Abstract
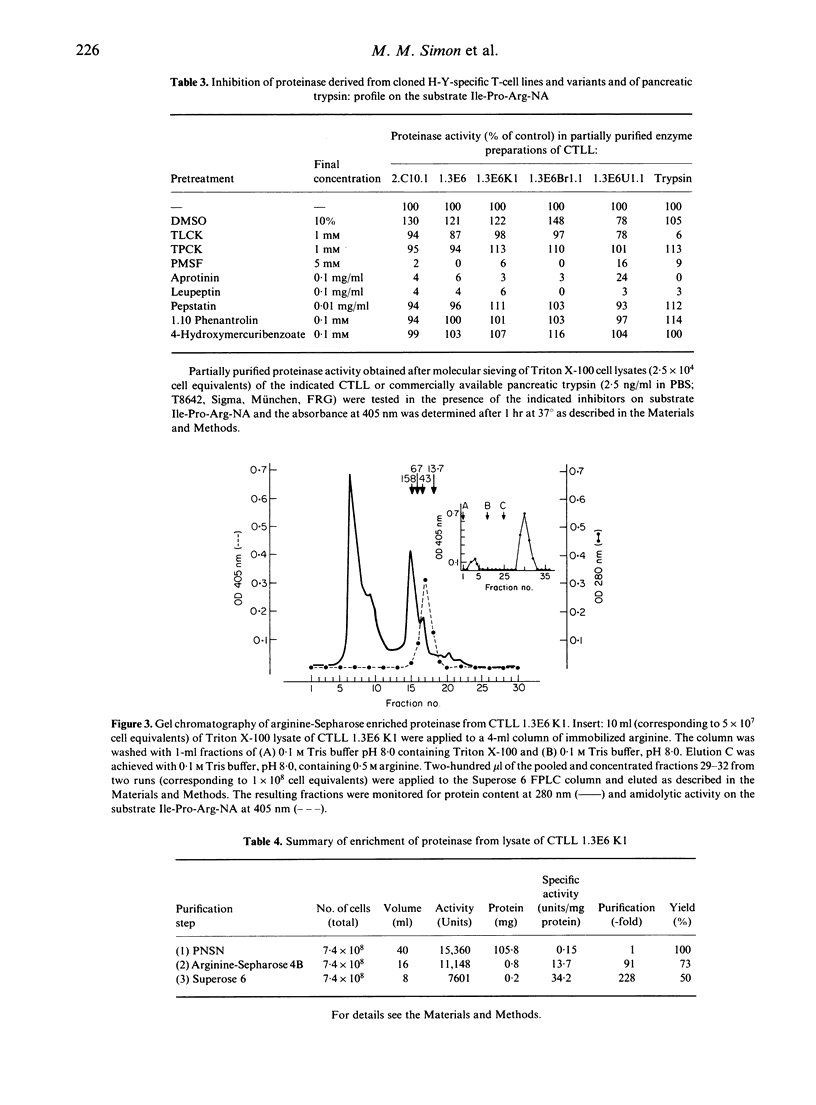
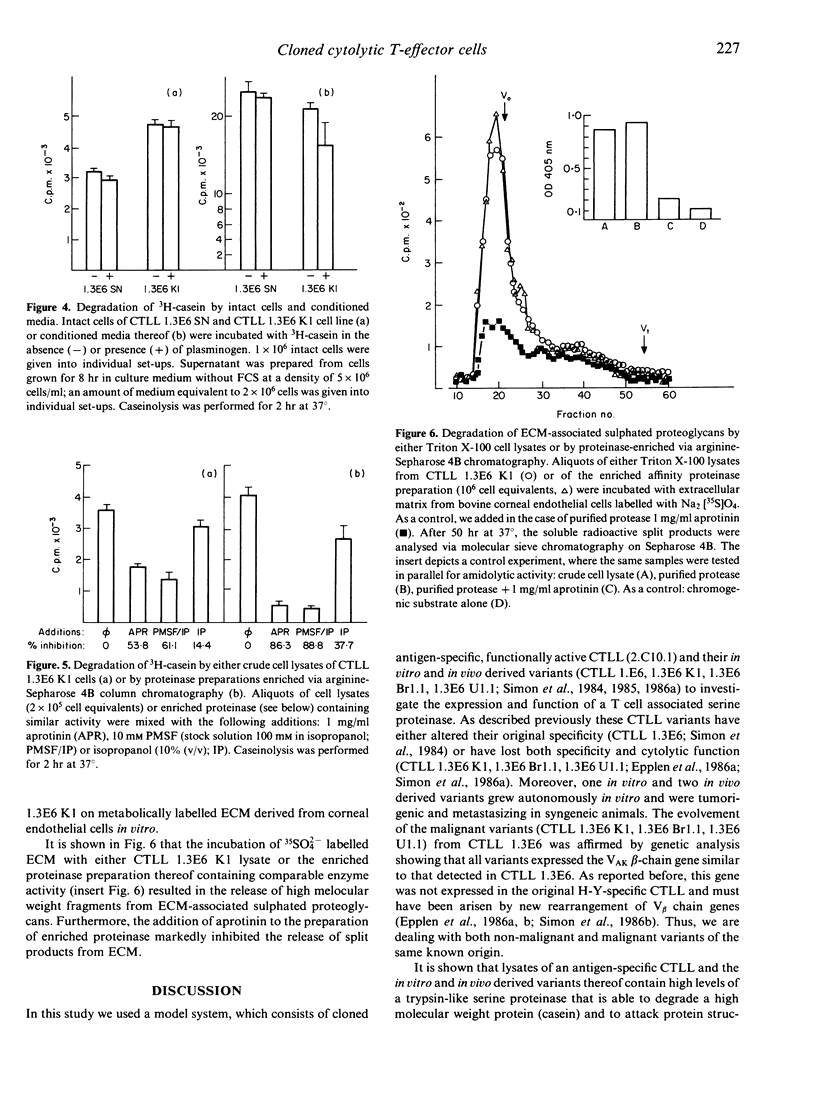
This report describes the distribution of a trypsin-like proteinase in defined homogeneous cytolytic T-cell lines (CTLL) and their in vitro and in vivo derived malignant T-lymphoma variants. By means of chromogenic peptide substrates, we found the enzyme to attack preferentially at the carboxy terminus of arginine, in particular when non-polar amino acids were present in the amino terminal neighbouring position. The enzyme was identified by means of various inhibitors as a serine type proteinase having a pH optimum around 8 X 5. Affinity chromatography in connection with molecular sieving resulted in a 200-fold purification and indicated a molecular weight (MW) of about 50,000 for the proteinase. The enzyme was found to be highly expressed in antigen-specific CTLL as well as in their tumorigenic variants. Both intact lymphocytes of all CTLL tested and Triton X-100 lysates or enriched proteinase preparations thereof were able to degrade a high molecular weight protein (casein) and to release high molecular weight split products from the sulphated proteoglycans in subendothelial extracellular matrix. The results are discussed with respect to the invasiveness of normal and malignant T lymphocytes and the proteinase is suggested to be crucially involved in the process of cellular migration in vivo.
Full text
PDF


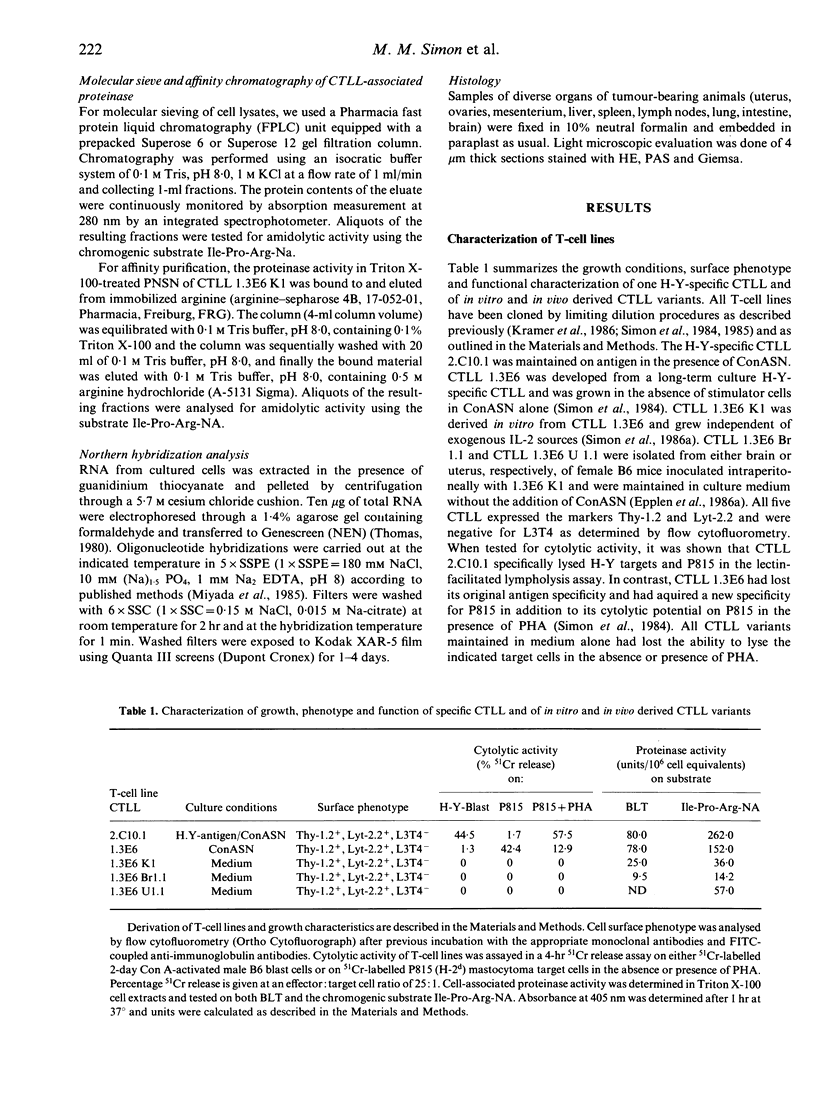

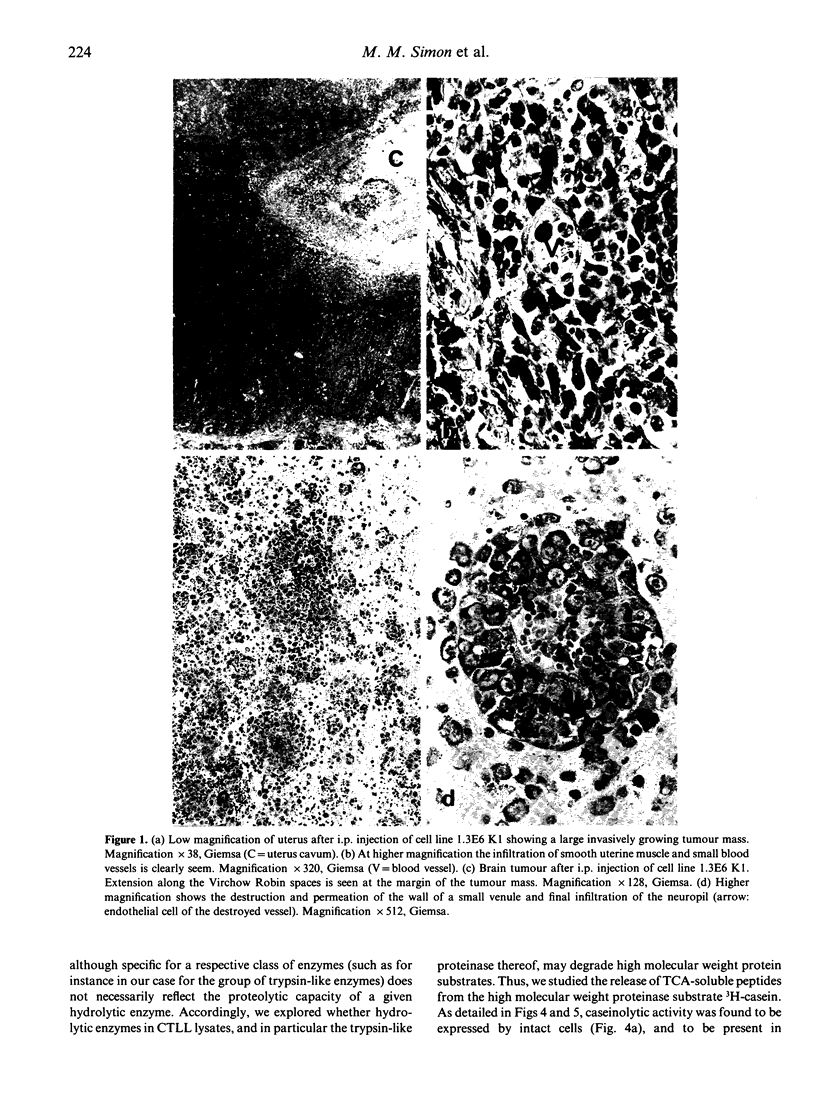
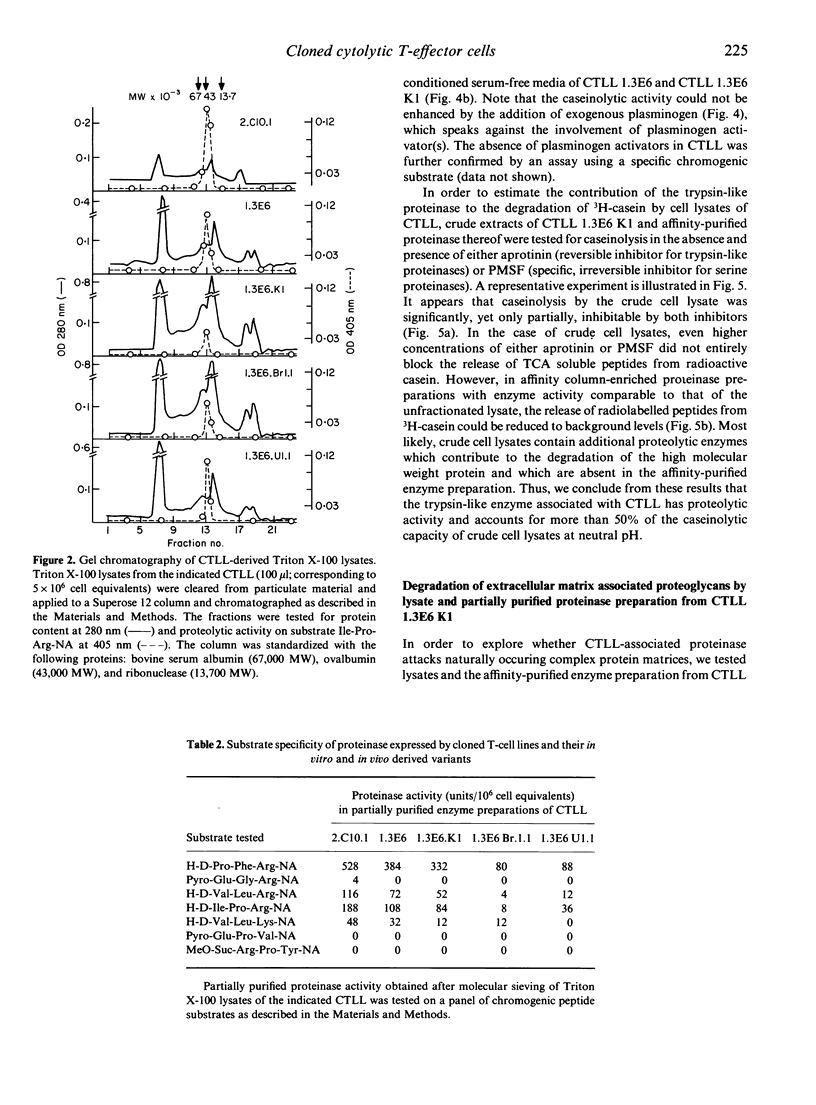



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Ner M., Kramer M. D., Schirrmacher V., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Sequential degradation of heparan sulfate in the subendothelial extracellular matrix by highly metastatic lymphoma cells. Int J Cancer. 1985 Apr 15;35(4):483–491. doi: 10.1002/ijc.2910350411. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Ts'o P. O. Quantitation of fibrinolytic activity of Syrian hamster fibroblasts using 3H-labeled fibrinogen prepared by reductive alkylation. Cancer Res. 1977 Apr;37(4):1182–1185. [PubMed] [Google Scholar]
- Bevan M. J., Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol. 1975 Feb;114(2 Pt 1):559–565. [PubMed] [Google Scholar]
- Epplen J. T., Ali S., Rinaldy A., Simon M. M. The change of specificity, karyotype, and antigen-receptor gene expression is correlated in cytotoxic T-cell lines. Curr Top Microbiol Immunol. 1986;126:69–74. doi: 10.1007/978-3-642-71152-7_9. [DOI] [PubMed] [Google Scholar]
- Fulton R. J., Hart D. A. Detection and partial characterization of lymphoid cell surface proteases. Cell Immunol. 1980 Oct;55(2):394–405. doi: 10.1016/0008-8749(80)90170-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Foidart J. M., Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981 May;107(2):171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A. L., Birdwell C. R. Stimulation of corneal endothelial cell proliferations in vitro by fibroblast and epidermal growth factors. Exp Eye Res. 1977 Jul;25(1):75–89. doi: 10.1016/0014-4835(77)90248-2. [DOI] [PubMed] [Google Scholar]
- Goutner A., Simmler M. C., Tapon J., Rosenfeld C. Modulation by alpha-2 macroglobulin of human lymphocyte proliferation in response to mitogens and antigen. Differentiation. 1976 Jun 4;5(2-3):171–173. doi: 10.1111/j.1432-0436.1976.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Thiobenzyl benzyloxycarbonyl-L-lysinate, substrate for a sensitive colorimetric assay for trypsin-like enzymes. Anal Biochem. 1979 Mar;93(2):223–226. doi: 10.1016/s0003-2697(79)80141-4. [DOI] [PubMed] [Google Scholar]
- Hatcher V. B., Oberman M. S., Lazarus G. S., Grayzel A. I. A cytotoxic proteinase isolated from human lymphocytes. J Immunol. 1978 Feb;120(2):665–670. [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Kammer G. M., Sapolsky A. I., Malemud C. J. Secretion of an articular cartilage proteoglycan-degrading enzyme activity by murine T lymphocytes in vitro. J Clin Invest. 1985 Aug;76(2):395–402. doi: 10.1172/JCI111985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. D., Binninger L., Schirrmacher V., Moll H., Prester M., Nerz G., Simon M. M. Characterization and isolation of a trypsin-like serine protease from a long-term culture cytolytic T cell line and its expression by functionally distinct T cells. J Immunol. 1986 Jun 15;136(12):4644–4651. [PubMed] [Google Scholar]
- Kramer M. D., Robinson P., Vlodavsky I., Barz D., Friberger P., Fuks Z., Schirrmacher V. Characterization of an extracellular matrix-degrading protease derived from a highly metastatic tumor cell line. Eur J Cancer Clin Oncol. 1985 Mar;21(3):307–316. doi: 10.1016/0277-5379(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Matzner Y., Bar-Ner M., Yahalom J., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985 Oct;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyada C. G., Klofelt C., Reyes A. A., McLaughlin-Taylor E., Wallace R. B. Evidence that polymorphism in the murine major histocompatibility complex may be generated by the assortment of subgene sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2890–2894. doi: 10.1073/pnas.82.9.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Dornand J., Kaplan J. G. Inhibition of lymphocyte transformation: effect of soy bean trypsin inhibitor and synthetic anti-proteases. Can J Biochem. 1975 Dec;53(12):1337–1341. doi: 10.1139/o75-182. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Schwartz D. E., Thonar E. J., Kuettner K. E. Tumor invasion and host extracellular matrix. Cancer Metastasis Rev. 1983;2(2):129–152. doi: 10.1007/BF00048966. [DOI] [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Fuks Z. Interaction of T lymphocytes and macrophages with cultured vascular endothelial cells: attachment, invasion, and subsequent degradation of the subendothelial extracellular matrix. J Cell Physiol. 1984 Feb;118(2):169–178. doi: 10.1002/jcp.1041180209. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Waller C. A. Quantitative determination of disseminated tumor cells by [3H]thymidine incorporation in vitro and by agar colony formation. Cancer Res. 1982 Feb;42(2):660–666. [PubMed] [Google Scholar]
- Simon M. M., Nerz G., Prester M., Moll H. Immunoregulation by mouse T cell clones. III. Cloned H-Y-specific cytotoxic T cells secrete a soluble mediator(s) that inhibits cytotoxic responses by acting on both Lyt-2- and L3T4- lymphocytes. Eur J Immunol. 1985 Aug;15(8):773–783. doi: 10.1002/eji.1830150807. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Weltzien H. U., Bühring H. J., Eichmann K. Aged murine killer T-cell clones acquire specific cytotoxicity for P815 mastocytoma cells. Nature. 1984 Mar 22;308(5957):367–370. doi: 10.1038/308367a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L. Stimulation of mouse B lymphocytes by trypsin. J Immunol. 1974 Jul;113(1):58–62. [PubMed] [Google Scholar]