Abstract
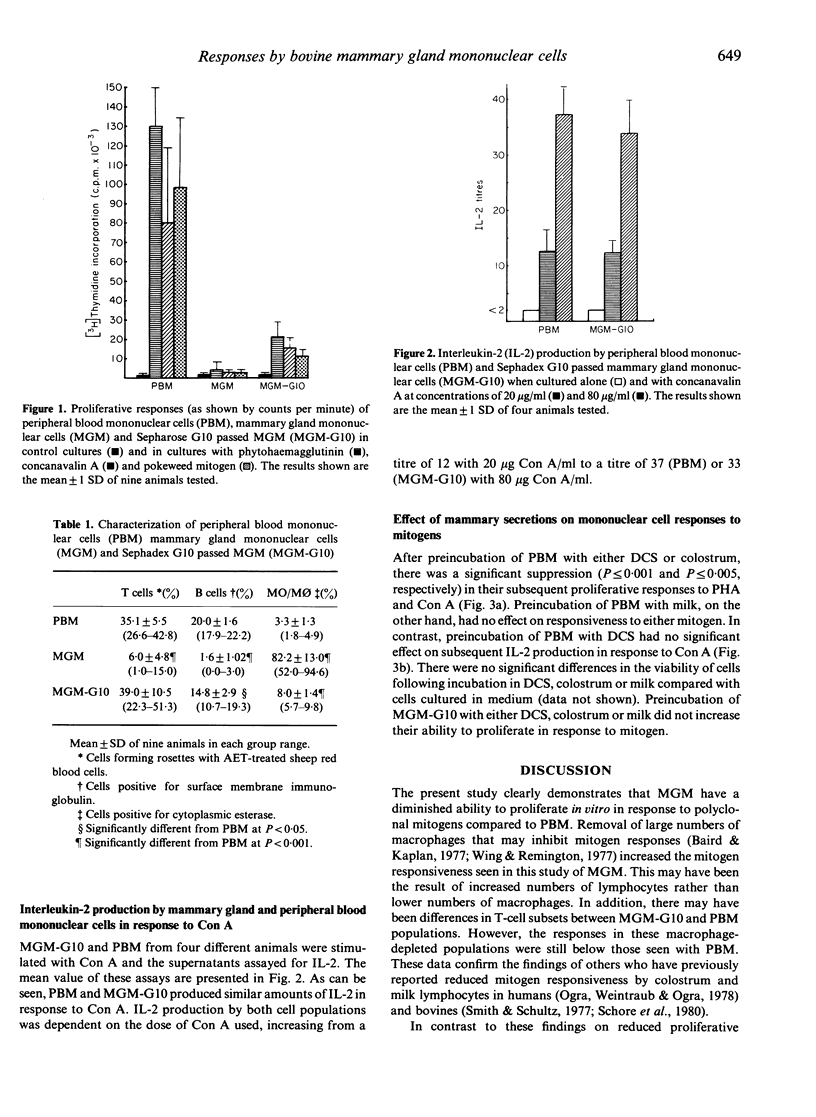
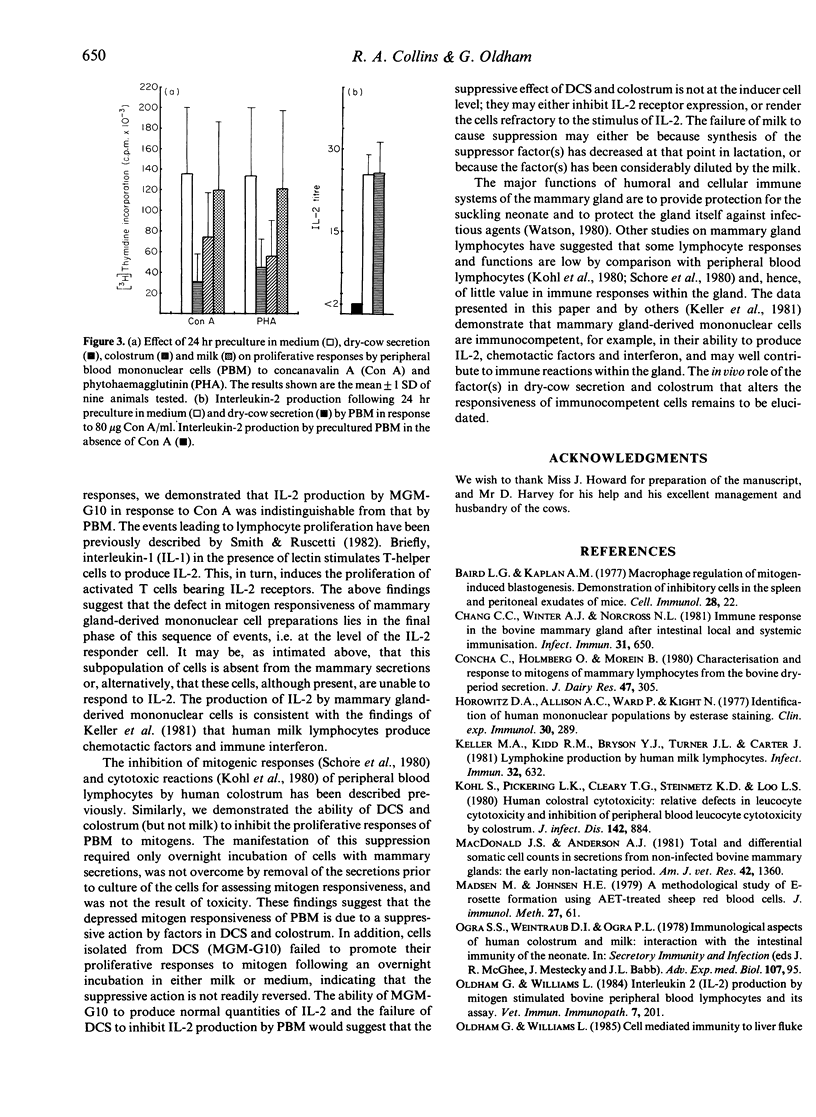
Mammary gland mononuclear cells (MGM) were compared with peripheral blood mononuclear cells (PBM) for their ability to proliferate in response to phytohaemagglutinin (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM), and to produce interleukin-2 (IL-2) when cultured with Con A. MGM proliferative responses to all three mitogens were at a level only just above background. Following removal of adherent cells by passage of the MGM population over Sephadex G10, their ability to proliferate increased but these responses were still significantly lower (P less than 0.001) than those of PBM. Mammary secretions from non-lactating cows and colostrum, but not milk, were found to suppress the proliferative responses to PHA and Con A of PBM following a 24-hr preincubation period. There was no difference in IL-2 production in response to Con A by Sephadex G10-treated MGM and PBM, and dry-cow secretions had no effect on IL-2 production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird L. G., Kaplan A. M. Macrophage regulation of mitogen-induced blastogenesis. I. Demonstration of inhibitory cells in the spleens and peritoneal exudates of mice. Cell Immunol. 1977 Jan;28(1):22–35. doi: 10.1016/s0008-8749(77)80003-8. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Winter A. J., Norcross N. L. Immune response in the bovine mammary gland after intestinal, local, and systemic immunization. Infect Immun. 1981 Feb;31(2):650–659. doi: 10.1128/iai.31.2.650-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha C., Holmberg O., Morein B. Characterization and response to mitogens of mammary lymphocytes from the bovine dry-period secretion. J Dairy Res. 1980 Oct;47(3):305–311. doi: 10.1017/s0022029900021191. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Keller M. A., Kidd R. M., Bryson Y. J., Turner J. L., Carter J. Lymphokine production by human milk lymphocytes. Infect Immun. 1981 May;32(2):632–636. doi: 10.1128/iai.32.2.632-636.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Pickering L. K., Cleary T. G., Steinmetz K. D., Loo L. S. Human colostral cytotoxicity. II. Relative defects in colostral leukocyte cytotoxicity and inhibition of peripheral blood leukocyte cytotoxicity by colostrum. J Infect Dis. 1980 Dec;142(6):884–891. doi: 10.1093/infdis/142.6.884. [DOI] [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E. A methodological study of E-rosette formation using AET-treated sheep red blood cells. J Immunol Methods. 1979 May 10;27(1):61–74. doi: 10.1016/0022-1759(79)90239-4. [DOI] [PubMed] [Google Scholar]
- McDonald J. S., Anderson A. J. Total and differential somatic cell counts in secretions from noninfected bovine mammary glands: the early nonlactating period. Am J Vet Res. 1981 Aug;42(8):1360–1365. [PubMed] [Google Scholar]
- Ogra S. S., Weintraub D. I., Ogra P. L. Immunologic aspects of human colostrum and milk: interaction with the intestinal immunity of the neonate. Adv Exp Med Biol. 1978;107:95–107. doi: 10.1007/978-1-4684-3369-2_12. [DOI] [PubMed] [Google Scholar]
- Oldham G., Williams L. Cell mediated immunity to liver fluke antigens during experimental Fasciola hepatica infection of cattle. Parasite Immunol. 1985 Sep;7(5):503–516. doi: 10.1111/j.1365-3024.1985.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Oldham G., Williams L. Interleukin 2 (IL-2) production by mitogen stimulated bovine peripheral blood lymphocytes and its assay. Vet Immunol Immunopathol. 1984 Oct;7(3-4):201–212. doi: 10.1016/0165-2427(84)90079-5. [DOI] [PubMed] [Google Scholar]
- Outteridge P. M., Lee C. S. Cellular immunity in the mammary gland with particular reference to T, B lymphocytes and macrophages. Adv Exp Med Biol. 1981;137:513–534. [PubMed] [Google Scholar]
- Smith J. W., Schultz R. D. Mitogen- and antigen-responsive milk lymphocytes. Cell Immunol. 1977 Mar 1;29(1):165–173. doi: 10.1016/0008-8749(77)90285-4. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Ruscetti F. W. T-cell growth factor and the culture of cloned functional T cells. Adv Immunol. 1981;31:137–175. doi: 10.1016/s0065-2776(08)60920-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Watson D. L. Immunological functions of the mammary gland and its secretion--comparative review. Aust J Biol Sci. 1980 Aug;33(4):403–422. doi: 10.1071/bi9800403. [DOI] [PubMed] [Google Scholar]


