Abstract
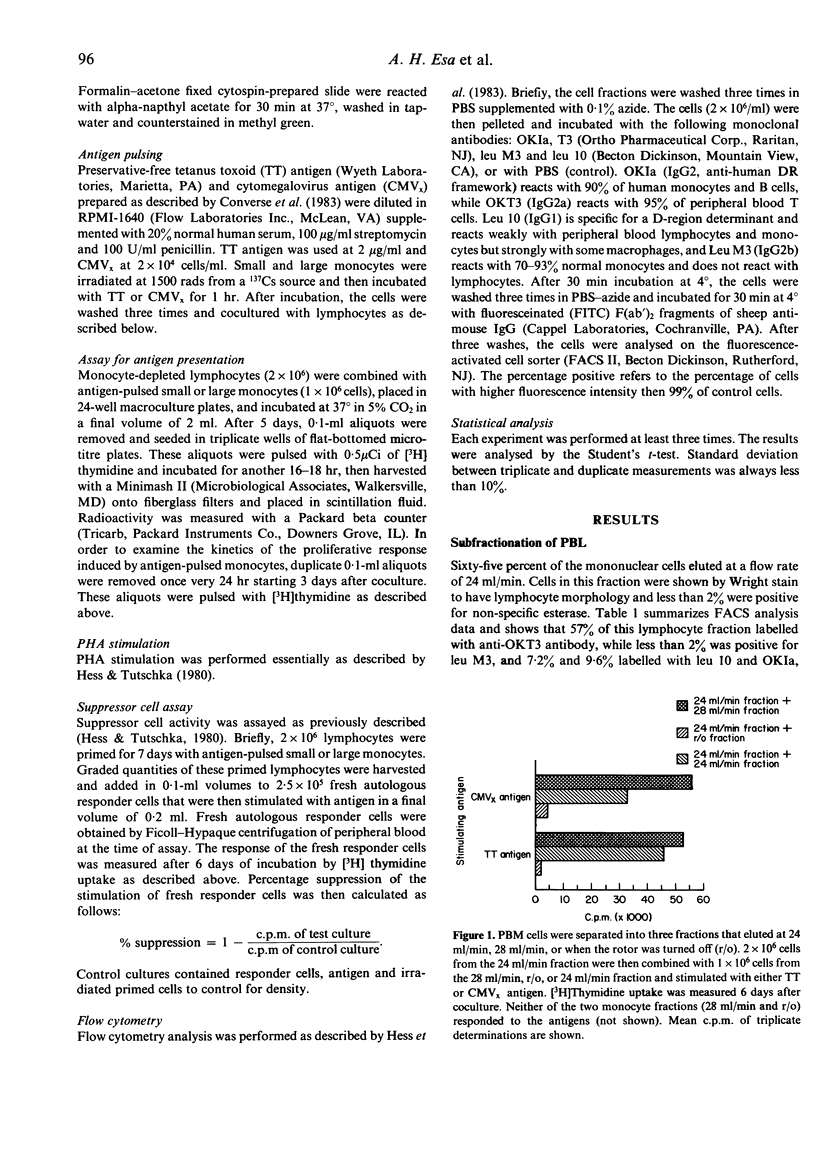
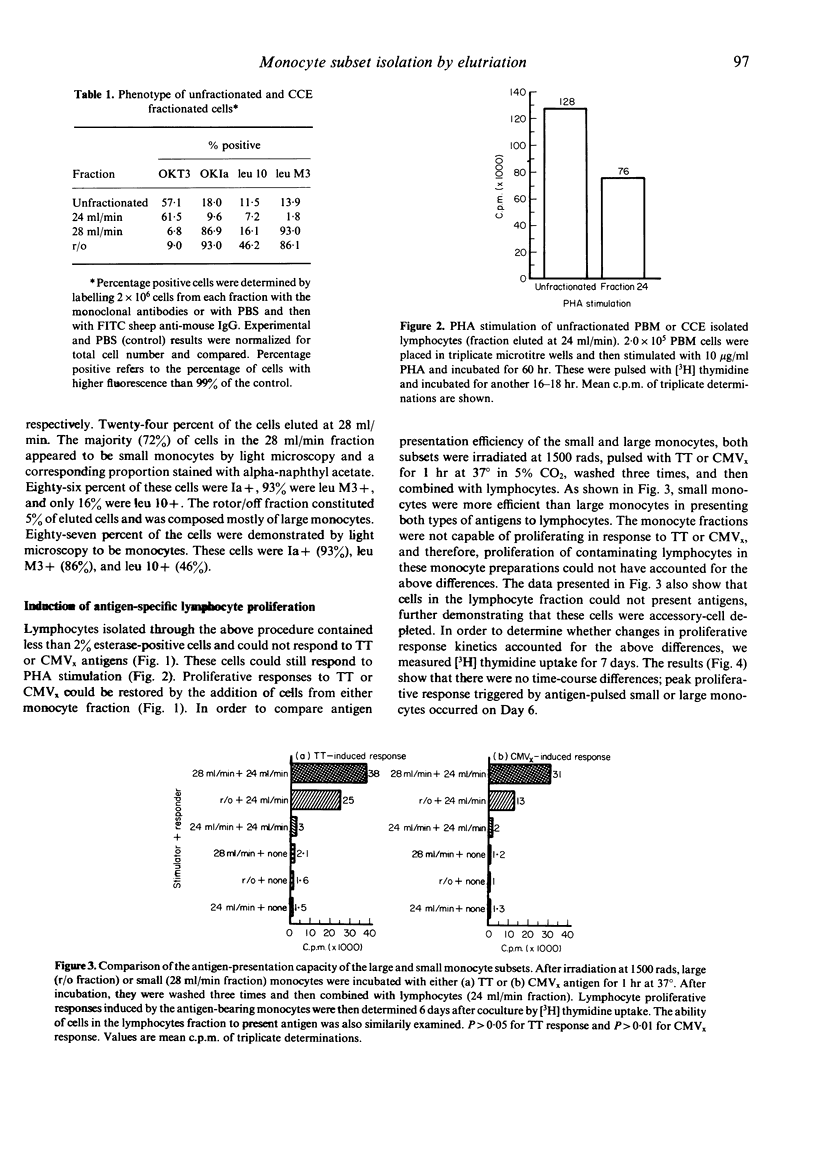
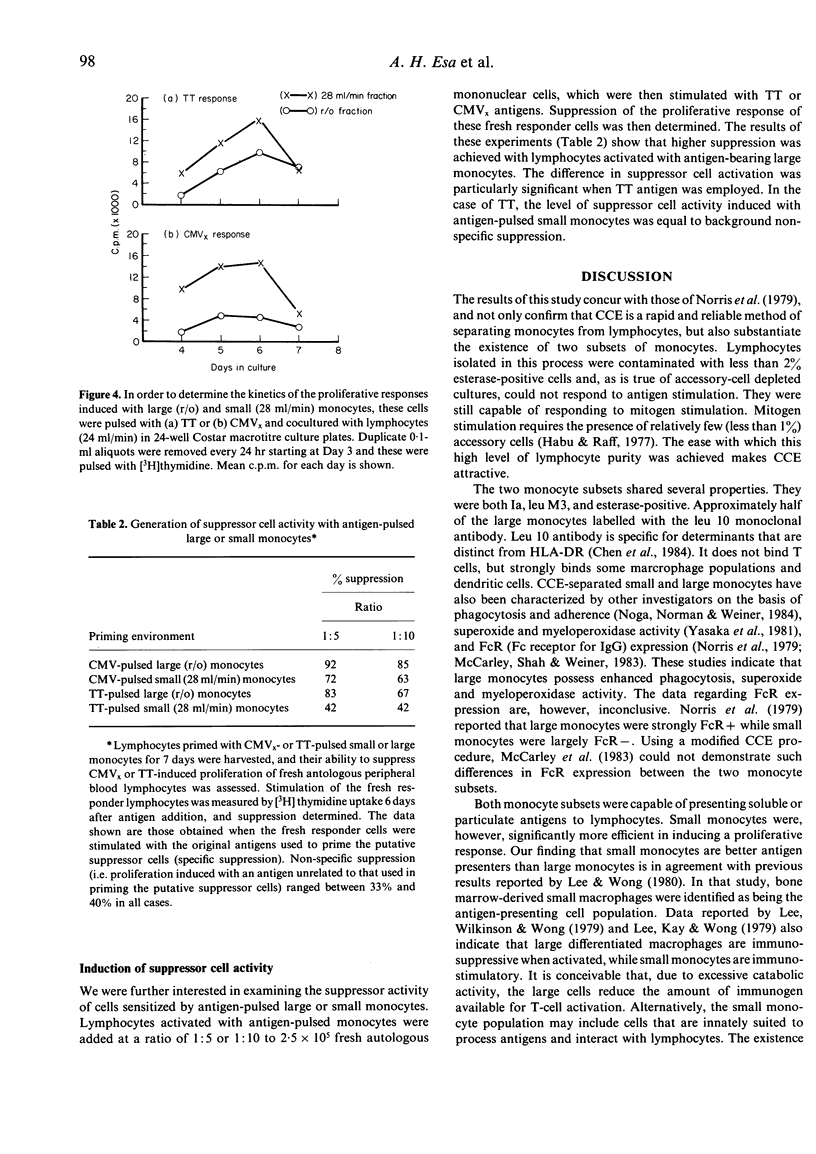
Human peripheral blood mononuclear cells were separated into three fractions by means of counterflow centrifugation elutriation (CCE). The first fraction, eluted at a flow rate of 24 ml/min, was composed of lymphocytes with less than 2% contaminating esterase-positive cells. The cells in this fraction were incapable of responding to either soluble antigen (tetanus toxoid) or particulate antigen (cytomegalovirus-infected fibroblasts) unless recombined with accessory cells. The second fraction, eluted at a flow rate of 28 ml/min, was composed predominantly (72%) of small Ia, leu M3, and esterase-positive monocytes, which stained weakly with leu 10 antibody. Cells in this fraction efficiently presented soluble and particulate antigens to monocyte-depleted lymphocytes. Of the remaining cells, 87% were large esterase-positive monocytes that labelled strongly with Ia, leu M3, and leu 10. These cells were less efficient in antigen presentation than the small monocytes. However, lymphocytes activated with antigen-pulsed large monocytes exhibited more suppressor cell activity than those activated with antigen-pulsed small monocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. X., Evans R. L., Pollack M. S., Lanier L. L., Phillips J. H., Rousso C., Warner N. L., Brodsky F. M. Characterization and expression of the HLA-DC antigens defined by anti-Leu 10. Hum Immunol. 1984 Aug;10(4):221–235. doi: 10.1016/0198-8859(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Converse P. J., Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporine on the response of normal human lymphocytes to cytomegalovirus in vitro. Infect Immun. 1983 Sep;41(3):1226–1233. doi: 10.1128/iai.41.3.1226-1233.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Habu S., Raff M. C. Accessory cell dependence of lectin-induced proliferation of mouse T lymphocytes. Eur J Immunol. 1977 Jul;7(7):451–457. doi: 10.1002/eji.1830070710. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Donnenberg A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. V. Analysis of responding T lymphocyte subpopulations in primary MLR with monoclonal antibodies. J Immunol. 1983 Feb;130(2):717–721. [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J. Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA allows for the expression of alloantigen-activated suppressor cells while preferentially inhibiting the induction of cytolytic effector lymphocytes in MLR. J Immunol. 1980 Jun;124(6):2601–2608. [PubMed] [Google Scholar]
- Lee K. C., Kay J., Wong M. Separation of functionally distinct subpopulations of Corynebacterium parvum-activated macrophages with predominantly stimulatory or suppressive effect on the cell-mediated cytotoxic T cell response. Cell Immunol. 1979 Jan;42(1):28–41. doi: 10.1016/0008-8749(79)90218-1. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Wilkinson A., Wong M. Antigen-specific murine T-cell proliferation: role of macrophage surface Ia and factors. Cell Immunol. 1979 Nov;48(1):79–90. doi: 10.1016/0008-8749(79)90101-1. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Wong M. Functional heterogeneity of culture-grown bone marrow-derived macrophages. I. Antigen presenting function. J Immunol. 1980 Jul;125(1):86–95. [PubMed] [Google Scholar]
- Mason R. R., Weiner R. S. Application of the Beckman JE6-B Elutriator System in the isolation of human monocyte subpopulations. Scand J Haematol. 1985 Jan;34(1):5–8. doi: 10.1111/j.1600-0609.1985.tb00735.x. [DOI] [PubMed] [Google Scholar]
- McCarley D. L., Shah V. O., Weiner R. S. Purified human monocyte subsets as effector cells in antibody-dependent cellular cytotoxicity (ADCC). J Immunol. 1983 Oct;131(4):1780–1783. [PubMed] [Google Scholar]
- Noga S. J., Normann S. J., Weiner R. S. Methods in laboratory investigation. Isolation of guinea pig monocytes and Kurloff cells: characterization of monocyte subsets by morphology, cytochemistry, and adherence. Lab Invest. 1984 Aug;51(2):244–252. [PubMed] [Google Scholar]
- Normann S. J., Weiner R. Cytotoxicity of human peripheral blood monocytes. Cell Immunol. 1983 Oct 15;81(2):413–425. doi: 10.1016/0008-8749(83)90248-4. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- Rosenson-Schloss R. S., Reinisch C. L. Selective activation of immunoregulatory T-cell circuits by an Ia+ antigen-presenting cell line. Cell Immunol. 1985 Jul;93(2):475–485. doi: 10.1016/0008-8749(85)90152-2. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Yasaka T., Mantich N. M., Boxer L. A., Baehner R. L. Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J Immunol. 1981 Oct;127(4):1515–1518. [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]


