Abstract
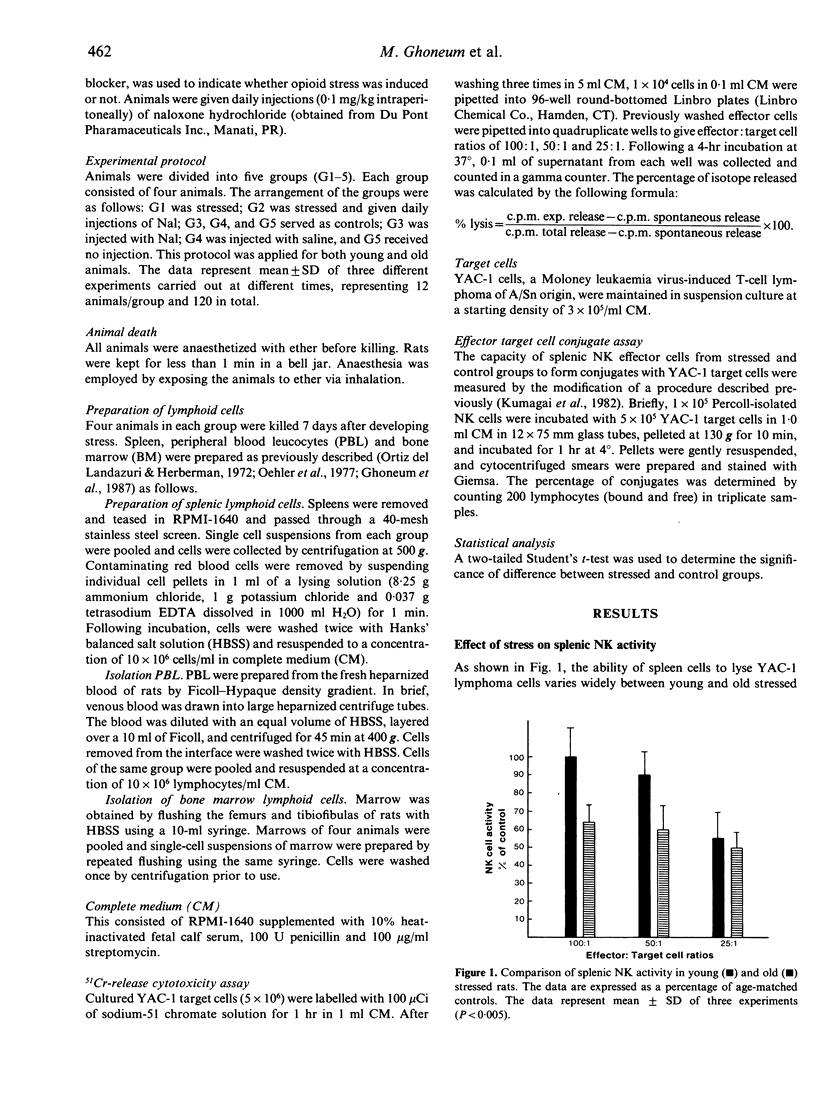
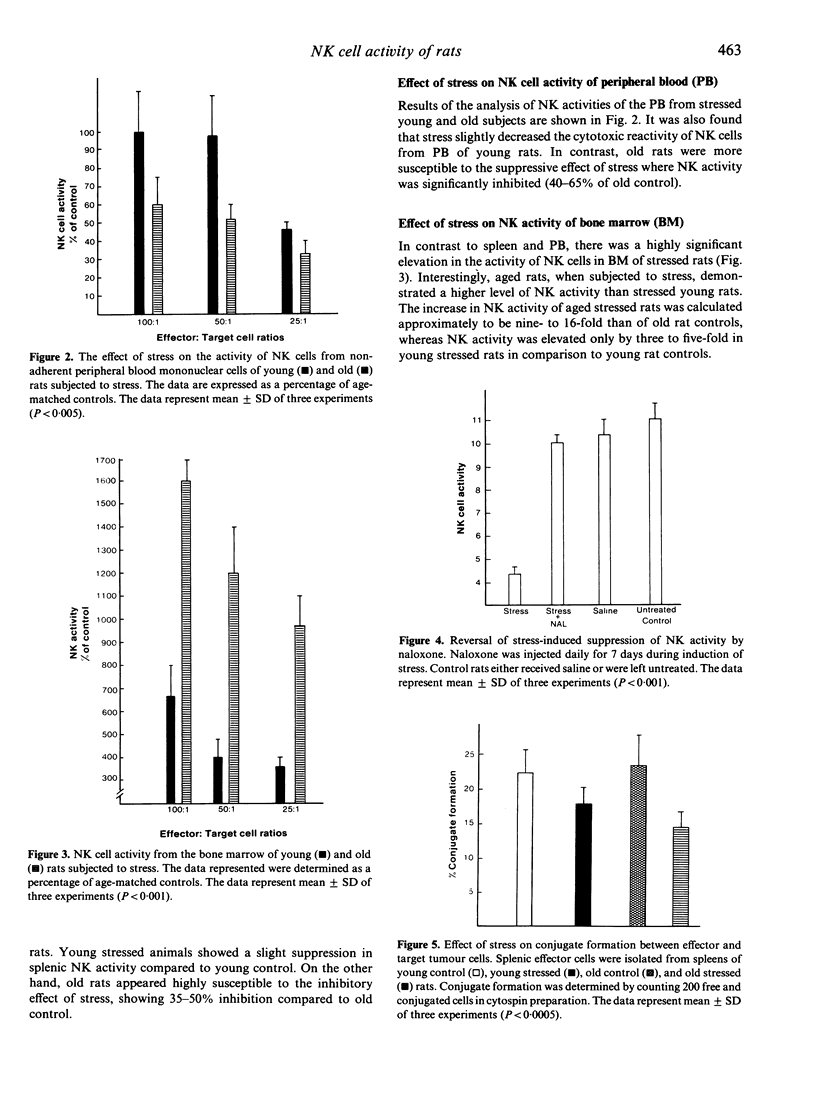
We determined an in vivo response of NK cells in young and old rats towards the suppressive effect of stress. Stress was developed by isolating rats in separate cages, but control littermates were kept together. Animals were subjected to stress for 7 days, and alterations of NK cell activities were examined in the spleen, peripheral blood (PB) and bone marrow (BM). The results showed that old rats subjected to stress had a remarkable decrease in splenic and PB-NK activity compared to old control rats, concomitant with a highly increased level of NK cell activity in BM. Suppression of the lytic activity in the spleen of stressed old rats was correlated with a decrease in the percentage of conjugate formation between splenic NK cells and target tumour cells. In contrast, stressed young rats demonstrated relatively unchanged activity of NK cells examined in different tissues compared to age-matched controls. We concluded that old animals are more sensitive to the suppressive effect of stress compared to young ones, and the mechanism of this suppression is probably due to the migration of large granular lymphocytes (LGL) from spleen and PB to other sites such as BM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarstad H. J., Gaudernack G., Seljelid R. Stress causes reduced natural killer activity in mice. Scand J Immunol. 1983 Nov;18(5):461–464. doi: 10.1111/j.1365-3083.1983.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Amkraut A., Solomon G. F. Stress and murine sarcoma virus (Moloney)-induced tumors. Cancer Res. 1972 Jul;32(7):1428–1433. [PubMed] [Google Scholar]
- Burchfield S. R., Woods S. C., Elich M. S. Pituitary adrenocortical response to chronic intermittent stress. Physiol Behav. 1980 Feb;24(2):297–302. doi: 10.1016/0031-9384(80)90090-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- De Landazuri M. O., Herberman R. B. Immune response to Gross virus-induced lymphoma. 3. Characteristics of the cellular immune response. J Natl Cancer Inst. 1972 Jul;49(1):147–154. [PubMed] [Google Scholar]
- De Souza E. B., Van Loon G. R. Differential plasma beta-endorphin, beta-lipotropin, and adrenocorticotropin responses to stress in rats. Endocrinology. 1985 Apr;116(4):1577–1586. doi: 10.1210/endo-116-4-1577. [DOI] [PubMed] [Google Scholar]
- Ebbesen P., Rask-Nielsen R. Influence of sex-segregated grouping and of inoculation with subcellular leukemic material on development of nonleukemic lesions in DBA/2, BALB/c, and CBA mice. J Natl Cancer Inst. 1967 Nov;39(5):917–932. [PubMed] [Google Scholar]
- Edwards E. A., Dean L. M. Effects of crowding of mice on humoral antibody formation and protection to lethal antigenic challenge. Psychosom Med. 1977 Jan-Feb;39(1):19–24. doi: 10.1097/00006842-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Corticosteroids and circulating lymphocytes. Transplant Proc. 1975 Mar;7(1):37–40. [PubMed] [Google Scholar]
- Fauci A. S. Immunosuppressive and anti-inflammatory effects of glucocorticoids. Monogr Endocrinol. 1979;12:449–465. doi: 10.1007/978-3-642-81265-1_24. [DOI] [PubMed] [Google Scholar]
- Friedman S. B., Glasgow L. A., Ader R. Psychosocial factors modifying host resistance to experimental infections. Ann N Y Acad Sci. 1969 Oct 14;164(2):381–393. doi: 10.1111/j.1749-6632.1969.tb14052.x. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. H., Egami N., Ijiri K., Cooper E. L. Effect of corticosteroids on the thymus of the fish Oryzias latipes. Dev Comp Immunol. 1986 Winter;10(1):35–44. doi: 10.1016/0145-305x(86)90042-x. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. M., Egami N. Age related changes in morphology of the thymus of the fish, Oryzias latipes. Exp Gerontol. 1982;17(1):33–40. doi: 10.1016/0531-5565(82)90006-7. [DOI] [PubMed] [Google Scholar]
- Henry J. P., Stephens P. M., Watson F. M. Force breeding, social disorder and mammary tumor formation in CBA/USC mouse colonies: a pilot study. Psychosom Med. 1975 May-Jun;37(3):277–283. doi: 10.1097/00006842-197505000-00006. [DOI] [PubMed] [Google Scholar]
- Itoh K., Suzuki R., Umezu Y., Hanaumi K., Kumagai K. Studies of murine large granular lymphocytes. II. Tissue, strain, and age distributions of LGL and LAL. J Immunol. 1982 Jul;129(1):395–405. [PubMed] [Google Scholar]
- Keller S. E., Weiss J. M., Schleifer S. J., Miller N. E., Stein M. Suppression of immunity by stress: effect of a graded series of stressors on lymphocyte stimulation in the rat. Science. 1981 Sep 18;213(4514):1397–1400. doi: 10.1126/science.6973822. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Madden J., 4th, Akil H., Patrick R. L., Barchas J. D. Stress-induced parallel changes in central opioid levels and pain responsiveness in the rat. Nature. 1977 Jan 27;265(5592):358–360. doi: 10.1038/265358a0. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Albright J. W., Good P. I., Peter C. P., Heidrick M. L. Reduced humoral immune activity in long-lived old mice: an approach to elucidating its mechanisms. Immunology. 1976 Dec;31(6):903–911. [PMC free article] [PubMed] [Google Scholar]
- Mansour A., Nelson D. S. Effect of hydrocortisone on the response of rat lymphocytes to phytohaemagglutinin. Aust J Exp Biol Med Sci. 1978 Jun;56(3):301–311. doi: 10.1038/icb.1978.32. [DOI] [PubMed] [Google Scholar]
- Mathews P. M., Froelich C. J., Sibbitt W. L., Jr, Bankhurst A. D. Enhancement of natural cytotoxicity by beta-endorphin. J Immunol. 1983 Apr;130(4):1658–1662. [PubMed] [Google Scholar]
- Newberry B. H., Gildow J., Wogan J., Reese R. L. Inhibition of Huggins tumors by forced restraint. Psychosom Med. 1976 May-Jun;38(3):155–162. doi: 10.1097/00006842-197605000-00001. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B. Natural cell-mediated cytotoxicity in rats. III. Effects of immunopharmacologic treatments on natural reactivity and on reactivity augmented by polyinosinic-polycytidylic acid. Int J Cancer. 1978 Feb 15;21(2):221–229. doi: 10.1002/ijc.2910210214. [DOI] [PubMed] [Google Scholar]
- Pavlidis N., Chirigos M. Stress-induced impairment of macrophage tumoricidal function. Psychosom Med. 1980 Jan;42(1):47–54. doi: 10.1097/00006842-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Riley V. Mouse mammary tumors: alteration of incidence as apparent function of stress. Science. 1975 Aug 8;189(4201):465–467. doi: 10.1126/science.168638. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Lewis J. W., Terman G. W., Gale R. P., Liebeskind J. C. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984 Jan 13;223(4632):188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Sklar L. S., Anisman H. Stress and cancer. Psychol Bull. 1981 May;89(3):369–406. [PubMed] [Google Scholar]
- Spry C. J. Inhibition of lymphocyte recirculation by stress and corticotropin. Cell Immunol. 1972 May;4(1):86–92. doi: 10.1016/0008-8749(72)90007-x. [DOI] [PubMed] [Google Scholar]
- WISTAR R., Jr, HILDEMANN W. H. Effect of stress on skin transplantation immunity in mice. Science. 1960 Jan 15;131(3394):159–160. doi: 10.1126/science.131.3394.159. [DOI] [PubMed] [Google Scholar]


