Abstract
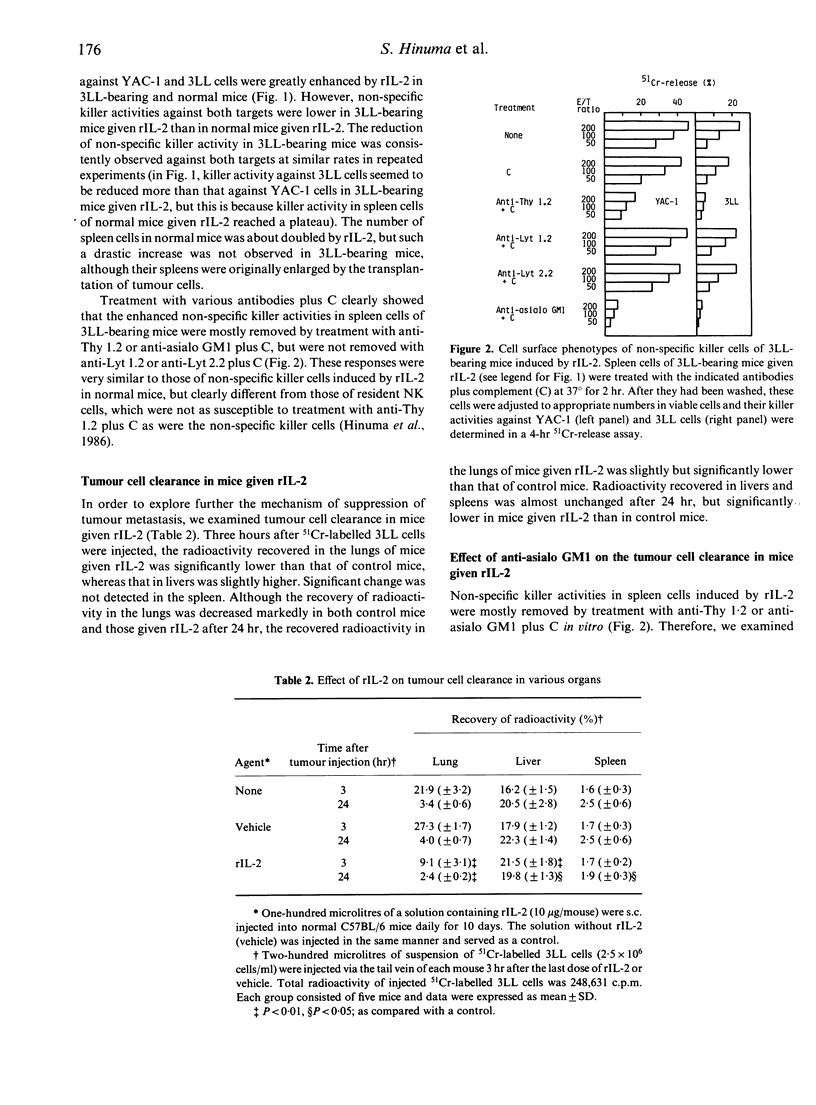
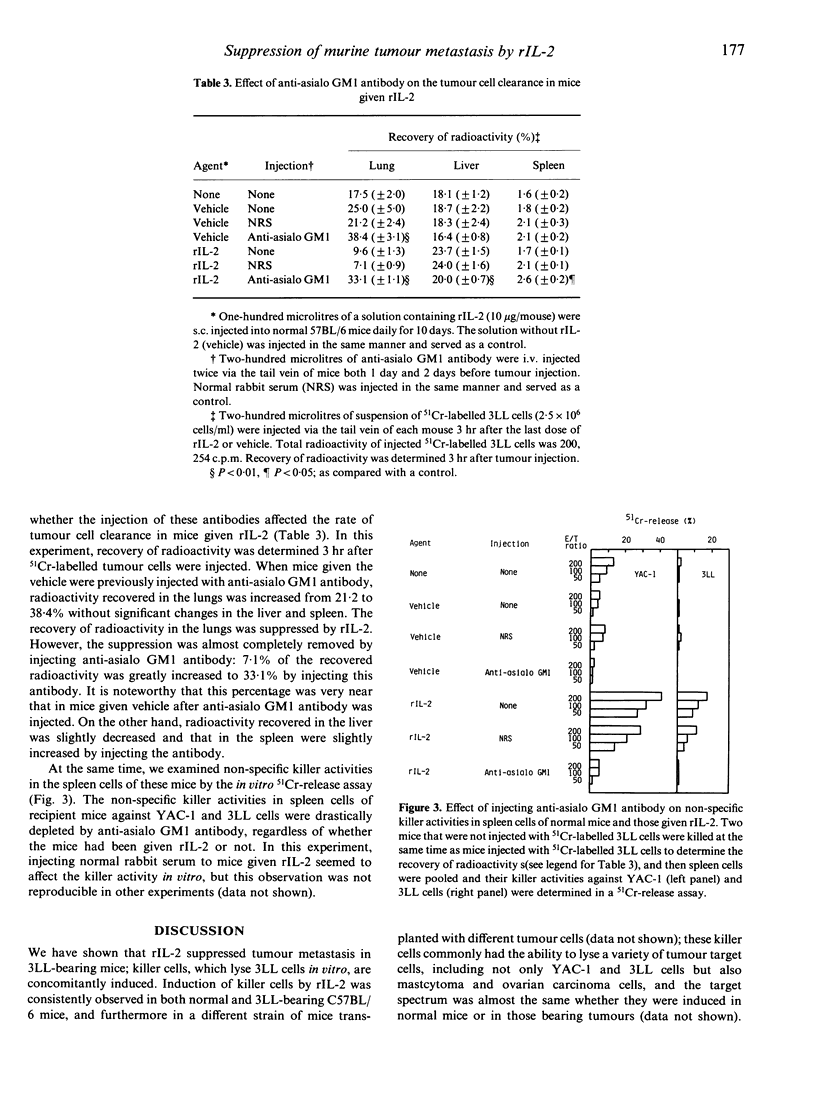
Recombinant human interleukin-2 (rIL-2) suppressed metastatic tumour colony formation in the lungs of C57BL/6 mice bearing Lewis lung carcinoma (3LL). In tumour-bearing mice given rIL-2, non-specific killer cells that were cytotoxic not only against natural killer-sensitive YAC-1 cells but also against 3LL cells in an in vitro 51Cr-release assay were concomitantly induced as tumour metastasis was suppressed. These non-specific killer cells were mostly removed by treatment with anti-Thy 1.2 or anti-asialo GM1 antibody plus complement (C) in vitro but not with anti-Lyt 1.2 or anti-Lyt 2.2 plus C, indicating that they were positive for Thy 1 and asialo GM1 but not for Lyt 1 and Lyt 2. In order to explore the mechanism by which rIL-2 suppressed tumour metastasis, we examined the clearance of intravenously injected 51Cr-labelled 3LL cells in the lungs of mice given rIL-2. The rate of tumour cell clearance was increased. This enhanced clearance was almost completely removed by injecting anti-asialo GM1 antibody. In addition, the injection of anti-asialo GM1 antibody also depleted most of the non-specific killer cells induced by administering rIL-2. These results indicate that asialo GM1-positive cells are not only cytotoxic in vitro but also play a critical role in the clearance of 3LL cells in the lungs in vivo. Our results indicate that asialo GM1-positive cells play an important role as anti-metastatic effector cells in suppressing the metastasis of 3LL cells in mice given rIL-2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlozzari T., Leonhardt J., Wiltrout R. H., Herberman R. B., Reynolds C. W. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985 Apr;134(4):2783–2789. [PubMed] [Google Scholar]
- Chang A. E., Hyatt C. L., Rosenberg S. A. Systemic administration of recombinant human interleukin-2 in mice. J Biol Response Mod. 1984 Oct;3(5):561–572. [PubMed] [Google Scholar]
- Donohue J. H., Lotze M. T., Robb R. J., Rosenstein M., Braziel R. M., Jaffe E. S., Rosenberg S. A. In vivo administration of purified Jurkat-derived interleukin 2 in mice. Cancer Res. 1984 Apr;44(4):1380–1386. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Hinuma S., Naruo K., Shiho O., Tsukamoto K. Characteristics of murine non-specific killer cells induced in vivo by recombinant human interleukin-2. Immunology. 1986 Oct;59(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Hinuma S., Onda H., Naruo K., Ichimori Y., Koyama M., Tsukamoto K. Translation of interleukin 2 mRNA from human peripheral blood leukocytes in Xenopus oocytes. Biochem Biophys Res Commun. 1982 Nov 30;109(2):363–369. doi: 10.1016/0006-291x(82)91729-6. [DOI] [PubMed] [Google Scholar]
- Kato K., Naruo K., Koyama M., Kawahara K., Hinuma S., Tada H., Sugino H., Tsukamoto K. Purification and partial sequence analysis of human interleukin-2 derived from peripheral blood leukocytes. Biochem Biophys Res Commun. 1985 Feb 28;127(1):182–190. doi: 10.1016/s0006-291x(85)80142-x. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamada T., Kawahara K., Onda H., Asano T., Sugino H., Kakinuma A. Purification and characterization of recombinant human interleukin-2 produced in Escherichia coli. Biochem Biophys Res Commun. 1985 Jul 31;130(2):692–699. doi: 10.1016/0006-291x(85)90472-3. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Lotze M. T., Frana L. W., Sharrow S. O., Robb R. J., Rosenberg S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985 Jan;134(1):157–166. [PubMed] [Google Scholar]
- Mazumder A., Rosenberg S. A. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984 Feb 1;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Naruo K., Hinuma S., Kato K., Koyama M., Tada H., Shiho O., Tsukamoto K. Comparison of the biological properties of purified natural and recombinant human interleukin-2. Biochem Biophys Res Commun. 1985 Apr 16;128(1):257–264. doi: 10.1016/0006-291x(85)91672-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R., Handa K., Itoh K., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). I. Proliferative response and establishment of cloned cells. J Immunol. 1983 Feb;130(2):981–987. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Gorelik E., Brunda M. J., Holden H. T., Herberman R. B. Assessment of in vivo natural antitumor resistance and lymphocyte. Migration in mice: comparison of 125I-iododeoxyuridine with 111indium-oxine and 51chromium as cell labels. Cancer Immunol Immunother. 1983;14(3):172–179. doi: 10.1007/BF00205356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Herberman R. B., Zhang S. R., Chirigos M. A., Ortaldo J. R., Green K. M., Jr, Talmadge J. E. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol. 1985 Jun;134(6):4267–4275. [PubMed] [Google Scholar]
- Wiltrout R. H., Santoni A., Peterson E. S., Knott D. C., Overton W. R., Herberman R. B., Holden H. T. Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J Leukoc Biol. 1985 May;37(5):597–614. doi: 10.1002/jlb.37.5.597. [DOI] [PubMed] [Google Scholar]
- Yamada T., Kato K., Kawahara K., Nishimura O. Separation of recombinant human interleukin-2 and methionyl interleukin-2 produced in Escherichia coli. Biochem Biophys Res Commun. 1986 Mar 28;135(3):837–843. doi: 10.1016/0006-291x(86)91004-1. [DOI] [PubMed] [Google Scholar]


