Abstract
The heat shock genes of Bacillus subtilis are assigned to four classes on the basis of their regulation mechanisms. While classes I and III are negatively controlled by two different transcriptional repressors, class II is regulated by the alternative sigma factor σB. All heat shock genes with unidentified regulatory mechanisms, among them htpG, constitute class IV. Here, we show that expression of htpG is under positive control. We identified a DNA sequence (GAAAAGG) located downstream of the σA-dependent promoter of htpG. The heat inducibility of the promoter could be destroyed by inversion, nucleotide replacements, or removal of this DNA sequence. Fusion of this sequence to the vegetative lepA promoter conferred heat inducibility. Furthermore, we were able to show that the heat induction factor is dependent on the absolute temperature rather than the temperature increment and that nonnative proteins within the cytoplasm fail to induce htpG.
The heat shock response is a mechanism found in all organisms to cope with a sudden increase in temperature and some other stresses. It consists of the transiently enhanced synthesis of a group of proteins termed heat shock proteins (HSPs) involved in coping with the stressful situation and in returning the stressed cell to the pre-heat shock level. Most prominent among these HSPs are molecular chaperones and ATP-dependent proteases which remove misfolded polypeptide chains either by refolding or by degradation (6, 9).
The heat shock response is genetically regulated to ensure a rapid increase in the rate of synthesis of HSPs followed by a turn-off. It is generally accepted that the sudden increase in temperature leads to the appearance of misfolded and partially unfolded proteins collectively termed nonnative proteins which are sensed by the cells and lead to the induction of the heat shock response. For bacteria, two classes of regulators have been described so far, alternative sigma factors and transcriptional repressors (20, 26). The best-studied example of an alternative sigma factor is σ32 of Escherichia coli, which controls expression of more than 30 genes of the σ32 regulon (35). The σ32 factor is kept at a low level in the absence of thermal stress due to a low translation rate of its mRNA and a short half-life of the protein. The latter parameter is controlled by the DnaK chaperone mechanism, which sequesters σ32 from the cytoplasm and presents it to proteases. Upon a heat shock, the DnaK chaperone mechanism is titrated by the nonnative proteins, thereby increasing the half-life of σ32, which in turn interacts with the RNA polymerase core enzyme to enhance expression of the genes of the σ32 regulon. As more nonnative proteins are removed, DnaK chaperone complexes become more available to bind σ32 molecules and mediate their degradation, thereby turning off the heat shock response (35).
The paradigm of transcriptional repressors is the HrcA-CIRCE system, analyzed most extensively for Bacillus subtilis (26, 27). It consists of the HrcA repressor protein, which binds to an operator sequence designated CIRCE (controlling inverted repeat of chaperone expression). In B. subtilis, the HrcA repressor regulates expression of the heptacistronic dnaK and the bicistronic groE operon. The activity of the HrcA repressor is assumed to be modulated by the GroE chaperonin system (16). Newly synthesized HrcA is inactive and has to interact with the GroE chaperonin mechanism to be converted to its active conformation, which is able to bind at CIRCE. Upon heat stress, the GroE machine is titrated by nonnative proteins, resulting in an increasing amount of inactive HrcA, which leads to the transient induction of both operons. Again, as more nonnative proteins are removed, GroE complexes become more available to activate HrcA. Quite recently, another study confirmed binding of HrcA to the CIRCE operator by DNase I footprinting and demonstrated that GroEL specifically interacts with immobilized HrcA, thereby underlining our GroEL titration model (23).
The objective of the present study was to identify the components involved in the regulation of the B. subtilis class IV heat shock gene htpG, which is induced about 10-fold upon a sudden temperature increase at the level of both transcription and translation (25). We were already able to show that htpG is not involved in the development of thermotolerance and that it does not act as a cellular thermometer (33). In E. coli, htpG is under the control of σ32, and deletion of htpG from the chromosome caused no change in phenotype, except a slight growth retardation at high temperatures (2, 31). To get a clue to the function of htpG, we decided first to elucidate the regulation mechanism of htpG. We were able to identify its regulatory sequence, which is located downstream from a σA-type promoter. Furthermore, we could show that the induction factor of htpG is influenced by the absolute temperature rather than by the temperature increment and that the appearance of nonnative proteins within the cytoplasm does not enhance transcription of htpG.
MATERIALS AND METHODS
Bacterial strains, plasmids, and DNA manipulations.
E. coli K-12 strains DH10B and DH5α (Gibco-BRL) were used as the general cloning hosts. B. subtilis strain 1012 (24) and its isogenic derivatives described below were used for the expression of transcriptional fusions. Strains SS01 and SS02 have already been described elsewhere (25). All strains were grown in Luria-Bertani (LB) broth supplemented with ampicillin (100 μg/ml), neomycin (10 μg/ml), or erythromycin (1 μg/ml) as appropriate. CaCl2-competent E. coli cells were used (13), and competent cells for B. subtilis transformation (1) were prepared.
Construction of transcriptional fusions.
All DNA fragments carrying different versions of the htpG or lepA promoter region were purchased as complementary oligonucleotides (Table 1), annealed, fused via BamHI/EcoRI restriction sites to the bgaB gene coding for a heat-stable β-galactosidase (10) by using the promoter test vector pBgaB (15), and confirmed by DNA sequencing of all inserts. All promoter-bgaB fusions were integrated ectopically at the amyE locus of the B. subtilis strain 1012, and integration at the correct position was verified by PCR.
TABLE 1.
Transcriptional fusions constructed in this study
| Strain | Oligonucleotide sequence, 5′→3′ |
|---|---|
| SV02 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AGA TGC TGA AAA GGG |
| 3′ AAT TCC CTT TTC AGC ATC TAT TTA TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV03 | 5′ GAT CCC TCG AGT TGA CAA TTT TTA TCT TAT GTG ATA AAT AGA TGC TGA AAA GGG |
| 3′ AAT TCC CTT TTC AGC ATC TAT TTA TCA CAT AAG ATA AAA ATT GTC AAC TCG AGG | |
| SV09 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AG |
| 3′ AAT TCT ATT TAT CAC ATA AGA TGA CAA TTG TCA ACT CGA GG | |
| SV16 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AGA AAA GGG |
| 3′ AAT TCC CTT TTC TAT TTA TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV17 | 5′ GAT CCC TCG AGG AAA AGG TTG ACA ATT GTC ATC TTA TGT GAT AAA TAG |
| 3′ AAT TCT ATT TAT CAC ATA AGA TGA CAA TTG TCA ACC TTT TCC TCG AGG | |
| SV18 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AGC GCC CGG |
| 3′ AAT TCC GGG CGC TAT TTA TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV19 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AGA CCA GGG |
| 3′ AAT TCC CTG GTC TAT TTA TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV20 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT ACT TTT CCG |
| 3′ AAT TCG GAA AAG TAT TTA TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV21 | 5′ GAT CCC TCG AGA TTG TCA TCT TAT GTG ATA AAT AGA AAA GGG |
| 3′ AAT TCC CTT TTC TAT TTA TCA CAT AAG ATG ACA ATC TCG AGG | |
| SV22 | 5′ GAT CCC TCG AGT TAA CAA TTG TCA TCT TAT GTG ATA AAT AGA AAA GGG |
| 3′ AAT TCC CTT TTC TAT TTA TCA CAT AAG ATG ACA ATT GTT AAC TCG AGG | |
| SV23 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG AAA AAT AGA AAA GGG |
| 3′ AAT TCC CTT TTC TAT TTT TCA CAT AAG ATG ACA ATT GTC AAC TCG AGG | |
| SV27 | 5′ GAT CCC TCG AGT TGA ATC TTT ACA ATC CTA TTG ATA TAA TG |
| 3′ AAT TCA TTA TAT CAA TAG GAT TGT AAA GAT TCA ACT CGA GG | |
| SV13 | 5′ GAT CCC TCG AGT TGA ATC TTT ACA ATC CTA TTG ATA TAA TGA TGC TGA AAA GGG |
| 3′ AAT TCC CTT TTC AGC ATC ATT ATA TCA ATA GGA TTG TAA AGA TTC AAC TCG AGG | |
| AW02 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AAA AAG GG |
| 3′ AAT TCC CTT TTT ATT TAT CAC ATA AGA TGA CAA TTG TCA ACT CGA GG | |
| AW03 | 5′ GAT CCC TCG AGT TGA CAA TTG TCA TCT TAT GTG ATA AAT AGA AAA GCC CG |
| 3′ AAT TCG GGC TTT TCT ATT TAT CAC ATA AGA TGA CAA TTG TCA ACT CGA GG | |
| AW05 | 5′ CTA GATAGA CTG AAA AGG |
| 3′ AAT TCC TTT TCA GTC TAT | |
| AW06 | 5′ CTA GAT AGA CTT GCA CTG AAA AGG |
| 3′ AAT TCC TTT TCA GTC CAA GTC TAT | |
| AW07 | 5′ CTA GAT AGA CTT GCA CTT AAC GCC TAG CTT GAACTC AAT CCG ACT TGG CTG AAA AGG |
| 3′ AAT TCC TTT TCA GCC AAG TCG GAT TGA GTT CAA GCT AGG CGT TAA GTG CAA GTC TAT |
β-Galactosidase assay.
Two cultures of each B. subtilis strain were grown in LB medium to an optical density at 578 nm of 0.7 at 37°C. One culture remained at 37°C, whereas the other one was heat shocked to 50°C. Aliquots were taken 30 min after reaching the optical density value. The activity of the β-galactosidase was assayed according to the method of Mogk et al. (15). At least three independent experiments were carried out.
Transcriptional analysis.
Preparation of total B. subtilis RNA, hybridization, and Northern blot analysis were performed as described previously (12). Hybridizations specific for htpG, groEL, grpE, and bgaB were carried out with digoxigenin (DIG)-labeled riboprobe RNAs synthesized in vitro with T3 RNA polymerase from PCR products equipped with a promoter recognized by that polymerase. In vitro RNA labeling was accomplished according to the manufacturer's instructions (DIG RNA labeling kit; Roche Diagnostics, Mannheim, Germany).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis.
Sample preparation for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis was performed as described previously (11). Five micrograms of total cellular protein was applied per lane. Polyclonal antibodies raised against HtpG and DnaK were used at a dilution of 1:10,000 each. A donkey anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Amersham) and a chemiluminescence reaction (ECL system; Amersham) were used for visualization of cross-reacting material.
RESULTS
Localization of the regulatory sequence involved in heat induction of htpG.
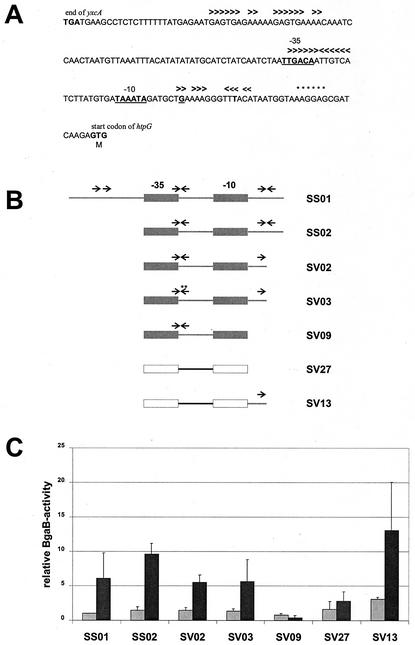
To identify the DNA sequences involved in transcriptional control of htpG, promoter fragments of different lengths were fused to the bgaB gene coding for a heat-stable β-galactosidase (10). Visual inspection of the DNA sequence around a σA-type promoter marked by its −35 and −10 regions located between the beginning of the coding region of htpG and its upstream gene yxcA revealed three repeats as potential binding sites for regulatory proteins, a direct one upstream of the −35 region, an indirect one located within the spacer region and overlapping the −35 sequence, and an imperfect inverted repeat downstream of the −10 region (Fig. 1A). Therefore, we asked whether one or more of these repeats were involved in the thermal regulation of htpG. To answer this question, we constructed transcriptional fusions between the promoter region and bgaB and then deleted or mutated the three different repeats as shown in Fig. 1B. In the presence of all three repeats the β-galactosidase activity increased about sixfold after heat stress (Fig. 1C, SS01). Removal of the upstream direct repeat resulted in about 10-fold induction of the reporter gene activity (SS02), while deletion of the right arm of the downstream inverted repeat (SV02) and introduction of two point mutations within the left arm of the inverted repeat overlapping with the −35 region (SV03) affected neither the basal level nor the induction factor (Fig. 1C). Hence, we conclude that none of the three repeat sequences is involved in thermal regulation of htpG.
FIG. 1.
Localization of the regulatory sequence involved in the heat shock regulation of htpG. (A) DNA sequence of the promoter region of htpG. One direct and two inverted repeats are indicated by arrowheads above the DNA sequence; the stop codon of the divergently transcribed yxcA and the putative start codon of htpG are indicated and given in boldface; the −35 and −10 regions of the potential σA-dependent promoter are indicated, given in boldface, and underlined; the transcriptional start site is underlined; the Shine-Dalgarno sequence of htpG is marked by asterisks above the DNA sequence. (B) Schematic representation of different promoter regions transcriptionally fused to the bgaB reporter gene. The repeats are indicated by arrows; the fusions in SS01, SS02, SV02, SV03, and SV09 contain htpG promoter regions (gray rectangles), and SV27 and SV13 contain the promoter of lepA (white rectangles). In strain SV13, part of the downstream region of htpG is fused to the lepA promoter. Asterisks mark the two point mutations. (C) Relative β-galactosidase activities of the transcriptional fusions presented in panel B. The enzymatic activity measured with the unstressed strain SS01 was set as one relative BgaB unit (equivalent to a specific activity of 0.010 ± 0.001 U/mg of protein). The reporter enzyme activities were measured from cultures without temperature shift (light gray columns) and 30 min after a shift from 37 to 50°C (dark gray columns).
Subsequently, the complete DNA sequence downstream of the −10 region was deleted. Analysis of the deletion derivative revealed that it lost its heat inducibility (Fig. 1C, SV09), indicating that at least part of the regulatory sequence is located within the 13 bp downstream of the −10 region. To confirm this result and to evaluate whether this regulatory sequence functions independently of the promoter, this 13-bp segment was fused to the lepA promoter, which is not subject to heat induction (11). While this promoter alone did not confer temperature inducibility (SV27), addition of the 13 bp from the htpG gene resulted in about fourfold induction of the β-galactosidase activity (SV13). To summarize these results, a DNA sequence located downstream of the htpG promoter acts as a regulatory sequence conferring heat inducibility not only on htpG but also on the unrelated σA-controlled lepA promoter. Since deletion of this DNA sequence abolished heat induction, it must act as a binding site for a transcriptional activator. We propose to designate this DNA sequence a downstream activating sequence (DAS) in accordance with a functional analogous sequence described by Belitsky and Sonenshein (3).
The regulatory sequence involved in thermal regulation of htpG acts in a location- and orientation-specific way.
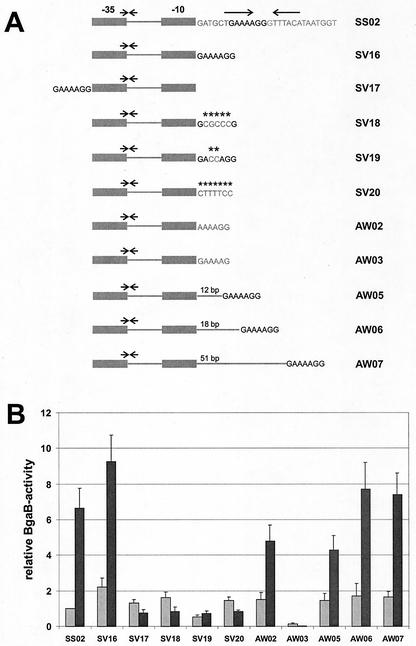
Next, we wanted to determine the exact sequence of the DAS within the 13-bp region and whether its orientation and position are important for its function. When the DNA sequence GAAAAGG was directly fused to the −10 region of the promoter, thereby deleting the 6 intervening bp (Fig. 2A, SV16), this fusion exhibited behavior comparable to that of the fusion containing the complete downstream region (Fig. 2B, SV16). When these 7 bp were moved upstream of the −35 region (Fig. 2A, SV17), this fusion lost its heat inducibility (Fig. 2B, SV17). Changing 5 or 2 bp within the DAS (SV18 and SV19, respectively) or inverting the whole sequence (SV20) abolished its heat inducibility completely (Fig. 2B). To check whether the two flanking nucleotides are important for the recognition by the transcriptional regulator, they were changed from G to A (AW02) and from G to C (AW03) within the sequence GAAAAGG (altered nucleotides underlined; also Fig. 2A). When cells carrying these transcriptional fusions with the two mutated DASs were assayed for reporter enzyme activity before and after a heat shock, it turned out that the flanking G residue on the left side was not important while the one on the right side exhibited an effect (Fig. 2B). In summary, all these results show that the DAS consists of a 6-bp (or less) DNA sequence (AAAAGG) and acts in a location- and orientation-specific way.
FIG. 2.
The regulatory sequence involved in heat shock regulation of htpG is located downstream of the promoter and acts in a location- and orientation-specific way. (A) Schematic representation of promoter-bgaB fusions. All fusions contain htpG promoter regions; the repeats are indicated by arrows; asterisks show nucleotide exchanges. (B) Relative β-galactosidase activities of the transcriptional fusions presented in panel A. The enzymatic activity measured with the unstressed strain SS02 was set as one relative BgaB unit (equivalent to a specific activity of 0.015 ± 0.002 U/mg of protein). The reporter enzyme activities were measured from cultures without temperature shift (light gray columns) and 30 min after a shift from 37 to 50°C (dark gray columns).
Next, we asked whether moving the DAS further downstream would affect the basal or/and the induction level. The DAS was separated by 12 bp (about one helical turn; AW05, Fig. 2A), 18 bp (one-and-a-half helical turns; AW06), and 51 bp from the −10 region of the putative promoter. With all three transcriptional fusions, the basal level of the β-galactosidase activity was slightly increased, and the postinduction level was within the range measured for the control strain SS02 (Fig. 2B). We conclude that the DAS can be moved at least 51 bp downstream of the promoter without significantly affecting either the basal or the heat-induced level.
Thermal induction of htpG depends on the σA-dependent promoter.
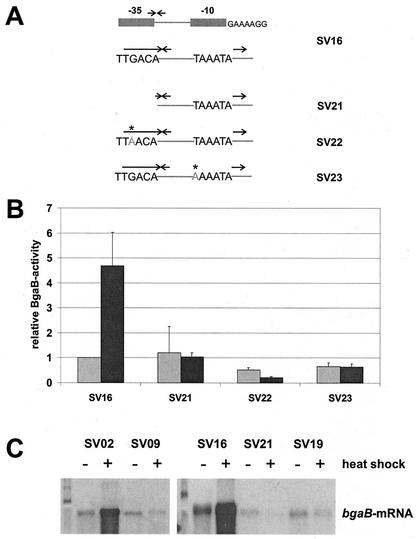
In bacteria, most of the growth-related and housekeeping genes expressed within the exponential phase of cell growth are transcribed by the RNA polymerase holoenzyme containing the major sigma factor, σA, in the case of B. subtilis. Visual inspection of the region before the coding sequence of htpG revealed DNA sequences typical of σA-dependent promoters. When the complete −35 region was deleted (Fig. 3A, SV21), the basal level was not severely reduced but this construct lost its heat inducibility (Fig. 3B, SV21). To confirm that sequences in the −10 and −35 regions of the htpG promoter constitute the key elements recognized by the σA-containing RNA polymerase, either the third G nucleotide in the putative −35 region (TTGACA) was mutated to an A or the first T nucleotide in the putative −10 region (TAAATA) was changed to A. While both mutations did not abolish the basal promoter activity (SV22 and SV23), they completely prevented heat induction (Fig. 3B). These data demonstrate that both nucleotide positions are important for the expression of htpG after challenge with heat stress.
FIG. 3.
Thermal induction of htpG depends on a σA-dependent promoter. (A) Schematic presentation of htpG-bgaB fusions; the repeats are indicated by arrows; point mutations are written in light gray letters and marked by asterisks. (B) Relative β-galactosidase activities were measured from cultures without temperature shift (light gray columns) and 30 min after a shift from 37 to 50°C (dark gray columns). The enzymatic activity measured with the unstressed strain SS16 was set as one relative BgaB unit (equivalent to a specific activity of 0.012 ± 0.001 U/mg of protein). (C) Northern blot analyses of different fusions as indicated. Total RNA was prepared before (−) and 10 min after (+) a heat shock from 37 to 50°C. Each lane contained 2 μg of total RNA. The bgaB transcript was visualized with DIG-labeled antisense bgaB RNA.
To confirm these data by an independent experimental approach, we performed Northern blot experiments with bgaB antisense RNA as a probe. Both fusions (SV02 and SV16) carrying the complete DAS downstream of the promoter showed heat inducibility (Fig. 3C), but in the absence of this regulatory sequence (SV09) or the −35 region (SV21) or with two point mutations within the DAS (SV19), the transcripts of all three operon fusions failed to exhibit an increase after a thermal upshock (Fig. 3C). All these results confirm that transcription of the htpG gene is directed by a σA-dependent promoter and that heat inducibility of this promoter is strictly dependent on the DAS located downstream of the promoter.
The htpG gene is not induced when cells are challenged with either ethanol or puromyin.
Besides heat shock, ethanol and puromycin are potent inducers of the heat shock response. While ethanol increases the error rate during translation (29) or even blocks the translation step (8), puromycin is recognized as a tRNA analog and causes chain termination and release of peptidyl puromycin from ribosomes (7, 28).
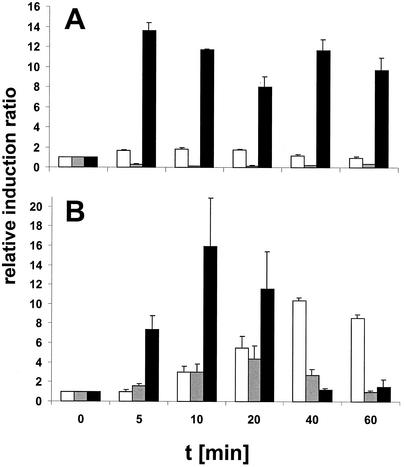
To determine whether ethanol and puromycin were able to induce expression of htpG, the level of htpG mRNA was determined by slot blot analysis. While heat shock triggered a rapid increase in the level of htpG transcript as expected, neither ethanol (as already reported [25]) nor puromycin caused a significant increase (Fig. 4A). As a control, we also determined the induction factors for the groEL transcript from the same cultures. While heat stress resulted in a rapid induction with about a 16-fold increase after 10 min (Fig. 4B), ethanol caused a 5-fold induction and puromycin caused a 12-fold induction (Fig. 4B); in the latter two cases, this increase occurred at later times as already noted for the class I heat shock genes (17). Since both ethanol and puromycin cause the accumulation of denatured proteins within the cytoplasm, nonnative proteins cannot be responsible for the induction of htpG. This conclusion raised the question whether the absolute temperature rather than the increment in temperature might affect the induction ratio.
FIG. 4.
Neither ethanol nor puromycin induces htpG. Three cultures of B. subtilis 1012 were grown to mid-exponential phase in LB medium at 37°C and then either subjected to a heat shock (50°C) or treated with ethanol (4%, vol/vol) or puromycin (20 μg/ml, final concentration). Aliquots were withdrawn from all three cultures immediately before (t = 0) and at different times after treatment with the stressor (t = 5, 10, 20, 40, and 60 min). Total RNA prepared from all aliquots was bound to a positively charged nylon membrane and hybridized with DIG-labeled antisense RNA probes specific for htpG (A) and groEL (B). The mRNA level in the control prior to stress (t = 0) was set to 1, and the induction ratios are shown. Black columns, 50°C; gray columns, 4% ethanol; white columns, 20 μg of puromycin per ml.
The induction ratio of htpG is dependent on the absolute temperature.
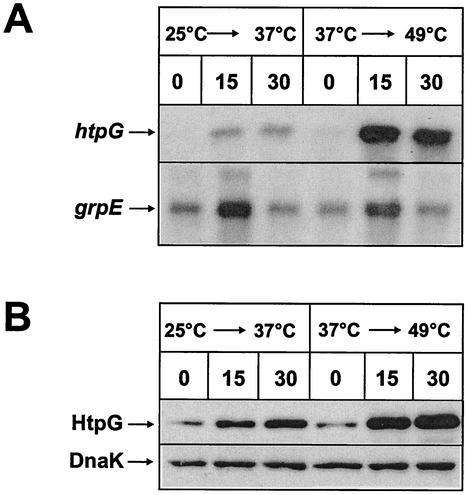
To investigate the assumption that the absolute temperature might dictate the induction factor of htpG, we pregrew B. subtilis 1012 at two different temperatures (25 and 37°C) to the exponential phase and subjected half of the culture to a heat shock by increasing the temperature in both cultures by 12°C, resulting in the final temperatures of 37 and 49°C, respectively. Aliquots were withdrawn immediately before and 15 and 30 min after heat stress for Northern and Western blot analysis. Whereas the amount of htpG transcript exhibited a modest increase after a heat shock from 25 to 37°C, this increase was dramatic after a temperature shift from 37 to 49°C (Fig. 5A). In contrast, the increases in the amounts of grpE transcript, a representative of class I heat shock genes, were comparable in the two heat shock experiments (Fig. 5A). To confirm these data, we also quantified the level of HtpG protein by immunoblotting. Again, the increase in the level of HtpG was significantly higher after the heat shock from 37 to 49°C than after that from 25 to 37°C (Fig. 5B), while the level of DnaK remained constant. It has to be stressed that the total amount of DnaK protein after a challenge with heat stress only doubles (A. Mogk, personal communication). These results strongly indicate that the heat induction factor for htpG seems to depend on the absolute temperature rather than the temperature increment.
FIG. 5.
The heat shock induction factor of htpG is dependent on the absolute temperature. Two cultures of B. subtilis 1012 were grown in LB medium to mid-exponential phase at 25 and 37°C followed by a sudden increase in growth temperature by 12°C to 37 and 49°C, respectively. Samples were taken immediately before the heat shock (t = 0) and 15 and 30 min after heat challenge, and the amounts of htpG- and grpE-specific transcripts and HtpG and DnaK proteins were assayed by Northern (A) and Western (B) blotting. Genes grpE and dnaK are both representatives of class I heat shock genes.
DISCUSSION
A tight regulation of the heat shock genes is critical for the survival of organisms in their natural environment. While the stress proteins produced as a response to high temperature and some other stresses, in particular molecular chaperones and ATP-dependent proteases, are remarkably similar in prokaryotes and eukaryotes, regulation of the heat shock response can be quite different. While E. coli uses only alternative sigma factors (35), B. subtilis has been reported elsewhere to regulate its heat shock response via two different repressors and one alternative sigma factor (21, 26, 27). The data presented in this paper show two important novel findings. (i) htpG is the second example of a bacterial heat shock gene being under positive control of a putative transcriptional activator. Quite recently, it has been reported that the heat shock gene lonD of Myxococcus xanthus is under positive control of a two-component signal transduction system (32). (ii) htpG seems not to be induced by nonnative proteins arising within the cytoplasm. Two additional findings are worth mentioning: the regulatory sequence recognized by the transcriptional activator is located downstream of the σA-dependent promoter, instead of upstream as is usually the case, and the absolute temperature rather than the increase in temperature affects the expression level.
Several years ago, it was reported that htpG is under negative control (25). This assumption was based on the observation that deletion of the complete upstream region of the putative htpG promoter did not abolish heat induction. Therefore, we reasoned that the regulatory sequence either overlaps with the promoter or is located downstream of it, in both cases pointing to a transcriptional repressor as the regulator of htpG. While the first part of the assumption could be verified, namely, location of the binding site downstream of the promoter, the second part turned out not to be correct. All our results clearly indicate that htpG is under positive control by either an alternative sigma factor or a transcriptional activator. The following three results point toward a transcriptional activator rather than an alternative sigma factor: (i) fusing the DAS to the lepA promoter conferred heat inducibility on that promoter, (ii) deletion of the −35 region or replacement of critical nucleotides within the −35 or the −10 region of the htpG promoter completely abolished heat induction, and (iii) neither deleting the 6-bp spacer region located between the −10 region and the DAS nor moving the DAS up to 51 bp downstream of the promoter prevented heat induction.
The presence of a functional binding site for a transcriptional activator immediately downstream of a σA-dependent promoter is unique to the B. subtilis htpG gene. In most cases, the corresponding binding sites are positioned upstream of the regulated promoters, but binding sites downstream have been reported for other prokaryotes. Efficient transcription of the nifLA promoter of Klebsiella pneumoniae requires binding of NifA to two sites, one upstream and the other downstream of the σ54-dependent promoter. Activation is assumed to occur via a loop in the intervening DNA (14). Regulation of flaN transcription in Caulobacter crescentus depends on a σ54 promoter and two binding sites located downstream of the promoter and recognized by the transcriptional activator FlbD (18). The MetR protein from Salmonella enterica serovar Typhimurium binds upstream and downstream of the metF promoter to counteract the activity of the repressor MetJ (5). The fourth example is phosphorylated PhoP, which activates the weak promoter of the pstS operon in B. subtilis. Here, binding sites upstream and downstream of the −10 region have been mapped by DNase I footprinting. Both sites are needed for PhoP∼P-dependent transcription in vitro (22). DnaA binds to a single site downstream of the bacteriophage λ promoter PR, from which it contacts the β subunit of the E. coli RNA polymerase (30). Another example is rocG of B. subtilis, whose expression depends on a sequence located 1.5 kb downstream of the σ54-dependent promoter to which RocR binds (3). The last example is the transcriptional activator Rns, required for expression of pilin genes of enterotoxigenic E. coli strains. Activation of its own gene requires one upstream and one downstream binding site (19).
What is the exact DNA sequence of the DAS and how could a transcriptional regulator activate transcription at the htpG promoter? From our data, the sequence GAAAAGG is sufficient to promote activation, but we cannot exclude the possibility that a smaller DNA sequence or a sequence with different flanking nucleotides could be recognized by the transcriptional activator. Indeed, while the 5′ flanking G can be replaced by a C residue without impairing its function, the 3′ flanking G is important. DNase I footprint experiments with the identified transcriptional activator will answer this question.
The distance between the −10 region and the DAS is 6 bp. We are able to show here that deletion of this 6-bp spacer region did not impair its function. We also asked whether increasing this distance would affect the functionality of the DAS. Increasing the spacer region to 12 bp (here, the DAS was moved to the other side of the DNA helix with respect to the −10 region), 18 bp, and 51 bp did not affect either the basal or the postinduction level significantly, allowing the conclusion that moving the DAS up to 51 bp downstream of the promoter region did not prevent binding of the transcriptional activator or its activity.
Neither ethanol nor puromycin, two classical inducers of the heat shock response in many organisms, was able to induce htpG. Such a failure of htpG induction was also seen after addition of the analogous amino acid norleucine to growing cells while the class I heat shock genes were induced (O. Sipos, unpublished results). Therefore, the inducing signal has to be generated outside the cytoplasm, either within the membrane or on the outside of the cell. This conclusion is strengthened by the finding that the absolute temperature after a heat shock rather than the temperature increment influences the induction factor. Vigh and coworkers have suggested that membranes can act as temperature sensors (34). Modification, by genetic manipulation, of the ratio of unsaturated to saturated fatty acids in Saccharomyces cerevisiae had a significant effect on the expression of the heat shock genes hsp70 and hsp82 (4). To test the hypothesis that the fluidity of the cytoplasmic membrane affects transcription of htpG after a heat shock, we treated B. subtilis cells with the fluidizer benzyl alcohol but were unable to measure any difference from the untreated control (A. Escher, unpublished data). Therefore, it remains an open question whether the cytoplasmic membrane acts as a sensor of heat stress for htpG. Identification of the transcriptional activator of htpG and study of the modulation of its activity might give a clue to this important question.
Last but not least, the classification of the heat shock genes has to be adapted to the new findings. Now, htpG is the first member of class IV, and all genes of the former class IV are now reclassified as class V.
Acknowledgments
We thank O. Sipos for her help with one experiment.
We thank the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie for financial support.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell, J. C. A., and E. A. Craig. 1988. Ancient heat shock gene is dispensable. J. Bacteriol. 170:2977-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belitsky, B. R., and A. L. Sonenshein. 1999. An enhancer element located downstream of the major glutamate dehydrogenase gene of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 96:10290-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrat, L., S. Franceschelli, C. L. Pardini, G. S. Kobayashi, I. Horvath, L. Vigh, and B. Maresca. 1996. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc. Natl. Acad. Sci. USA 93:3870-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan, J. M., M. L. Urbanus, M. Talmi, and G. V. Stauffer. 1993. Regulation of the Salmonella typhimurium metF gene by the MetR protein. J. Bacteriol. 175:5862-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgopoulos, C., and W. J. Welch. 1993. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 9:601-634. [DOI] [PubMed] [Google Scholar]
- 7.Goff, S. A., and A. L. Goldberg. 1985. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell 41:587-595. [DOI] [PubMed] [Google Scholar]
- 8.Halogoua, S., and M. Inouye. 1979. Translocation and assembly of outer membrane proteins in Escherichia coli. J. Mol. Biol. 130:39-61. [DOI] [PubMed] [Google Scholar]
- 9.Hendrick, J. P., and F.-U. Hartl. 1993. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62:349-384. [DOI] [PubMed] [Google Scholar]
- 10.Hirata, H., S. Negoro, and H. Okada. 1985. High production of thermostable β-galactosidase of Bacillus stearothermophilus in Bacillus subtilis. Appl. Environ. Microbiol. 49:1547-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homuth, G., M. Heinemann, U. Zuber, and W. Schumann. 1996. The genes lepA and hemN form a bicistronic operon in Bacillus subtilis. Microbiology 142:1641-1649. [DOI] [PubMed] [Google Scholar]
- 12.Homuth, G., S. Masuda, A. Mogk, Y. Kobayashi, and W. Schumann. 1997. The dnaK operon of Bacillus subtilis is heptacistronic. J. Bacteriol. 179:1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandel, M., and A. Higa. 1970. Calcium dependent bacteriophage DNA infection. J. Mol. Biol. 53:154-162. [DOI] [PubMed] [Google Scholar]
- 14.Minchin, S. D., S. Austin, and R. A. Dixon. 1988. The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol. Microbiol. 2:433-442. [DOI] [PubMed] [Google Scholar]
- 15.Mogk, A., R. Hayward, and W. Schumann. 1996. Integrative vectors for constructing single-copy transcriptional fusions between Bacillus subtilis promoters and various reporter genes encoding heat-stable enzymes. Gene 182:33-36. [DOI] [PubMed] [Google Scholar]
- 16.Mogk, A., G. Homuth, C. Scholz, L. Kim, F. X. Schmid, and W. Schumann. 1997. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16:4579-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogk, A., A. Völker, S. Engelmann, M. Hecker, W. Schumann, and U. Völker. 1998. Nonnative proteins induce expression of the Bacillus subtilis CIRCE regulon. J. Bacteriol. 180:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullin, D. A., and A. Newton. 1993. A σ54 promoter and downstream sequence elements ftr2 and ftr3 are required for regulated expression of divergent transcription units flaN and flbG in Caulobacter crescentus. J. Bacteriol. 175:2067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munson, G. P., and J. R. Scott. 2000. Rns, a virulence regulator within the AraC family, requires binding sites upstream and downstream of its own promoter to function as an activator. Mol. Microbiol. 36:1391-1402. [DOI] [PubMed] [Google Scholar]
- 20.Narberhaus, F. 1999. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Price, C. W. 2002. General stress response, p. 369-384. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 22.Qi, Y., and F. M. Hulett. 1998. PhoP-P and RNA polymerase σA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol. Microbiol. 28:1187-1197. [DOI] [PubMed] [Google Scholar]
- 23.Reischl, S., T. Wiegert, and W. Schumann. 2002. Isolation and analysis of mutant alleles of the Bacillus subtilis HrcA repressor with reduced dependency on GroE function. J. Biol. Chem. 277:32659-32667. [DOI] [PubMed] [Google Scholar]
- 24.Saito, H., T. Shibata, and T. Ando. 1979. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol. Gen. Genet. 170:117-122. [DOI] [PubMed] [Google Scholar]
- 25.Schulz, A., S. Schwab, S. Versteeg, and W. Schumann. 1997. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J. Bacteriol. 10:3103-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumann, W. 2000. Function and regulation of temperature-inducible bacterial proteins on the cellular metabolism. Adv. Biochem. Eng. Biotechnol. 67:1-33. [DOI] [PubMed] [Google Scholar]
- 27.Schumann, W., M. Hecker, and T. Msadek. 2002. Regulation and function of heat-inducible genes in Bacillus subtilis, p. 359-368. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 28.Smith, J. D., R. R. Traut, G. M. Blackburn, and R. E. Monroe. 1965. Action of puromycin in polyadenylic acid-directed polylysine synthesis. J. Mol. Biol. 13:617-628. [Google Scholar]
- 29.So, A., and E. W. Davie. 1964. The effects of organic solvents on protein biosynthesis and their influence on the amino acid code. Biochemistry 3:1165-1169. [DOI] [PubMed] [Google Scholar]
- 30.Szalewska-Palasz, A., A. Wegrzyn, A. Blaszczak, and K. Taylor. 1998. DnaA-stimulated transcriptional activation of oriλ: Escherichia coli RNA polymerase β subunit as a transcriptional activator. Proc. Natl. Acad. Sci. USA 95:4241-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, J. G., and F. Baneyx. 2000. ClpB and HtpG facilitate de novo protein folding in stressed Escherichia coli cells. Mol. Microbiol. 36:1360-1370. [DOI] [PubMed] [Google Scholar]
- 32.Ueki, T., and S. Inouye. 2002. Transcriptional activation of a heat-shock gene, lonD, of Myxococcus xanthus by a two component histidine-aspartate phosphorelay system. J. Biol. Chem. 277:6170-6177. [DOI] [PubMed] [Google Scholar]
- 33.Versteeg, S., A. Mogk, and W. Schumann. 1999. The Bacillus subtilis htpG gene is not involved in thermal stress management. Mol. Gen. Genet. 261:582-588. [DOI] [PubMed] [Google Scholar]
- 34.Vigh, L., B. Maresca, and J. L. Harwood. 1998. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23:369-374. [DOI] [PubMed] [Google Scholar]
- 35.Yura, T., M. Kanemori, and M. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress response. ASM Press, Washington, D.C.