Abstract
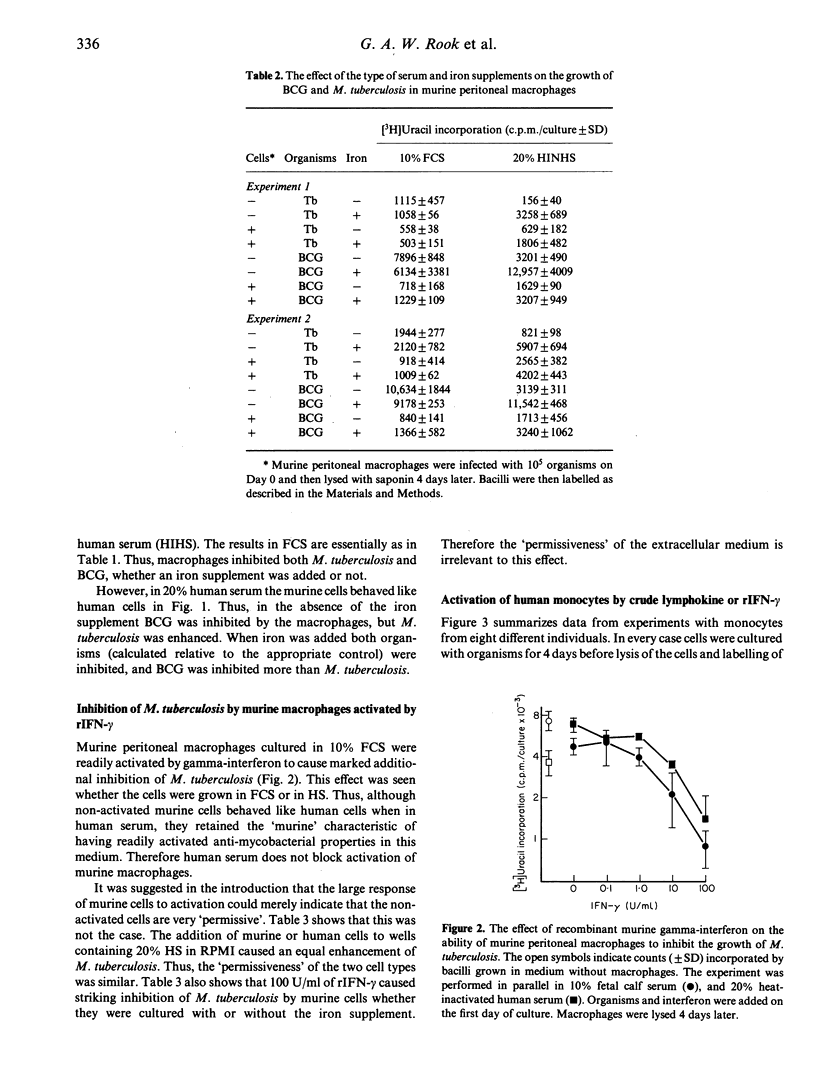
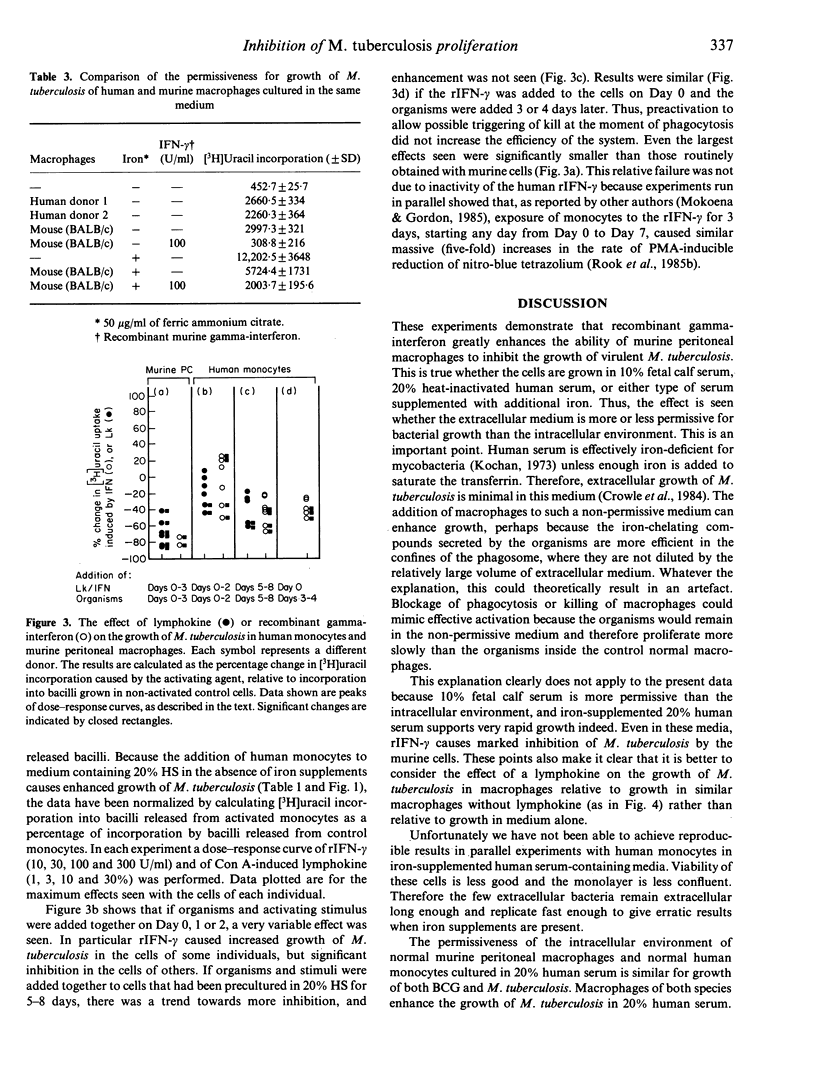
When cultured in 20% heat-inactivated human serum, human monocytes from seven donors were not on average significantly different from non-activated murine peritoneal cells (cultured simultaneously and in an identical manner) in their ability to inhibit BCG and, when calculated relative to growth of bacilli in the same medium without macrophages, to enhance the growth of Mycobacterium tuberculosis. Recombinant gamma-interferon caused marked inhibition of virulent M. tuberculosis by murine (BALB/c) peritoneal macrophages. This effect was seen, whether the cells were cultured in 10% fetal calf serum or in 20% heat-inactivated normal human serum, with or without the addition of iron supplements. However, unlike murine cells, the addition of crude lymphokine or recombinant gamma-interferon to human monocytes caused only weak inhibition of M. tuberculosis, and in some instances, gamma-interferon caused enhancement of growth of the bacilli. Monocytes were only slightly more effective if precultured for 4-8 days before the addition of the activating stimulus. This relative failure to develop anti-mycobacterial mechanisms occurred in spite of the activation of the cells as shown by a massive increase in reduction of nitro-blue tetrazolium inducible by phorbol myristate acetate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981 Jan;31(1):453–464. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Sbarbaro J. A., Judson F. N., Douvas G. S., May M. H. Inhibition by streptomycin of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1984 Nov;130(5):839–844. doi: 10.1164/arrd.1984.130.5.839. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Legrand L., Rivat-Perran L., Huttin C., Dausset J. HLA-and Gm-linked genes affecting the degradation rate of antigens (sheep red blood cells) endocytized by macrophages. Hum Immunol. 1982 Feb;4(1):1–13. doi: 10.1016/0198-8859(82)90045-3. [DOI] [PubMed] [Google Scholar]
- Mokoena T., Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985 Feb;75(2):624–631. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson R. A. Human monocyte maturation/differentiation during in vitro culture. Surv Immunol Res. 1984;3(2-3):138–141. doi: 10.1007/BF02918780. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]


