteriol. 179:122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi, J., S. Mitsuhashi, M. Hayashi, S. Ando, H. Yokoi, K. Ochiai and M. Ikeda. 2002. A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl. Microbiol. Biotechnol. 58:217-223. [DOI] [PubMed] [Google Scholar]
- 34.Porankiewicz, J., J. Wang, and A. K. Clarke. 1999. New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol. Microbiol. 32:449-458. [DOI] [PubMed] [Google Scholar]
- 35.Pouch, M.-N., B. Cournoyer, and W. Baumeister. 2000. Characterization of the 20S proteasome from the actinomycete Frankia. Mol. Microbiol. 35:368-377. [DOI] [PubMed] [Google Scholar]
- 36.Ruepp, A., C. Eckerskorn, M. Bogyo, and W. Baumeister. 1998. Proteasome function is dispensable under normal but not under heat shock conditions in Thermoplasma acidophilum. FEBS Lett. 425:87-90. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., and D. W. Russell. 2001. Molecular cloning. A laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sousa, M. C., C. B. Trame, H. Tsuruta, S. M. Wilbanks, V. S. Reddy, and D. B. McKay. 2000. Crystal and solution structure of an HslUV protease-chaperone complex. Cell 103:633-643. [DOI] [PubMed] [Google Scholar]
- 39.Stackebrandt, E., F. A. Rainey, and N. L. Ward-Rainey. 1997. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int. J. Syst. Bacteriol. 47:479-491. [Google Scholar]
- 40.Tamura, N., F. Lottspeich, W. Baumeister, and T. Tamura. 1998. The role of tricorn protease and its aminopeptidase-interacting factors in cellular protein degradation. Cell 95:637-648. [DOI] [PubMed] [Google Scholar]
- 41.Tamura, N., G. Pfeifer, W. Baumeister, and T. Tamura. 2001. Tricorn protease in bacteria: characterization of the enzyme from Streptomyces coelicolor. Biol. Chem. 382:449-458. [DOI] [PubMed] [Google Scholar]
- 42.Tamura, T., I. Nagy, A. Lupas, F. Lottspeich, Z. Cejka, G. Schoofs, K. Tanaka, R. De Mot, and W. Baumeister. 1995. The first characterization of a eubacterial proteasome: the 20S complex of Rhodococcus. Curr. Biol. 5:766-774. [DOI] [PubMed] [Google Scholar]
- 43.Tamura, T., N. Tamura, Z. Cejka, R. Hegerl, F. Lottspeich, and W. Baumeister. 1996. Tricorn protease—the core of a modular proteolytic system. Science 274:1385-1389. [DOI] [PubMed] [Google Scholar]
- 44.Tamura, T., N. Tamura, F. Lottspeich, and W. Baumeister. 1996. Tricorn protease (TRI) interacting factor 1 from Thermoplasma acidophilum is a proline iminopeptidase. FEBS Lett. 398:101-105. [DOI] [PubMed] [Google Scholar]
- 45.Todd, A. E., C. A. Orengo, and J. A. Thornton. 2001. Evolution of function in protein superfamilies, from a structural perspective. J. Mol. Biol. 307:1113-1143. [DOI] [PubMed] [Google Scholar]
- 46.Vanhoof, G., F. Goossens, I. De Meester, D. Hendriks, and S. Scharpé. 1995. Proline motifs in peptides and their biological processing. FASEB J. 9:736-744. [PubMed] [Google Scholar]
- 47.Viala, J., G. Rapoport, and P. Mazodier. 2000. The clpP multigenic family in Streptomyces lividans: conditional expression of the clpP3 clpP4 operon is controlled by PopR, a novel transcriptional activator. Mol. Microbiol. 38:602-612. [DOI] [PubMed] [Google Scholar]
- 48.Vitale, L. 1999. Aminopeptidases of the genus Streptomyces. Food Technol. Biotechnol. 37:29-37. [Google Scholar]
- 49.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68:1015-1068. [DOI] [PubMed] [Google Scholar]
- 50.Wang, J., J. J. Song, M. C. Franklin, S. Kamtekar, Y. J. Im, S. H. Rho, I. S. Seong, C. S. Lee, C. H. Chung, and S. H. Eom. 2001. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9:177-184. [DOI] [PubMed] [Google Scholar]
- 51.Wei, S., S. Segura, J. Vendrell, F. X. Aviles, E. Lanoue, R. Day, Y. Feng, and L. D. Fricker. 2002. Identification and characterization of three members of the human metallocarboxypeptidase gene family. J. Biol. Chem. 277:14954-14964. [DOI] [PubMed] [Google Scholar]
- 52.Wickner, S., M. R. Maurizi, and S. Gottesman. 1999. Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888-1893. [DOI] [PubMed] [Google Scholar]