Abstract
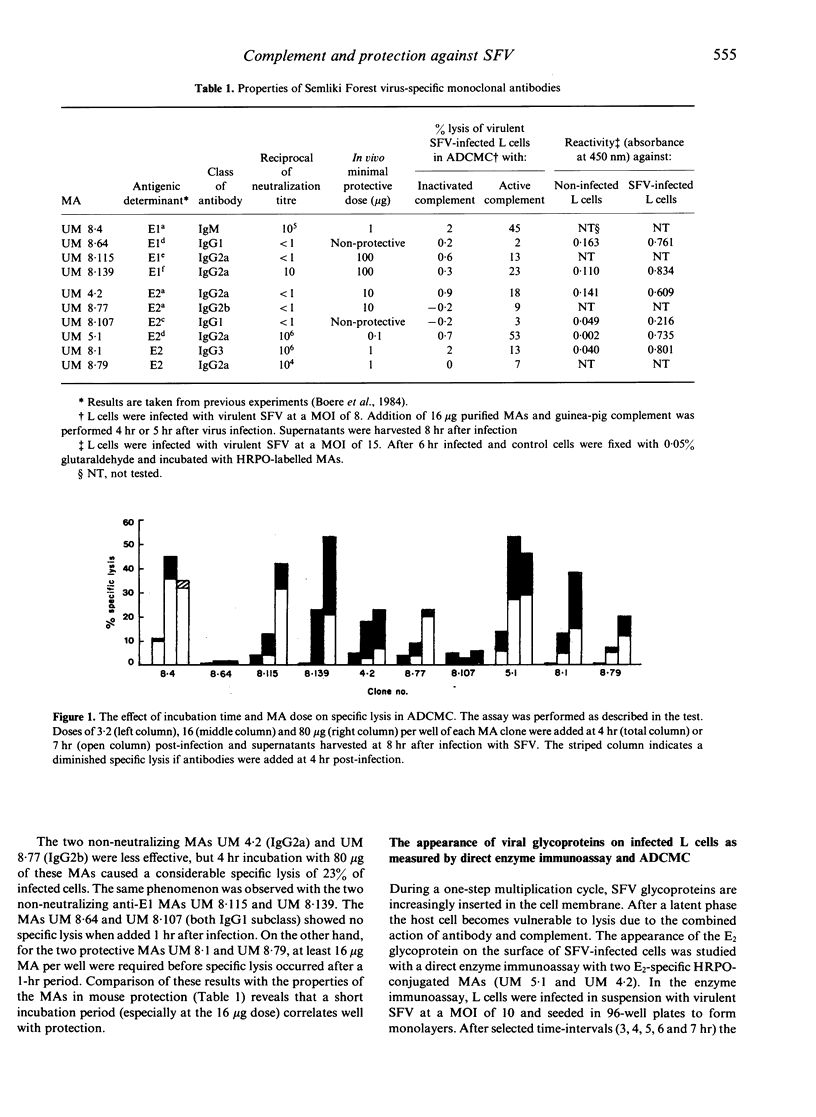
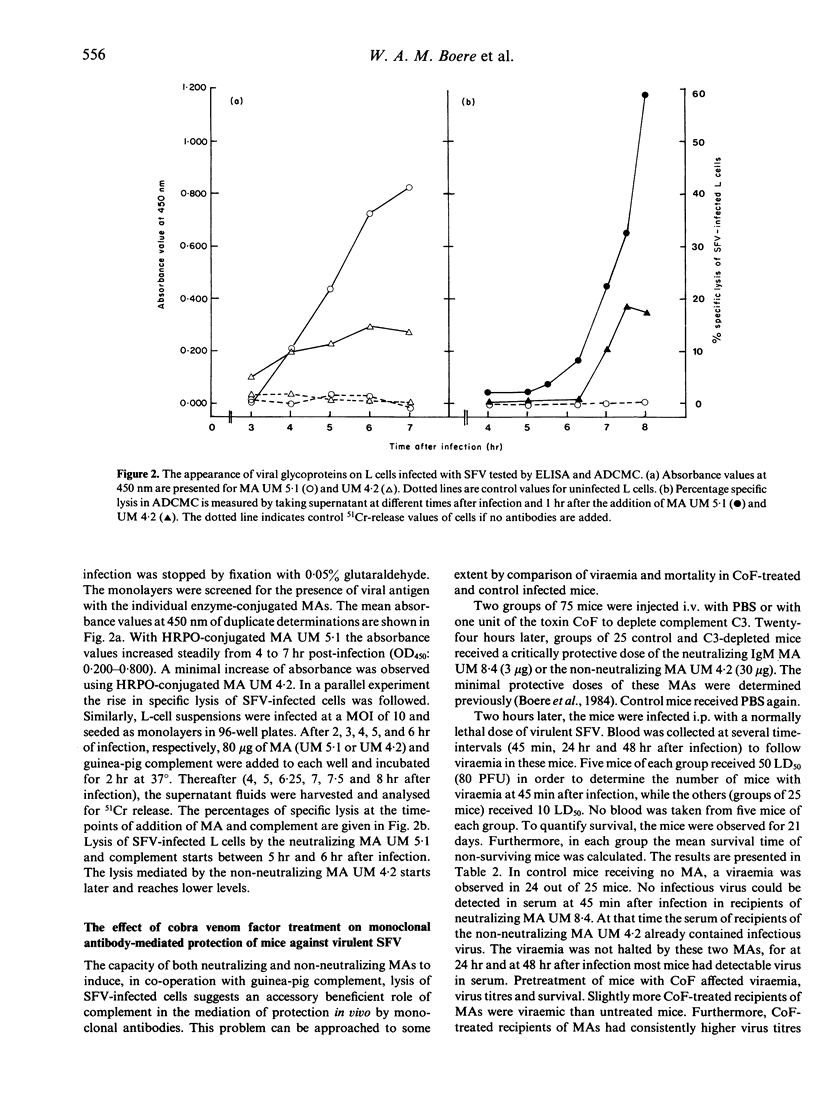
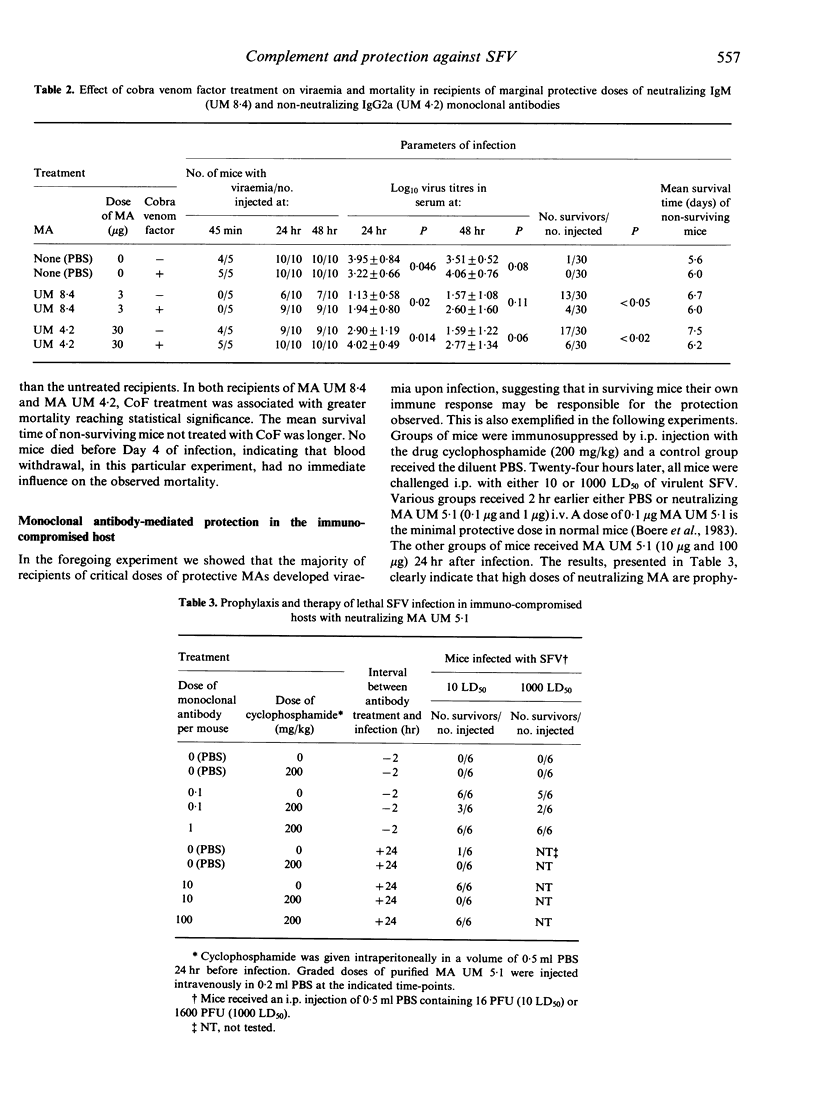
Monoclonal antibodies (MAs), specific for either the E1 or E2 glycoproteins of Semliki Forest virus (SFV), and belonging to various immunoglobulin subclasses (IgM, IgG2a, IgG2b and IgG3), effected lysis of SFV-infected L cells in co-operation with guinea-pig complement. In this antibody-dependent complement-mediated cytolysis (ADCMC) test, IgG1 MAs were not effective although these antibodies recognize the viral antigens on the surface of SFV-infected L cells. The latter was shown with horseradish peroxidase (HRPO)-labelled MAs in a direct enzyme immunoassay. The binding reactivities of HRPO-labelled MAs to infected L cells at selected time-intervals after infection correlated well with the amount of cytolysis in a parallel ADCMC test. Cytolysis was dependent on the duration of incubation with antibodies: more cytolysis was measured after a 4-hr incubation period with MA, starting at 4 hr after infection, compared to a 1-hr incubation period starting after 7 hr of infection. However, in the latter case (1-hr period) the amount of cytolysis measured correlated better to neutralization and/or protection by MAs than after the extended period (4 hr) of incubation. Complement (C3) depletion by cobra venom factor treatment led to a higher mortality and viraemia of mice prophylactically injected with critically protective doses of either the neutralizing MA UM 8.4 (IgM) or the non-neutralizing MA UM 4.2 (IgG2a). The results suggest a co-operative role of MA with complement in mediating protection against SFV. Passive immunization by administration of low amounts (0.1 micrograms/mouse) of neutralizing MA UM 5.1 resulted in protection of normal mice against a lethal infection with SFV. Mice immunosuppressed by cyclophosphamide were not protected by these doses. If the doses were increased however, these mice were protected both prophylactically and therapeutically. These results indicate that, using critical doses of MAs, an intact immune system ensures survival in normal mice after infection with virulent SFV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaskov J. G., Hadding U., Bitter-Suermann D. Interaction of Ross River virus with the complement system. J Gen Virol. 1985 Jan;66(Pt 1):121–129. doi: 10.1099/0022-1317-66-1-121. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. M., Almeida J. D. The morphological and biological effects of various antisera on avian infectious bronchitis virus. J Gen Virol. 1968 Jul;3(1):97–102. doi: 10.1099/0022-1317-3-1-97. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J Virol. 1985 May;54(2):546–551. doi: 10.1128/jvi.54.2.546-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Fitzgeorge R. Immunomodification and the expression of virulence in mice by defined strains of Semliki Forest virus: the effects of cyclophosphamide. J Gen Virol. 1975 Aug;28(2):225–237. doi: 10.1099/0022-1317-28-2-225. [DOI] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Gates M. C., Sheahan B. J., Atkins G. J. The pathogenicity of the M9 mutant of Semliki Forest virus in immune-compromised mice. J Gen Virol. 1984 Jan;65(Pt 1):73–80. doi: 10.1099/0022-1317-65-1-73. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Metselaar D., Kirya G. B., Timms G. L. Investigations into yellow fever virus and other arboviruses in the northern regions of Kenya. Bull World Health Organ. 1970;42(5):787–795. [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The effect of complement depletion on the course of Sindbis virus infection in mice. J Immunol. 1978 Oct;121(4):1276–1278. [PubMed] [Google Scholar]
- Hirsch R. L. The complement system: its importance in the host response to viral infection. Microbiol Rev. 1982 Mar;46(1):71–85. doi: 10.1128/mr.46.1.71-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Wust C. J., Brown A. Antibody-dependent, complement-mediated homologous and cross-cytolysis of togavirus-infected cells. J Immunol. 1977 Oct;119(4):1289–1292. [PubMed] [Google Scholar]
- Klerx J. P., Beukelman C. J., Van Dijk H., Willers J. M. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J Immunol Methods. 1983 Oct 14;63(2):215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Harmsen M., Khader Boutahar-Trouw B. Delayed-type hypersensitivity against Semliki Forest virus in mice. Infect Immun. 1979 Feb;23(2):219–223. doi: 10.1128/iai.23.2.219-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth G. J., 3rd, Appleton J. A. Passive protection of mice and sheep against bluetongue virus by a neutralizing monoclonal antibody. Infect Immun. 1983 Jan;39(1):208–212. doi: 10.1128/iai.39.1.208-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J Immunol. 1984 Mar;132(3):1533–1537. [PubMed] [Google Scholar]
- Oi V. T., Vuong T. M., Hardy R., Reidler J., Dangle J., Herzenberg L. A., Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984 Jan 12;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of the allergic response. Nat New Biol. 1972 May 31;237(74):157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Kokubun K. M., Cole G. A. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983 Oct 15;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B. Antibody-mediated destruction of virus-infected cells. Adv Immunol. 1980;29:209–260. doi: 10.1016/S0065-2776(08)60045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V. Immune lysis of Sindbis virus. Virology. 1975 Aug;66(2):620–624. doi: 10.1016/0042-6822(75)90235-4. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tiel F. H., Boere W. A., Vinjé J., Harmsen T., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Detection of Semliki Forest virus in cell culture by use of an enzyme immunoassay with peroxidase-labeled monoclonal antibodies specific for glycoproteins E1 and E2. J Clin Microbiol. 1984 Sep;20(3):387–390. doi: 10.1128/jcm.20.3.387-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


