Abstract
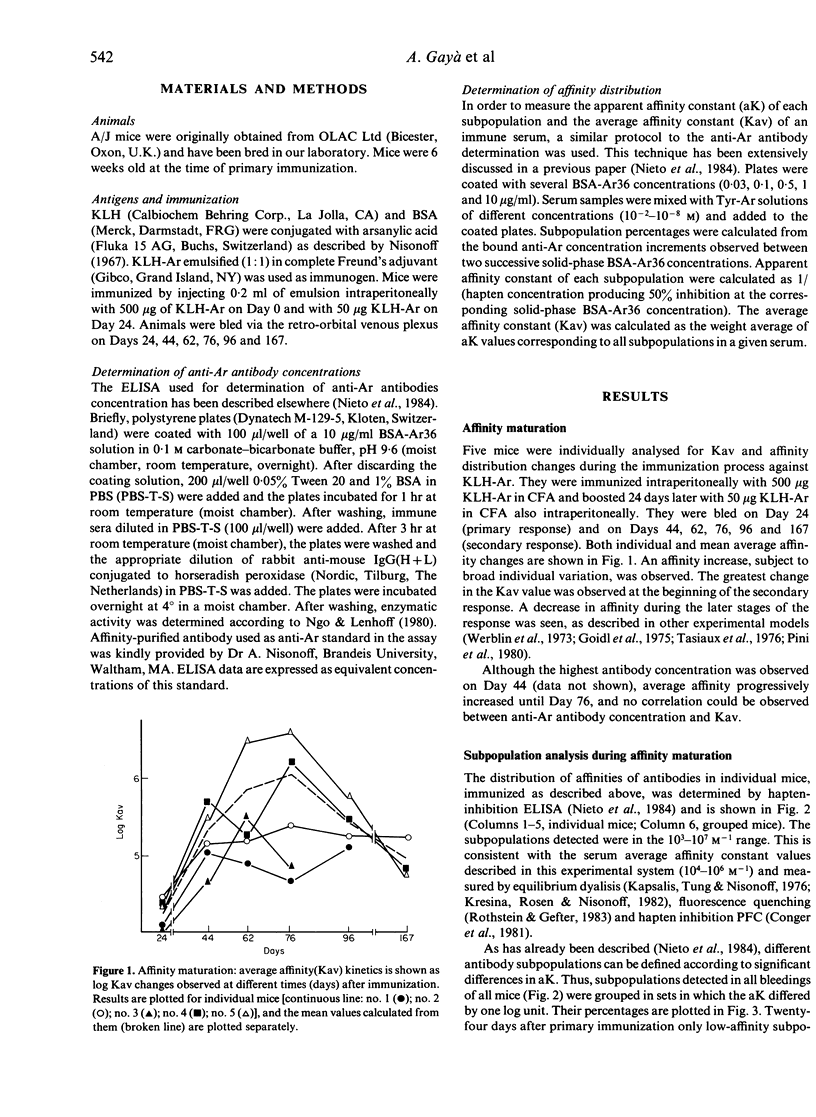
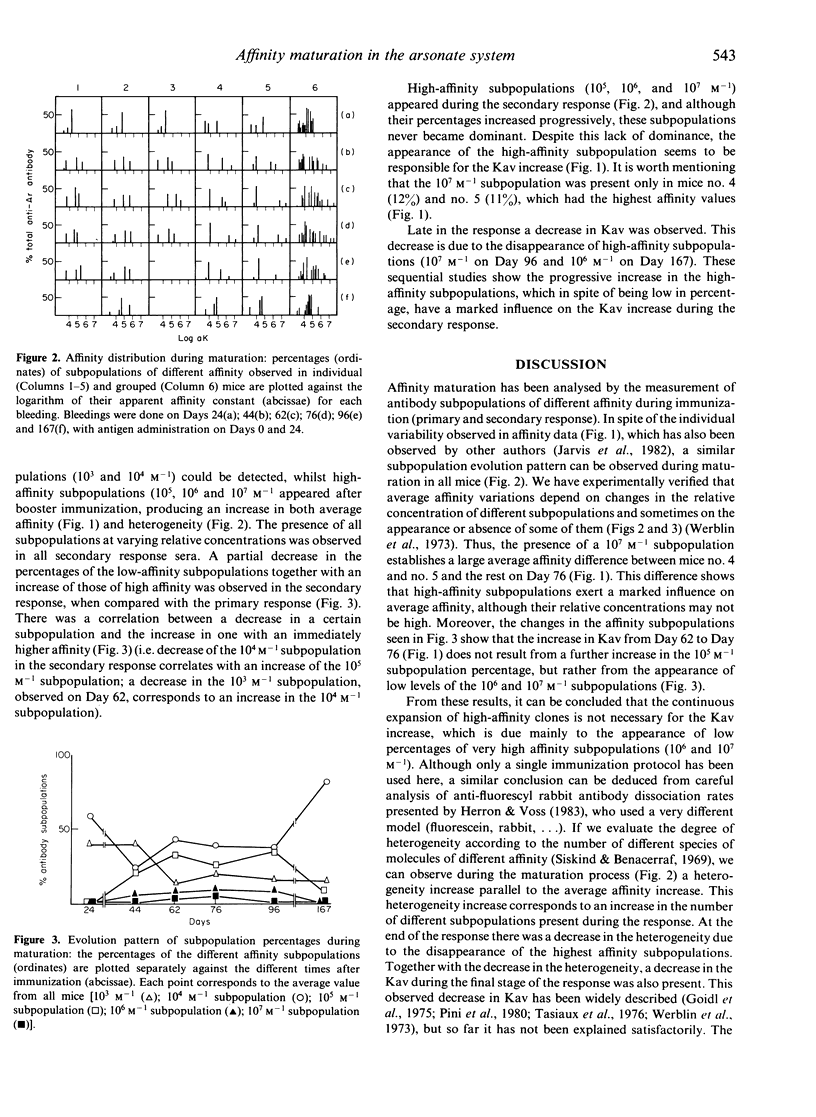
Affinity maturation was studied by the analysis of the kinetics of the appearance of antibody subpopulations with different affinities during the immune response, using an hapten-inhibition ELISA. The immune response in KLH-Ar-immunized A/J mice was used as a model system. Five antibody subpopulations of different affinity (10(3)-10(7) M-1) could be detected, the relative concentrations of which changed during affinity maturation. The high-affinity antibody subpopulations did not represent the major fraction at any stage during affinity maturation. The appearance of the highest affinity subpopulation (10(7) M-1), despite exhibiting relative concentrations no higher than 12%, produced an important increase in average affinity. On the other hand, its disappearance at the end of the maturation process could explain the average affinity decrease observed at this stage. Our results indicate that affinity maturation cannot be explained by the dominance of high-affinity clones, as proposed by Siskind & Benacerraf (1969). The increase in affinity could rather be due to the progressive appearance of low percentages of high-affinity clones, which are not present in the primary response and never become dominant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Lewis G. K., Goodman J. W. Idiotype profile of an immune response. I. Contrasts in idiotypic dominance between primary and secondary responses and between IgM and IgG plaque-forming cells. J Exp Med. 1981 May 1;153(5):1173–1186. doi: 10.1084/jem.153.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Barondess J. J., Siskind G. W. Studies on the control of antibody synthesis. VII. Change in affinity of direct and indirect plaque-forming cells with time after immunization in the mouse: loss of high affinity plaques late after immunization. Immunology. 1975 Oct;29(4):629–641. [PMC free article] [PubMed] [Google Scholar]
- Herron J. N., Voss E. W., Jr Analysis of heterogeneous dissociation kinetics in polyclonal populations of rabbit anti-fluorescyl-IgG antibodies. Mol Immunol. 1983 Dec;20(12):1323–1332. doi: 10.1016/0161-5890(83)90163-3. [DOI] [PubMed] [Google Scholar]
- Jarvis M. R., Casperson G. F., Kranz D. M., Voss E. W., Jr Affinity maturation of NZB and BALB/cV mice anti-fluorescyl response. Mol Immunol. 1982 Apr;19(4):525–533. doi: 10.1016/0161-5890(82)90220-6. [DOI] [PubMed] [Google Scholar]
- Kapsalis A. A., Tung A. S., Nisonoff A. Relative combining affinities of anti-p-azophenylarsonate antibodies bearing a cross-reactive idiotype. Immunochemistry. 1976 Sep;13(9):783–787. doi: 10.1016/0019-2791(76)90201-9. [DOI] [PubMed] [Google Scholar]
- Kresina T. F., Rosen S. M., Nisonoff A. Degree of heterogeneity of binding specificities of antibodies to the phenylarsonate group that share a common idiotype. Mol Immunol. 1982 Nov;19(11):1433–1439. doi: 10.1016/0161-5890(82)90190-0. [DOI] [PubMed] [Google Scholar]
- Ngo T. T., Lenhoff H. M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem. 1980 Jul 1;105(2):389–397. doi: 10.1016/0003-2697(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Nieto A., Gaya A., Jansa M., Moreno C., Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol Immunol. 1984 Jun;21(6):537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- Pini C., Di Felice G., Neri R., Mancini C., Vicari G., Doria G. Oscillations of IgM antibody affinity at the level of single immunocytes. J Immunol. 1980 Sep;125(3):1349–1354. [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Tasiaux N., Leuwenkroon R., Bruyns C., Urbain J. Possible occurrence and meaning of lymphocytes bearing autoanti-idiotypic receptors during the immune response. Eur J Immunol. 1978 Jul;8(7):464–468. doi: 10.1002/eji.1830080704. [DOI] [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Siskind G. W. Distribution of antibody affinities: technique of measurement. Immunochemistry. 1972 Oct;9(10):987–1011. doi: 10.1016/0019-2791(72)90110-3. [DOI] [PubMed] [Google Scholar]


