Abstract
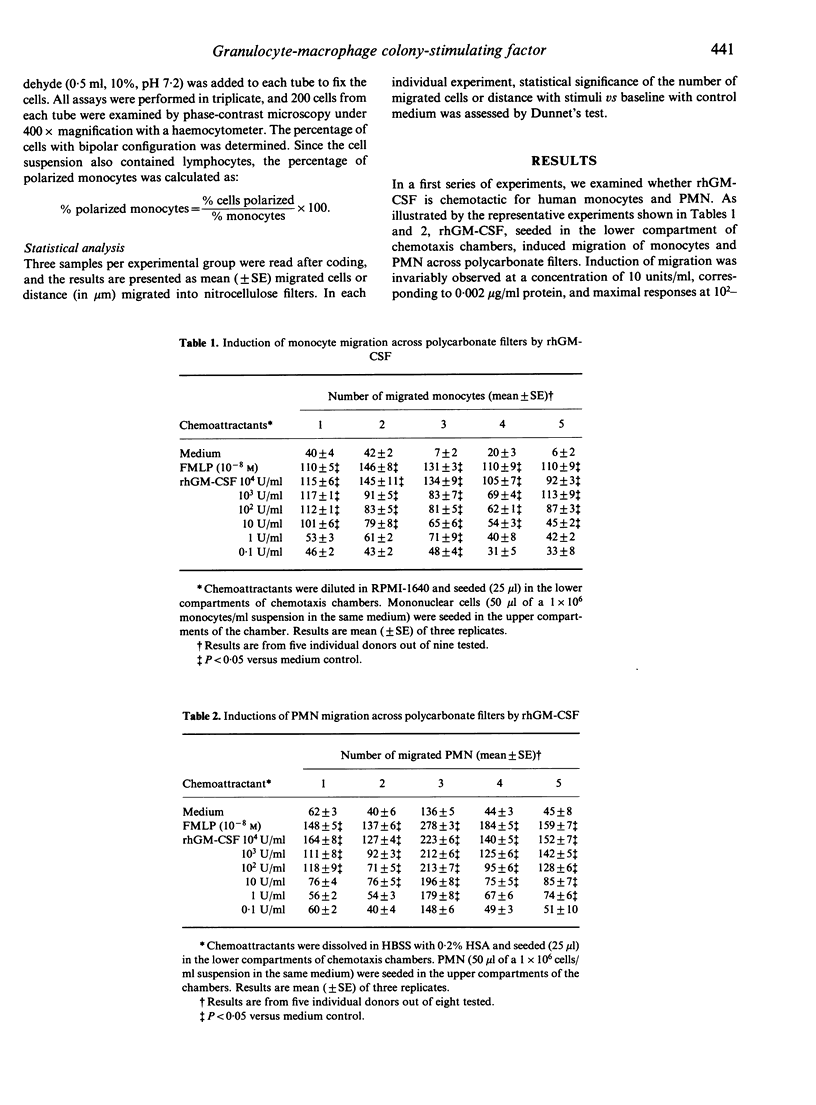
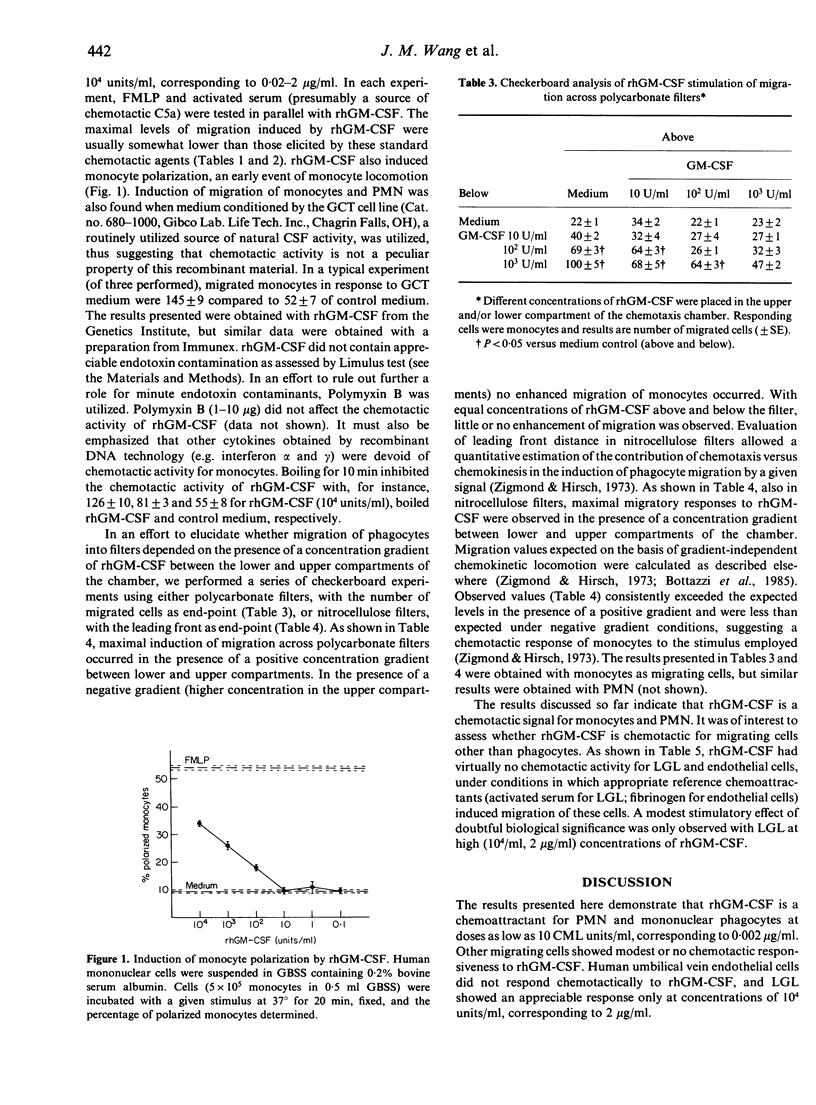
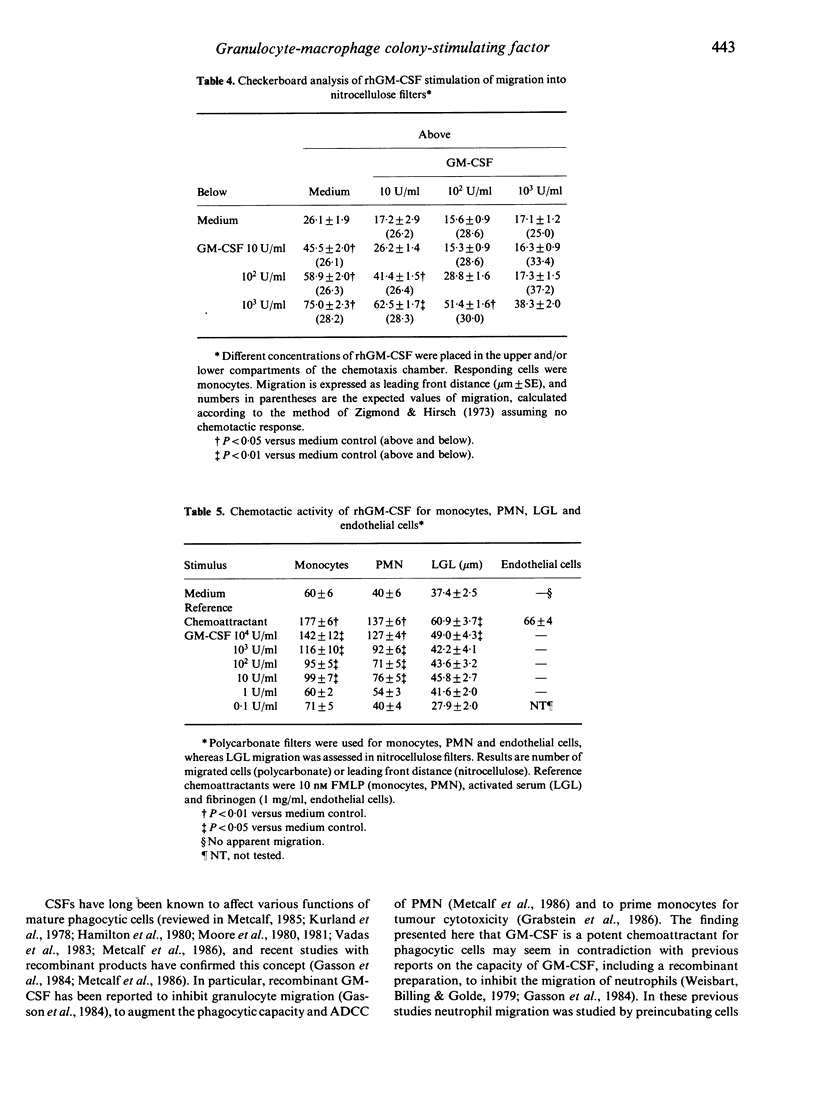
Recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) seeded in the lower compartment of chemotaxis chambers induced migration of human peripheral blood monocytes and polymorphonuclear phagocytes. rhGM-CSF was active in inducing phagocyte migration at concentrations as low as 10 CML units/ml (corresponding to 0.002 microgram), and maximal activity was observed at 10(2)-10(4)/ml. Checkerboard analysis performed by seeding different concentrations of rhGM-CSF above and below the filter revealed that maximal induction of migration required a positive concentration gradient between the lower and upper compartment, and that rhGM-CSF elicited an actual chemotactic response in phagocytes. Human umbilical vein endothelial cells and blood large granular lymphocytes (LGL) responded poorly or not at all (endothelial cells) to rhGM-CSF under conditions in which appropriate reference chemoattractants were active. The chemotactic activity of rhGM-CSF may serve to recruit phagocytic cells from the blood compartment to amplify resistance against noxious agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bottazzi B., Introna M., Allavena P., Villa A., Mantovani A. In vitro migration of human large granular lymphocytes. J Immunol. 1985 Apr;134(4):2316–2321. [PubMed] [Google Scholar]
- Cianciolo G., Hunter J., Silva J., Haskill J. S., Snyderman R. Inhibitors of monocyte responses to chemotaxins are present in human cancerous effusions and react with monoclonal antibodies to the P15(E) structural protein of retroviruses. J Clin Invest. 1981 Oct;68(4):831–844. doi: 10.1172/JCI110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Polentarutti N., Balconi G., Ryckewaert J. J., Larrieu M. J., Donati M. B., Mantovani A., Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985 Jan;75(1):11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Stanley E. R., Burgess A. W., Shadduck R. K. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol. 1980 Jun;103(3):435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. S., Broxmeyer H. E., Moore M. A. Limitation of excessive myelopoiesis by the intrinsic modulation of macrophage-derived prostaglandin E. Science. 1978 Feb 3;199(4328):552–555. doi: 10.1126/science.304600. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- Vadas M. A., Nicola N. A., Metcalf D. Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol. 1983 Feb;130(2):795–799. [PubMed] [Google Scholar]
- Weisbart R. H., Billing R., Golde D. W. Neutrophil migration-inhibition activity produced by a unique T lymphoblast cell line. J Lab Clin Med. 1979 Apr;93(4):622–626. [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


