Abstract
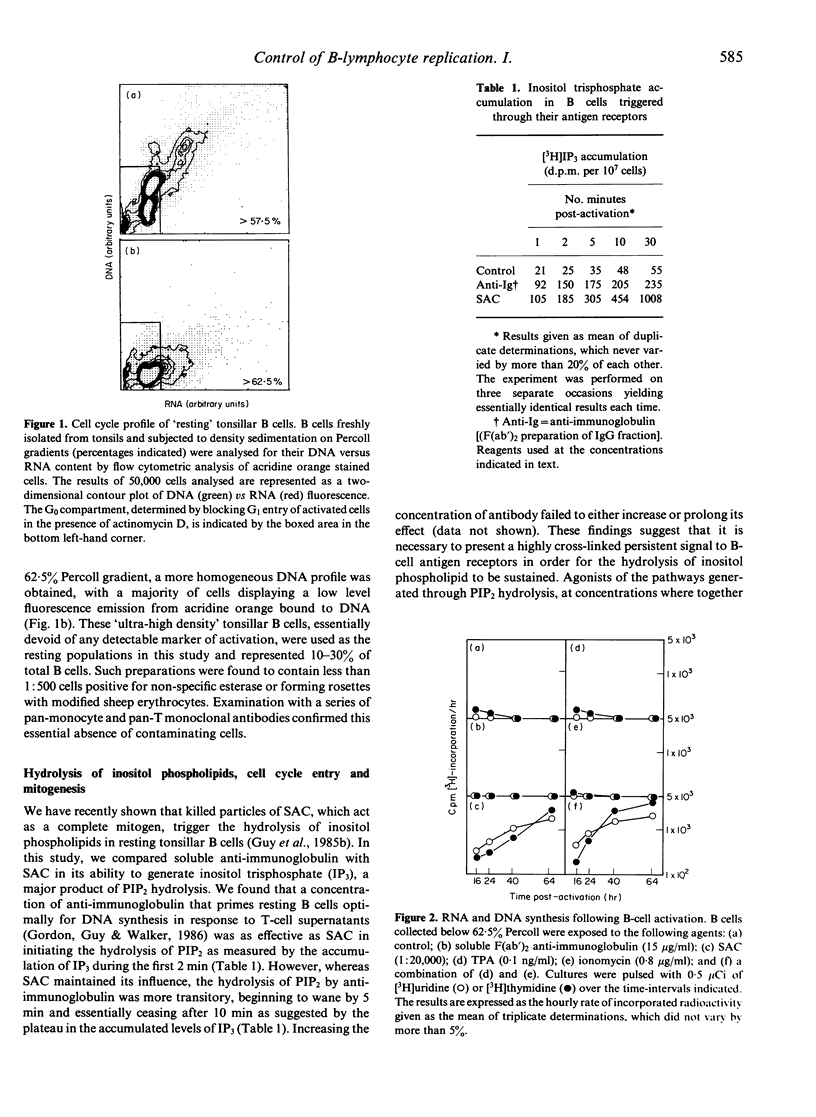
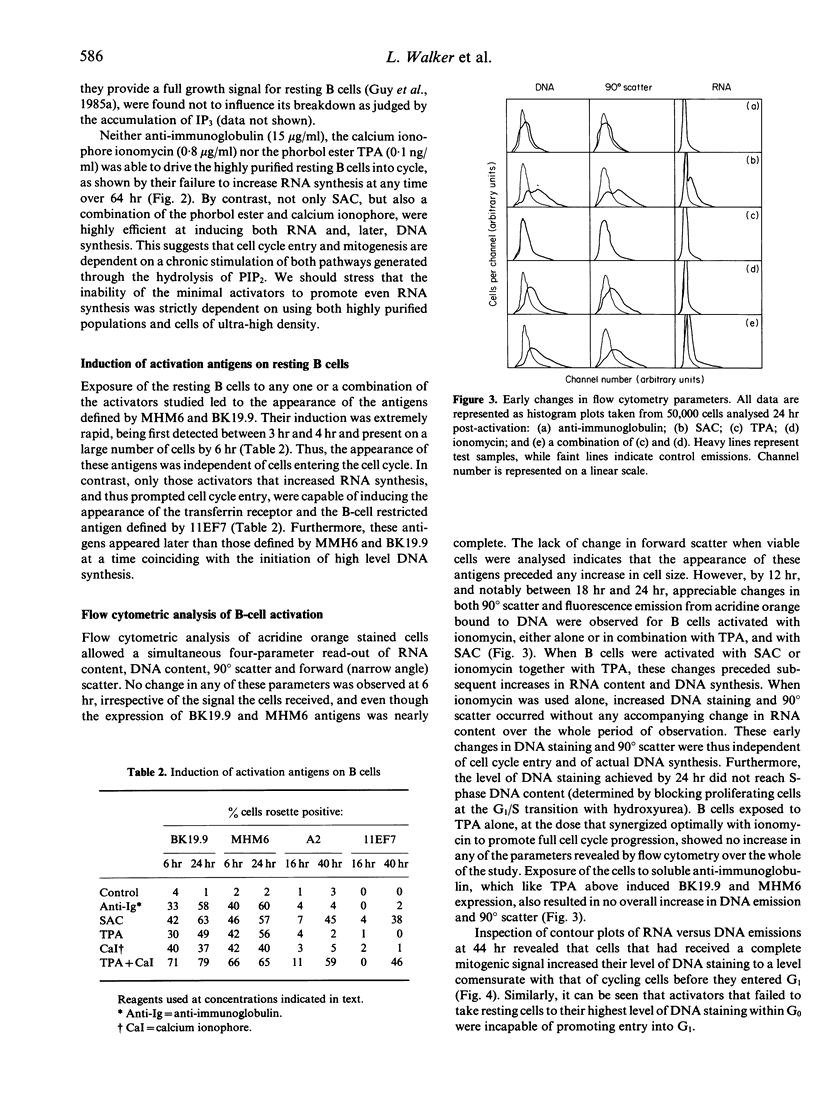
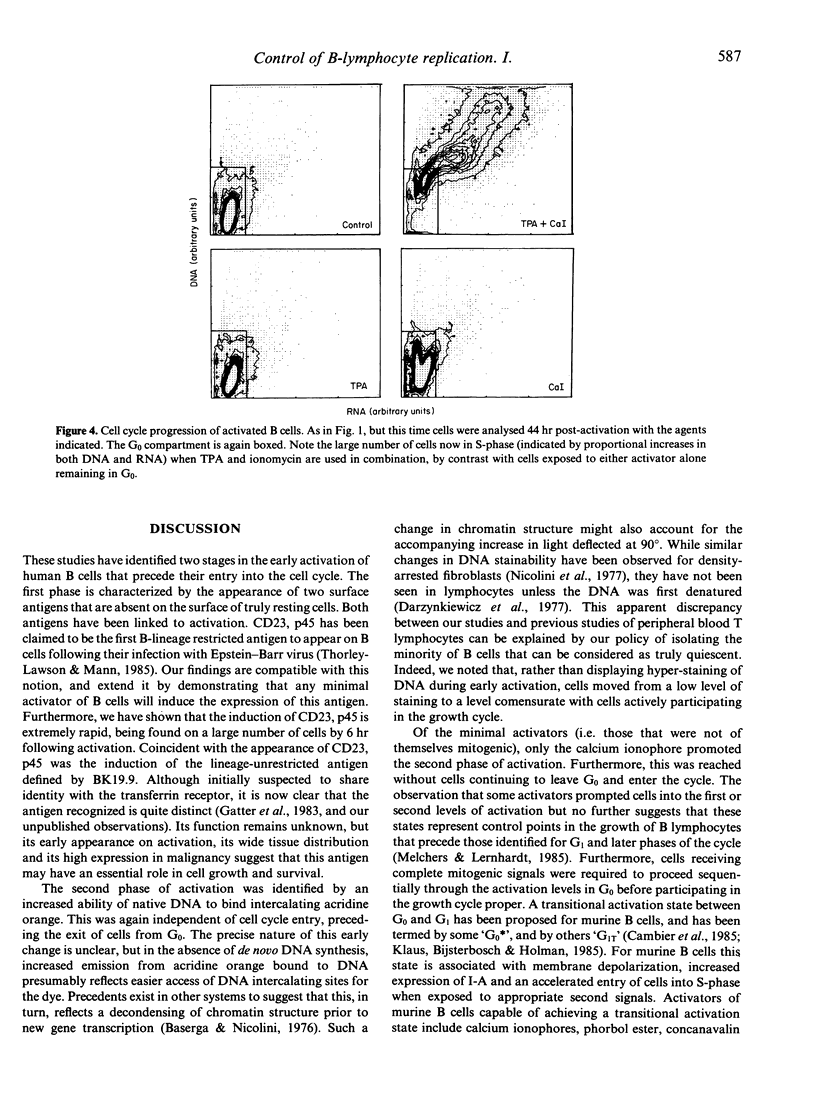
Tonsillar B lymphocytes of a particularly high buoyant density were prepared essentially free of contaminating monocytes and T cells. When exposed to anti-immunoglobulin, such cells initiated the hydrolysis of inositol phospholipids. This provides a postulated 'dual signal' for growth through the liberation of intracellular calcium stores and the activation of protein kinase C. Nevertheless, neither anti-immunoglobulin nor direct agonists of this bifurcating pathway (respectively, calcium ionophore and the phorbol ester TPA) were capable, when used alone, of driving cells out of G0 and into RNA synthesis. All three agents did, however, induce two activation antigens at the surface of G0 B cells, which included CD23, p45 and a lineage-unrestricted antigen identified by the monoclonal antibody BK.19.9. Cells that had been exposed to calcium ionophore, but not those activated with either TPA or anti-immunoglobulin, revealed further change indicated by an increased accessibility of their native DNA for the intercalating dye acridine orange. Cells receiving full mitogenic signals in the form of Staphylococcus aureus Cowan Strain I (SAC) or a combination of TPA and calcium ionophore showed the same initial sequelae but continued to enter the cell cycle and progress through to DNA synthesis. The observations identify two phases in the early activation of human B cells, both in terms of various temporal events, and the signals required to promote each activation state. before entering the proliferative cycle. Thus, the exit of human B cells from G0 appears subject to multiple controls that precede those associated with G1 and later phases of the cell cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Gordon J., Lewin N., Nordström M., Ehlin-Henriksson B., Klein G., Carstensson A. Surface marker characterization of EBV target cells in normal blood and tonsil B lymphocyte populations. J Immunol. 1985 Oct;135(4):2362–2367. [PubMed] [Google Scholar]
- Baserga R., Nicolini C. Chromatin structure and function in proliferating cells. Biochim Biophys Acta. 1976 Apr 30;458(1):109–134. doi: 10.1016/0304-419x(76)90016-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Clevers H. C., Versteegen J. M., Logtenberg T., Gmelig-Meyling F. H., Ballieux R. E. Synergistic action of A23187 and phorbol ester on human B cell activation. J Immunol. 1985 Dec;135(6):3827–3830. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Sharpless T., Staiano-Coico L., Melamed M. R. Subcompartments of the G1 phase of cell cycle detected by flow cytometry. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6696–6699. doi: 10.1073/pnas.77.11.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T. K., Melamed M. R. Cell cycle-related changes in nuclear chromatin of stimulated lymphocytes as measured by flow cytometry. Cancer Res. 1977 Dec;37(12):4635–4640. [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Guy G., Walker L. Autocrine models of B-lymphocyte growth. I. Role of cell contact and soluble factors in T-independent B-cell responses. Immunology. 1985 Oct;56(2):329–335. [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Guy G., Walker L. Autocrine models of B-lymphocyte growth. II. Interleukin-1 supports the proliferation of transformed lymphoblasts but not the stimulation of resting B cells triggered through their receptors for antigen. Immunology. 1986 Mar;57(3):419–423. [PMC free article] [PubMed] [Google Scholar]
- Grupp S. A., Harmony J. A. Increased phosphatidylinositol metabolism is an important but not an obligatory early event in B lymphocyte activation. J Immunol. 1985 Jun;134(6):4087–4094. [PubMed] [Google Scholar]
- Guy G. R., Bunce C. M., Gordon J., Michell R. H., Brown G. A combination of calcium ionophore and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) stimulates the growth of purified resting B cells. Scand J Immunol. 1985 Nov;22(5):591–596. doi: 10.1111/j.1365-3083.1985.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Gordon J., Michell R. H., Brown G. Synergism between diacylglycerols and calcium ionophore in the induction of human B cell proliferation mimics the inositol lipid polyphosphate breakdown signals induced by crosslinking surface immunoglobulin. Biochem Biophys Res Commun. 1985 Aug 30;131(1):484–491. doi: 10.1016/0006-291x(85)91828-5. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Keeler K. D., Klaus G. G. Activation and proliferation signals in mouse B cells. I. A comparison of the capacity of anti-Ig antibodies or phorbol myristic acetate to activate B cells from CBA/N or normal mice into G1. Eur J Immunol. 1984 Mar;14(3):244–250. doi: 10.1002/eji.1830140308. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Klaus G. G. Activation and proliferation signals in mouse B cells. IV. Concanavalin A stimulates B cells to leave G0, but not to proliferate. Immunology. 1984 Dec;53(4):703–711. [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Bijsterbosch M. K., Holman M. Activation and proliferation signals in mouse B cells. VII. Calcium ionophores are non-mitogenic polyclonal B-cell activators. Immunology. 1985 Oct;56(2):321–327. [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Hawrylowicz C. M., Holman M., Keeler K. D. Activation and proliferation signals in mouse B cells. III. Intact (IGG) anti-immunoglobulin antibodies activate B cells but inhibit induction of DNA synthesis. Immunology. 1984 Dec;53(4):693–701. [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Lernhardt W. Three restriction points in the cell cycle of activated murine B lymphocytes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7681–7685. doi: 10.1073/pnas.82.22.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini C., Kendall F., Baserga R., Dessaive C., Clarkson B., Fried J. The G0-G1 transition of WI38 cells. I. Laser flow microfluorimetric studies. Exp Cell Res. 1977 Apr;106(1):111–118. doi: 10.1016/0014-4827(77)90247-6. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Hildreth J. E., Rickinson A. B., Epstein M. A. Monoclonal antibodies to Epstein-Barr virus-induced, transformation-associated cell surface antigens: binding patterns and effect upon virus-specific T-cell cytotoxicity. Int J Cancer. 1982 Apr 15;29(4):373–381. doi: 10.1002/ijc.2910290403. [DOI] [PubMed] [Google Scholar]
- Subbarao B., Mosier D. E. Activation of B lymphocytes by monovalent anti-Lyb-2 antibodies. J Exp Med. 1984 Jun 1;159(6):1796–1801. doi: 10.1084/jem.159.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]



