Abstract
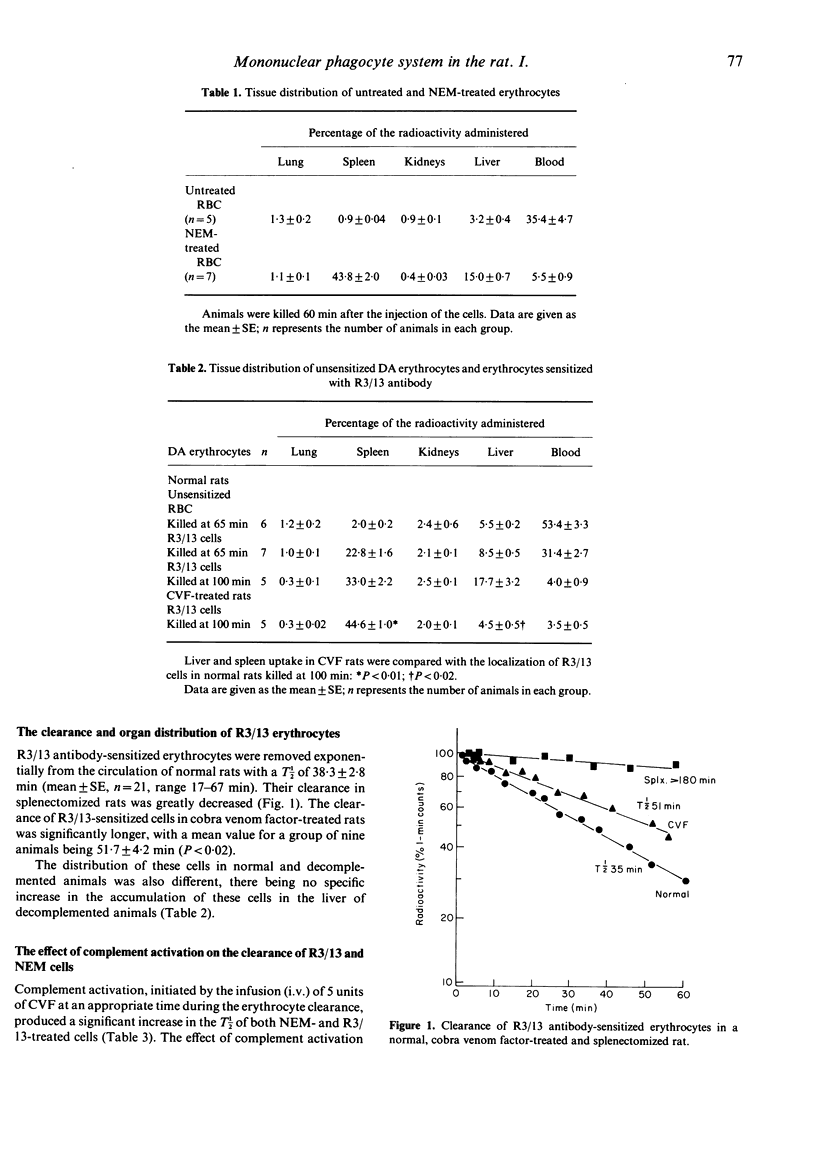
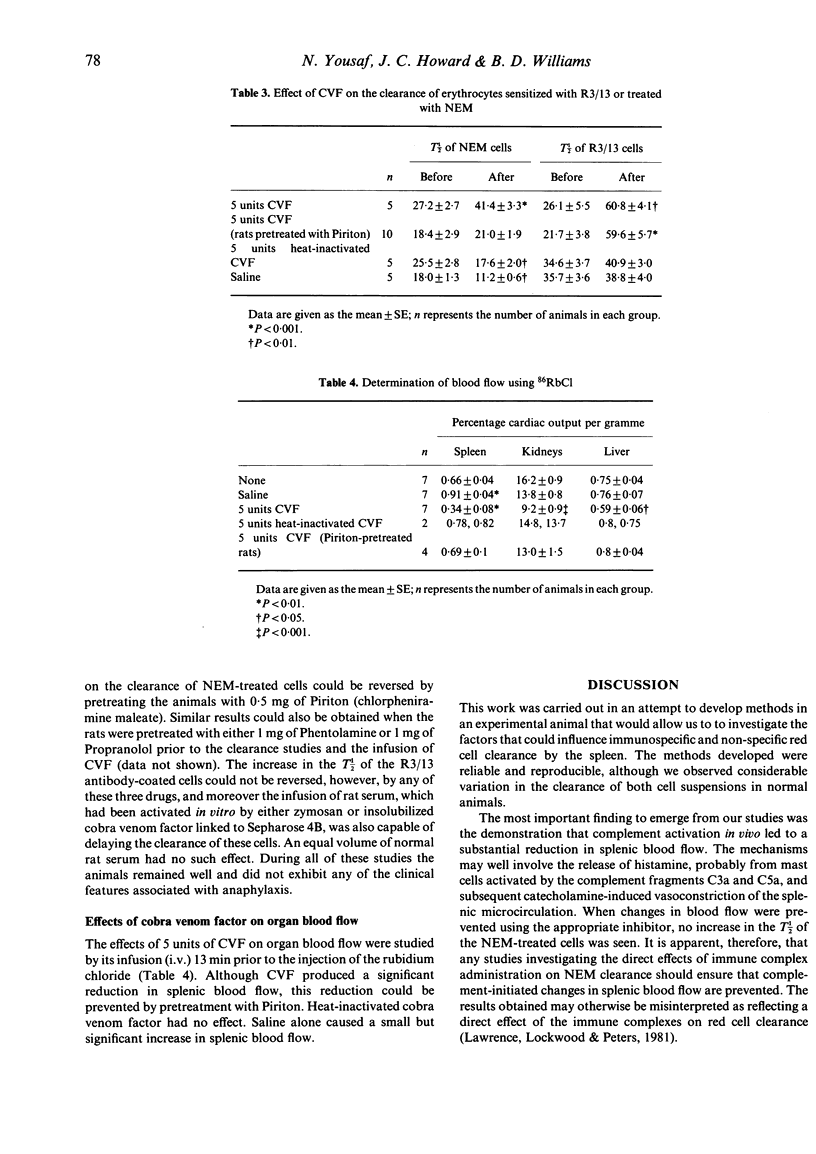
The splenic component of the mononuclear phagocyte system (MPS) was investigated in the rat using N-ethylmaleimide-treated erythrocytes (NEM) and erythrocytes coated with a monoclonal IgG2b antibody (R3/13) directed against the rat RT1Aa major histocompatibility antigen. Both cell suspensions were removed by the spleen, and their clearance times were significantly longer in splenectomized animals. The mean clearance times for the NEM-treated cells in both normal and cobra venom-treated rats were similar (19.1 +/- 1.1 min and 19.0 +/- 1.0 min, respectively) but differences were seen between the clearance of R3/13 antibody-sensitized cells in these two groups (normal rats 38.3 +/- 2.8 min and CVF-treated rats 51.7 +/- 4.2 min, P less than 0.02). Different receptors were also involved in the removal of these cells; in normal animals recognition entailed interaction with complement receptors, whereas in CVF-treated animals this was implemented by Fc receptors. Complement activation prolonged the clearance rates of both R3/13 cells and NEM cells in normal animals, but the effect of complement activation on the clearance of NEM-treated cells was achieved via changes in splenic blood flow. When this was prevented from taking place no effect was seen on the clearance of NEM cells, although the clearance of R3/13 cells was inhibited by the complement fragments generated by complement activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Massoni R. J. The effect of complement in adherent immune complexes on Fc and C3 receptor expression in human monocytes. Immunology. 1981 Dec;44(4):717–725. [PMC free article] [PubMed] [Google Scholar]
- Berger M., Gaither T. A., Hammer C. H., Frank M. M. Lack of binding of human C3, in its native state, to C3b receptors. J Immunol. 1981 Oct;127(4):1329–1334. [PubMed] [Google Scholar]
- Eden A., Bianco C., Nussenzweig V., Mayer M. M. C3 split products inhibit the binding of antigen-antibody-complement complexes to B lymphocytes. J Immunol. 1973 May;110(5):1452–1453. [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Lawley T. J., Hamburger M. I., Brown E. J. NIH Conference: Immunoglobulin G Fc receptor-mediated clearance in autoimmune diseases. Ann Intern Med. 1983 Feb;98(2):206–218. doi: 10.7326/0003-4819-98-2-218. [DOI] [PubMed] [Google Scholar]
- Howard J. C., Butcher G. W., Galfrè G., Milstein C., Milstein C. P. Monoclonal antibodies as tools to analyze the serological and genetic complexities of major transplantation antigens. Immunol Rev. 1979;47:139–174. doi: 10.1111/j.1600-065x.1979.tb00292.x. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Halbwachs L., Gewurz A., Gewurz H. Purification of cobra venom factor from phospholipase A contaminant. Immunology. 1976 Dec;31(6):961–968. [PMC free article] [PubMed] [Google Scholar]
- Lawrence S., Lockwood C. M., Peters D. K. Studies on NEM-treated erythrocyte clearance in the rabbit, with special reference to the effects of circulating immune complexes. Clin Exp Immunol. 1981 May;44(2):433–439. [PMC free article] [PubMed] [Google Scholar]
- Lockwood C. M., Worlledge S., Nicholas A., Cotton C., Peters D. K. Reversal of impaired splenic function in patients with nephritis or vasculitis (or both) by plasma exchange. N Engl J Med. 1979 Mar 8;300(10):524–530. doi: 10.1056/NEJM197903083001003. [DOI] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit J. E. Spleen function. Clin Haematol. 1977 Oct;6(3):639–656. [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Pussell B. A., Lockwood C. M., Cotton C. Defective reticuloendothelial system function in rheumatoid arthritis. Lancet. 1979 Jun 23;1(8130):1311–1314. doi: 10.1016/s0140-6736(79)91947-0. [DOI] [PubMed] [Google Scholar]


