Abstract
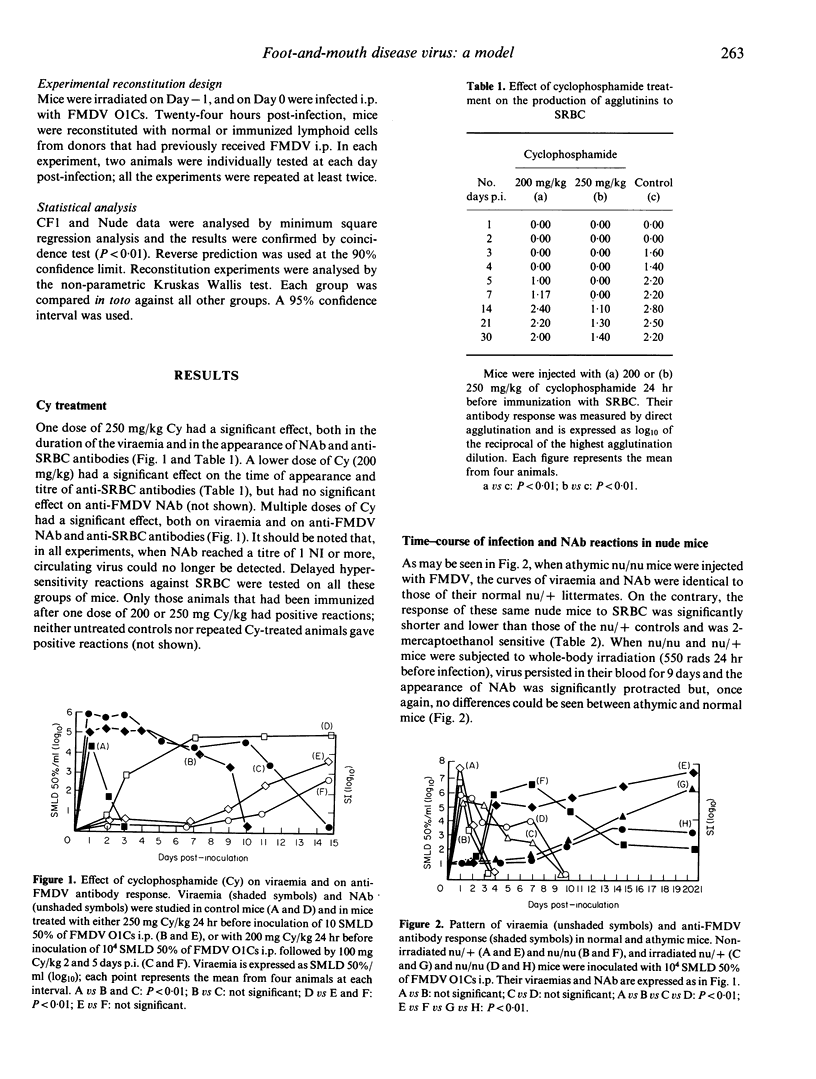
The immune response against foot-and-mouth disease virus (FMDV) was studied in a murine model. In untreated control mice, the inoculation of 10,000 suckling mouse 50% lethal doses of Ol Campos FMDV i.p. was followed by a burst of viraemia that disappeared in less than 4 days, i.e. when the neutralizing antibodies (NAb) reached titres above one neutralizing unit. In mice treated with cyclophosphamide, the curves of viraemia and NAb were significantly delayed. Nu/nu mice injected with FMDV had curves of viraemia and NAb identical to those of their nu/t littermates. We then studied the secondary (memory) immune reaction in the same model. In order to investigate which preimmunized cells participate in the elimination of actively replicating FMDV, mice were irradiated, then infected with FMDV, and 24 hr later repopulated with cells obtained from either donor mice that had been previously immunized by infection with live virus, or non-infected controls. The transfer of control (non-immunized) lymphoid cells was unable to eliminate the viraemia in recipient animals at times significantly different from those observed with irradiated recipients receiving no cells, while repopulation of recipients with 10(8) immune lymphoid cells (obtained from pooled thymus, blood, peritoneal exudate, spleen and lymph nodes of preinfected donor mice) led to undetectable titres of viraemia at Day 5 post-infection (p.i.). High doses of thymus cells were totally inactive, while a few as 10(7) donor spleen cells were able to abort viraemia at 6 days p.i. When enriched preparations of B or T spleen cells were adoptively transferred, only B cells were able to abort viraemia in irradiated recipients. It is concluded that, in the murine model of FMDV infection, B cells are mainly responsible for primary response and short-term immunological memory. In both cases the protective immune reaction is T-independent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. A., Campbell C. H. Experimental placental transfer of foot-and-mouth disease virus in mice. Am J Vet Res. 1976 May;37(5):585–589. [PubMed] [Google Scholar]
- Borca M. V., Fernández F. M., Sadir A. M., Schudel A. A. Reconstitution of immunosuppression mice with mononuclear cells from donors sensitized to foot-and-mouth disease virus (FMDV). Vet Microbiol. 1984 Dec;10(1):1–11. doi: 10.1016/0378-1135(84)90051-8. [DOI] [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CAMPBELL C. H. Antibody response of adult mice to virus of foot-and-mouth disease. Proc Soc Exp Biol Med. 1959 Jun;101(2):286–288. doi: 10.3181/00379727-101-24913. [DOI] [PubMed] [Google Scholar]
- CUNHA R. G., EICHHORN E. A. Influence of cortisone on susceptibility of adult mice to foot-and-mouth-disease virus. Am J Vet Res. 1954 Jan;15(54):149–151. [PubMed] [Google Scholar]
- CUNLIFFE H. R. OBSERVATIONS ON THE DURATION OF IMMUNITY IN CATTLE AFTER EXPERIMENTAL INFECTION WITH FOOT-AND-MOUTH DISEASE VIRUS. Cornell Vet. 1964 Oct;54:501–510. [PubMed] [Google Scholar]
- Gorhe D. S. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund's complete adjuvant. Nature. 1967 Dec 23;216(5121):1242–1244. doi: 10.1038/2161242a0. [DOI] [PubMed] [Google Scholar]
- Knudsen R. C., Groocock C. M., Andersen A. A. Immunity to foot-and-mouth disease virus in guinea pigs: clinical and immune responses. Infect Immun. 1979 Jun;24(3):787–792. doi: 10.1128/iai.24.3.787-792.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLATT H. The occurrence of pancreatic lesions in adult mice infected with the virus of foot-and-mouth disease. Virology. 1959 Nov;9:484–486. doi: 10.1016/0042-6822(59)90139-4. [DOI] [PubMed] [Google Scholar]
- SUBAK-SHARPE H. The effect of passage history, route of inoculation, virus strain and host strain on the susceptibility of adult mice to the virus of foot-and-mouth disease. Arch Gesamte Virusforsch. 1961;11:373–399. doi: 10.1007/BF01249593. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Chapman W. G., Garland A. J. A blastogenic test for foot-and-mouth disease. J Hyg (Lond) 1979 Dec;83(3):507–512. doi: 10.1017/s0022172400026358. [DOI] [PMC free article] [PubMed] [Google Scholar]


