Abstract
Localization and immunophenotype of lymphocyte subsets in fetal human spleens were studied by employing a panel of monoclonal antibodies (McAb) in an immunoperoxidase staining procedure on frozen tissue sections. Spleens varied from 15 weeks of gestational age (gestational weeks, gw) to newborn. The white pulp consisted of intermediate-sized lymphocytes; no separate compartments could be discerned. Germinal centre development was not observed. Dendritic cells stained for B2, HB5, aC3bR, anti-DRC and OKIa, but in most cases not for immunoglobulin. Although low cellular immunity is observed in neonates, T cells showed adult phenotypes in proportions comparable to the adult situation; immature OKT6(+) lymphocytes were rarely seen. Very few cells stained with anti-NK cell antibody Leu7. B cells all expressed B1, Leu14, aC3bR, T10 and OKIa, were strongly positive for BA1, and mostly stained very weakly for B2 and HB5. Almost all B cells expressed IgM and IgD simultaneously, and very few expressed IgG. IgA-positive cells were absent. At 15 gw a considerable number of IgM(+) B cells showed Leu1 staining, but this decreased during development. These cells may represent the normal counterpart of Leu1(+) IgM(+) cells observed in B-CLL and immunocytic and centrocytic malignant lymphomas. After 25 gw only very few Leu1(+) IgM(+) cells were seen. Altogether, the morphology and immunophenotype of white pulp B cells were different from the predominating adult B-cell subsets, at least until birth. These 'immature' splenic B cells may be precursors for adult splenic B-cell subsets. Considering the presumed role of the marginal zone in the immunity against TI-2 antigens, the absence of a marginal zone at birth may be a main factor in the defective immunity against these antigens in neonates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Miller C. A., Gartland G. L., Balch C. M. Differentiation stages of human natural killer cells in lymphoid tissues from fetal to adult life. J Exp Med. 1983 Jan 1;157(1):273–284. doi: 10.1084/jem.157.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlot P. L., Grennan D., Humphrey J. H. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985 May;15(5):508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- Amlot P. L., Hayes A. E. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985 May 4;1(8436):1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- Anderson K. C., Bates M. P., Slaughenhoupt B. L., Pinkus G. S., Schlossman S. F., Nadler L. M. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984 Jun;63(6):1424–1433. [PubMed] [Google Scholar]
- Andersson B., Skoglund A. C., Rönnholm M., Lindsten T., Lamon E. W., Collisson E. W., Walia A. S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Antin J. H., Emerson S. G., Martin P., Gadol N., Ault K. A. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986 Jan;136(2):505–510. [PubMed] [Google Scholar]
- Asma G. E., Langlois van den Bergh R., Vossen J. M. Development of pre-B and B lymphocytes in the human fetus. Clin Exp Immunol. 1984 May;56(2):407–414. [PMC free article] [PubMed] [Google Scholar]
- Baley J. E., Schacter B. Z. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985 May;134(5):3042–3048. [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Buckley P. J., Dickson S. A., Walker W. S. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985 Apr;134(4):2310–2315. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-2,4,6-trinitrophenyl (TNP) antibody-forming cells in the murine spleen. II. Suppressed antibody response to TNP-Ficoll after elimination of marginal zone cells. Eur J Immunol. 1986 May;16(5):492–497. doi: 10.1002/eji.1830160505. [DOI] [PubMed] [Google Scholar]
- Delia D., Cattoretti G., Bonati A., Villa S., De Braud F., Buscaglia M. Detection of the common acute lymphoblastic leukaemia antigen (CALLA) on B cells from human fetal tissues. A multiple phenotypic characterization. Clin Exp Immunol. 1985 Feb;59(2):305–314. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A. Ontogenetic development of T- and B-lymphocytes and non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 1983;229(2):351–363. doi: 10.1007/BF00214978. [DOI] [PubMed] [Google Scholar]
- Fujimoto J., Stewart S. J., Levy R. Immunochemical analysis of the released Leu-2 (T8) molecule. J Exp Med. 1984 Jul 1;160(1):116–124. doi: 10.1084/jem.160.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathings W. E., Kubagawa H., Cooper M. D. A distinctive pattern of B cell immaturity in perinatal humans. Immunol Rev. 1981;57:107–126. doi: 10.1111/j.1600-065x.1981.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Naiem M., Mason D. Y., Stein H. Human complement (C3b) receptors defined by a mouse monoclonal antibody. Immunology. 1982 Apr;45(4):645–653. [PMC free article] [PubMed] [Google Scholar]
- Gray D., Chassoux D., MacLennan I. C., Bazin H. Selective depression of thymus-independent anti-DNP antibody responses induced by adult but not neonatal splenectomy. Clin Exp Immunol. 1985 Apr;60(1):78–86. [PMC free article] [PubMed] [Google Scholar]
- Harris N. L., Bhan A. K. B-cell neoplasms of the lymphocytic, lymphoplasmacytoid, and plasma cell types: immunohistologic analysis and clinical correlation. Hum Pathol. 1985 Aug;16(8):829–837. doi: 10.1016/s0046-8177(85)80255-0. [DOI] [PubMed] [Google Scholar]
- Harris N. L., Nadler L. M., Bhan A. K. Immunohistologic characterization of two malignant lymphomas of germinal center type (centroblastic/centrocytic and centrocytic) with monoclonal antibodies. Follicular and diffuse lymphomas of small-cleaved-cell type are related but distinct entities. Am J Pathol. 1984 Nov;117(2):262–272. [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R. Development of lymphocyte responses and interactions in the human fetus and newborn. Immunol Rev. 1981;57:39–60. doi: 10.1111/j.1600-065x.1981.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Kamps W. A., Cooper M. D. Microenvironmental studies of pre-B and B cell development in human and mouse fetuses. J Immunol. 1982 Aug;129(2):526–531. [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Pizzolo G., Semenzato G., Chilosi M., Morittu L., Ambrosetti A., Warner N., Bofill M., Janossy G. Distribution and heterogeneity of cells detected by HNK-1 monoclonal antibody in blood and tissues in normal, reactive and neoplastic conditions. Clin Exp Immunol. 1984 Jul;57(1):195–206. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Visser L., De Leij L. Reactivity of presumed anti-natural killer cell antibody Leu 7 with intrafollicular T lymphocytes. Clin Exp Immunol. 1983 Dec;54(3):834–837. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P., Rimm I. J., Umiel T., Griffin J. D., Osathanondh R., Schlossman S. F., Nadler L. M. Ontogeny of human hematopoietic cells: analysis utilizing monoclonal antibodies. J Immunol. 1983 Jul;131(1):232–237. [PubMed] [Google Scholar]
- Ruben F. L., Hankins W. A., Zeigler Z., Norden C. W., Harrison A., Winkelstein A., Herrmann D. J. Antibody responses to meningococcal polysaccharide vaccine in adults without a spleen. Am J Med. 1984 Jan;76(1):115–121. doi: 10.1016/0002-9343(84)90759-9. [DOI] [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
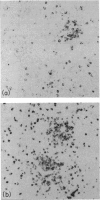
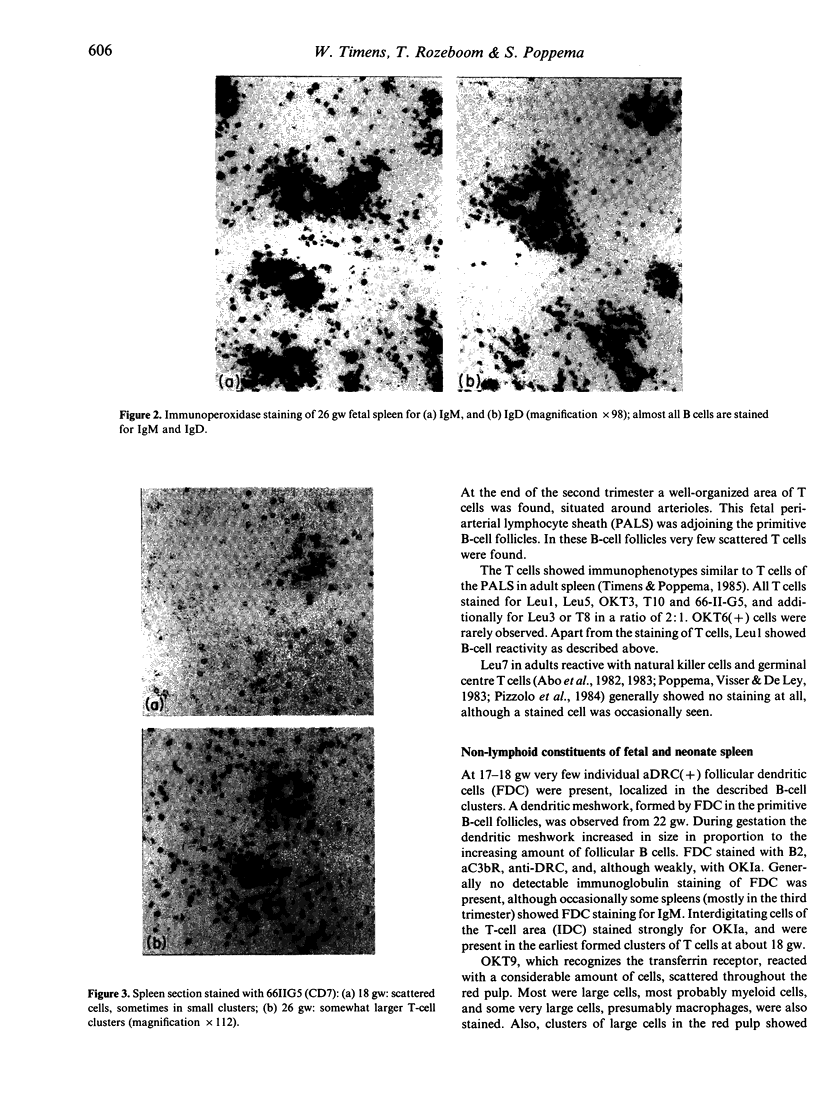
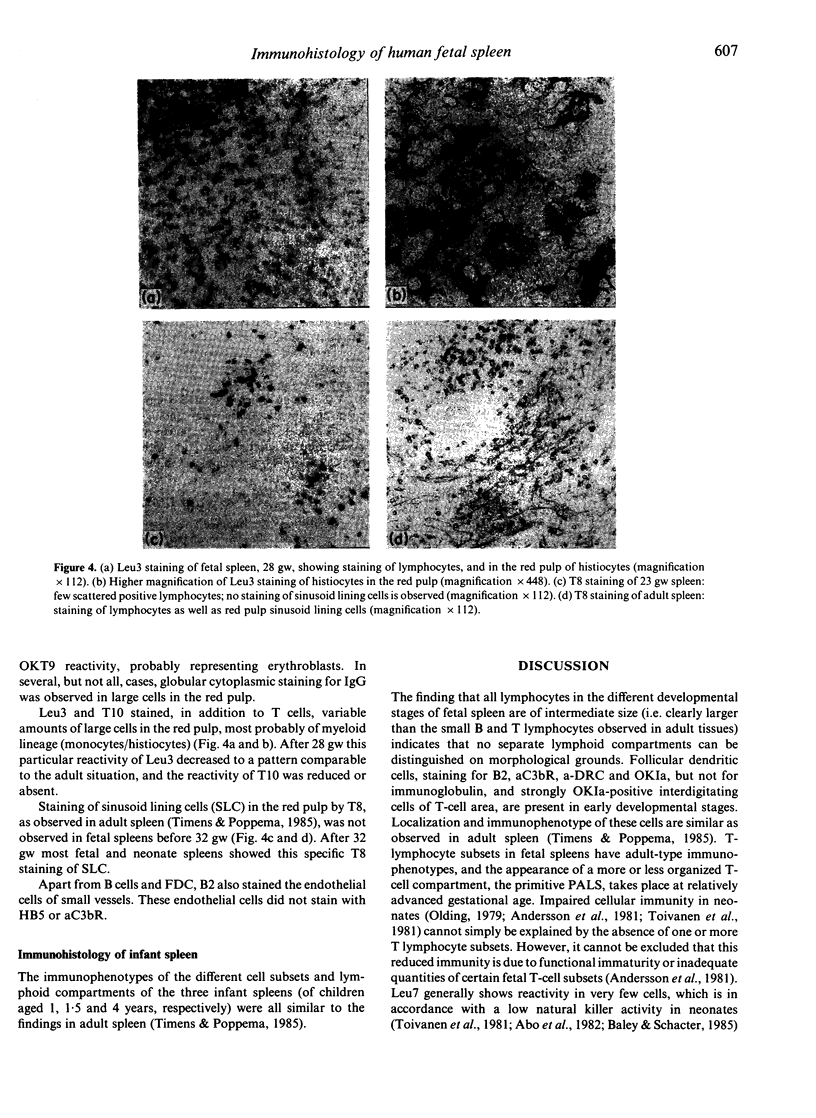
- Timens W., Poppema S. Lymphocyte compartments in human spleen. An immunohistologic study in normal spleens and uninvolved spleens in Hodgkin's disease. Am J Pathol. 1985 Sep;120(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- Toivanen P., Uksila J., Leino A., Lassila O., Hirvonen T., Ruuskanen O. Development of mitogen responding T cells and natural killer cells in the human fetus. Immunol Rev. 1981;57:89–105. doi: 10.1111/j.1600-065x.1981.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Tedder T. F., Fearon D. T. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]