Abstract
The thermoalkaliphilic Bacillus sp. strain TA2.A1 was able to grow in pH-controlled batch culture containing a nonfermentable growth substrate from pH 7.5 to 10.0 with no significant change in its specific growth rate, demonstrating that this bacterium is a facultative alkaliphile. Growth at pH 10.0 was sensitive to the protonophore carbonyl cyanide m-chlorophenylhydrazone, suggesting that a proton motive force (Δp) generated via aerobic respiration was an obligate requirement for growth of strain TA2.A1. Strain TA2.A1 exhibited intracellular pH homeostasis as the external pH increased from 7.5 to 10.0; however, the maximum ΔpH generated over this pH range was only 1.1 units at an external pH of 9.5. The membrane potential (Δψ) was maintained between −114 mV and −150 mV, and little significant change was observed over the pH range for growth. In contrast, the Δp declined from −164 mV at pH 7.5 to approximately −78 mV at pH 10.0. An inwardly directed sodium motive force (ΔpNa+) of −100 mV at pH 10.0 indicated that cellular processes (i.e., solute transport) dependent on a sodium gradient would not be affected by the adverse Δp. The phosphorylation potential of strain TA2.A1 was maintained between −300 mV and −418 mV, and the calculated H+/ATP stoichiometry of the ATP synthase increased from 2.0 at pH 7.5 to 5.7 at pH 10.0. Based on these data, vigorous growth of strain TA2.A1 correlated well with the ΔpNa+, phosphorylation potential, and the ATP/ADP ratio, but not with Δp. This communication represents the first report on the bioenergetics of an extremely thermoalkaliphilic aerobic bacterium.
The exploration of extreme environments has led to the isolation of microorganisms that are capable of surviving and growing under extreme conditions. One group of microorganisms that thrive at extremes of high pH are the alkaliphiles. Alkaliphilic bacteria grow over the pH range from 7.5 to 11.5 and can be divided into two groups, obligate alkaliphiles (e.g., Bacillus alcalophilus), which grow between pH 9.0 and pH 11.5, and facultative alkaliphiles (e.g., Bacillus pseudofirmus OF4), which grow between pH 7.5 and 11.2 (11). The intracellular pH of alkaliphilic bacteria is maintained at values more acidic (i.e., approximately 2 pH units) than their external environment (11, 12).
Because the total proton motive force (Δp) is the sum of the membrane potential (Δψ; positive out) and the pH gradient (ΔpH; acid out in neutrophiles), a large ΔpH generated in the opposite direction results in suboptimal Δp values for ATP synthesis (10-12). Despite the low Δp, ATP synthesis by the membrane-bound ATP synthase in these bacteria is still coupled to protons (7, 8).
The isolation of bacteria that can grow at extremes of temperature and high pH (i.e., thermoalkaliphiles) has been described, but these are predominantly anaerobic bacteria (30). To our knowledge, no bacteria that are able to grow at extremes of alkaline pH and temperature under aerobic growth conditions have been reported. We recently isolated a Bacillus species, designated strain TA2.A1, that is capable of vigorous growth at pH 10.0 and 65°C. Previous studies have shown that strain TA2.A1 has an obligate growth requirement for sodium (>5 mM) and uses a sodium motive force (ΔpNa+) to transport glutamate and sucrose into the cell (19, 20). Growth of strain TA2.A1 in pH-uncontrolled batch culture at pH 9.2 was shown to be sensitive to the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) and to the F-type ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD) (19, 20), but the bioenergetics of growth under these conditions has not been reported.
The aims of the current study were to determine the ability of Bacillus sp. strain TA2.A1 to regulate internal pH and elucidate the values for Δp and the phosphorylation potential (ΔGp) in order to investigate the roles of these parameters in ATP synthesis under conditions of high temperature and pH. This communication represents the first report on the bioenergetics of an extremely thermoalkaliphilic aerobic bacterium.
MATERIALS AND METHODS
Abbreviations, chemicals, and radiochemicals.
Δp, proton motive force; ΔpNa+, sodium motive force; ΔpH, pH gradient; ZΔpH, transmembrane proton gradient; Δψ, membrane potential; ΔGp, phosphorylation potential; CCCP, carbonyl cyanide m-chlorophenylhydrazone. ATP (sodium salt), valinomycin, nigericin, and CCCP were obtained from Sigma. The following radiochemicals were obtained from NEN Life Science Products, Inc.: [3H]methyltriphenylphosphonium iodide (TPP+) (30 to 60 Ci/mmol), [14C]methylamine hydrochloride (56 mCi/mmol), [7-14C]benzoic acid (10 to 25 mCi/mmol), [3H]water (25 mCi/g). d-Glucose-[1-14C]lactose (55 mCi/mmol) was obtained from Amersham Pharmacia (Amersham Laboratories). Luciferin, d-luciferase (EC 1.13.12.7), and pyruvate kinase (EC 2.7.1.40) were purchased from Sigma.
Media, culture conditions, and gas chromatography.
Bacillus sp. strain TA2.A1 was routinely grown in an alkaline basal medium that contained the following (per liter): 0.5 g of Na2SO4, 0.1 g of (NH4)2SO4, 0.1 g of MgSO4 · 7H2O, 0.2 g of K2HPO4, 9.0 g of NaHCO3, 10 g of tryptone peptone (Difco), 5 ml of Dictyoglomus trace elements (25), and 2 g of l-glutamate as the oxidizable carbon and energy source. The pH of the medium was adjusted to 9.5 at 65°C. Unless otherwise stated, pHs of all media and buffers are those measured at 65°C. Cells were grown with aeration by shaking at 120 rpm. For pH-controlled batch culture, cells were grown in a fermentor (500 ml) equipped with an automatic pH control unit (Cole-Parmer 5656-05) with a 1% inoculum from an overnight culture. The pH value being studied was maintained throughout growth by the automatic addition of sterile 2 N NaOH or 2 N HCl. Constant stirring (120 rpm) was maintained during the entire experiment.
To monitor culture growth and conduct bioenergetic measurements, samples were withdrawn aseptically as required, and culture optical density at 600 nm (OD600) was measured with a Beckman DU-64 spectrophotometer (1-cm light path length). The growth rate constant (k) for the log phase of growth was determined by plotting the log10 of the optical density against time (21).
End products from bacterial growth were measured by gas chromatography with a Hewlett-Packard HP5890 series II gas chromatograph fitted with a wide-bore Carbowax capillary column (Econocap 19659; Alltech Associates) with the conditions and procedures described previously (3). Cellular protein was determined by the method of Markwell et al. (16). The relationship between optical density and cell protein was 345 mg of protein/liter/turbidity unit.
Determination of bioenergetic parameters.
For the determination of Δp, Δψ, and ΔpH, two types of cell cultures were studied. The first determination was carried out on exponentially growing pH-controlled batch cultures (pH 7.5 to pH 10.0). In these experiments, cells (1 ml) were taken directly from the fermentor at the respective pHs and added to a glass tube containing one of the following radioisotopes: [3H]TPP+ (2.4 nM final concentration), [14C]methylamine (1.8 μM, pKa = 10.6) at pH values of 8 and above, or [7-14C]benzoate (11 μM, pKa = 4.2) at pH values below 8.0.
For the determination of Δψ, no significant variation in the Δψ was noted whether the final concentration of [3H]TPP+ used was 2.4 nM or 1 μM (data not shown). d-Glucose-[1-14C]lactose (20 μM) and [3H]water (1 mM) were used to determine intracellular volume. Lactose was shown to be nonmetabolizable by strain TA2.A1. After incubation for 5 min at 65°C, the cultures (450 μl in duplicate) were filtered rapidly through a 0.45-μm cellulose-acetate filter (Sartorius). This method was adapted from that of Zilberstein et al. (31). Oxygen consumption measurements of these cultures indicated that they remained energized throughout the course of the 5-min incubation. The filters were washed twice with 2 ml of 25 mM Na2CO3 (corresponding to the pH of the sample) and dried for 60 min at 40°C. Filters were resuspended in 2 ml of scintillation liquid, and counts per minute (cpm) were determined with an LKB Wallac 1214 Rackbeta liquid scintillation counter.
In the second set of experiments, Δp, Δψ, and ΔpH were determined on mid-log-phase cultures (grown at pH 9.5) that were harvested by centrifugation (8,000 × g, 20 min, 4°C) and washed twice in 25 mM Na2CO3 (pH values of 7.5 to 10.0). Cells were resuspended to a final OD600 of 1.0 at the external pH being studied in a volume of 1 ml, energized for 15 min with d-glucose (10 mM final concentration), and the bioenergetic parameters were measured as outlined above. The oxygen consumption rate of these energized cell suspensions was 303 nmol of O2/min/mg of protein. It should be noted that the silicon oil centrifugation method commonly employed for the determination of Δp was not successful with strain TA2.A1 due to the filamentous growth of strain TA2.A1, which made it difficult to obtain reproducible cell pellets after centrifugation through silicon oil.
The intracellular volume (3.90 ± 0.40 μl/mg of protein) was estimated from the difference between the partitioning of [3H]water and d-glucose-[-1-14C]lactose. The Δψ across the cell membrane was calculated from the uptake of [3H]TPP+ according to the Nernst relationship. Nonspecific TPP+ binding was estimated from cells which had been treated with valinomycin and nigericin (10 μM each) for 10 min. These inhibitors have been used previously with strain TA2.A1 and are membrane active with this bacterium (19). The ΔpH was determined from the distribution of either [14C]methylamine or [7-14C]benzoate with the Henderson-Hasselbach equation (22), and ZΔpH was determined by 67 mV times the ΔpH. The uptake of [14C]methylamine at a ΔpH of 0 was negligible, indicating that strain TA2.A1 did not metabolize or transport this compound.
Measurements of intracellular ATP, ADP, and inorganic phosphate concentrations.
Adenine nucleotides were extracted from the cells by perchloric acid and KOH-NaHCO3 treatment after separation of the cells from the growth medium as previously described (4) and frozen at −70°C. Prior to analysis, samples were thawed, and the potassium perchlorate was removed by centrifugation (13,000 × g, 5 min, 22°C). The ATP and ADP concentrations ([ATP] and [ADP], respectively) were determined by the luciferin-luciferase method (13). ATP was determined directly, whereas ADP was measured after enzymatic conversion to ATP with pyruvate kinase. The enzymatic conversion of ADP to ATP was carried out by incubating the adenylate-containing extract in 20 mM Tris-acetate buffer (pH 7.75)-1 mM MgCl2-0.1 mM phosphoenolpyruvate-2 U of pyruvate kinase. After incubation at 25°C for 2 min, the samples were boiled for 30 s and rapidly cooled on ice (9). This allowed complete conversion of ADP to ATP. The samples (50 μl) were then diluted in 400 μl of 40 mM Tris-acetate buffer (pH 7.75) containing 2 mM EDTA and 50 mM MgCl2.
The luciferase reaction was initiated by adding 50 μl of a purified luciferin-luciferase mix to 450 μl of the diluted extract according to the supplier's recommendations (Sigma). Light output was immediately measured with a luminometer (model LB953, EG & G Berthold) with ATP as a standard. The determination of intracellular inorganic phosphate concentration was carried out in cell extracts based on the formation of a phosphomolybdate complex as previously described (17). Standard curves were constructed with KH2PO4 solution (0 to 1 mM). All cell manipulations were carried out with acid-washed glassware, and determinations were carried out in triplicate. The phosphorylation potential (ΔGp) was calculated with the following equation: ΔGp = ΔGo + 2.3RT log[ATP]/[ADP][Pi]. The value used for ΔG° at 65 °C was 33.3 kJ/mol, or the equivalent of −347 mV. The values for ΔGp were calculated with values of ΔG° reported for the measured cytoplasmic pH and assuming an Mg2+ concentration of 1 to 25 mM (24).
Measurements of intracellular sodium and potassium concentrations.
Cells were harvested from pH-controlled batch culture, washed twice with an equal volume of distilled water, and resuspended in distilled water to give a final protein concentration of 100 to 150 mg of protein/ml. Then 20 μl of the cell-free supernatant was taken for the determination of external potassium and sodium. For sodium analysis, the washed cell suspension was passed through a Dowex-50W (K+ form) column and washed twice with 10 ml of distilled water. The effluent from the column was collected and centrifuged at 16,000 × g for 15 min, and the cell pellet was dried at 40°C. For all samples, the cell pellets and supernatant samples were digested at room temperature for 24 h in 3 N HNO3. Sodium and potassium concentrations were determined by flame photometry as previously described (3). Corrections were made for extracellular contamination of the cell pellet by Na+ and K+. The sodium motive force (ΔpNa+) was equal to 67 × log ([Na+]in/[Na+]out), where in and out refer to the concentrations inside and outside the membrane, respectively.
RESULTS AND DISCUSSION
Effect of external pH on the growth of Bacillus sp. strain TA2.A1 in pH-controlled batch culture.
Strain TA2.A1 was able to grow in the pH range from 7.5 to 10.0 in pH-controlled batch culture with no significant change in the specific growth rate, indicating that strain TA2.A1 was a facultative alkaliphile (Fig. 1A). At external pH values of 7.0 and 10.2, there was a 50% decline in the growth rate, and beyond these limits, no growth was observed. Under the growth conditions used in this study, all ATP was derived from oxidative phosphorylation, as supported by the lack of fermentation end products (i.e., acetate) accumulated during exponential growth. This result is consistent with l-glutamate's being metabolized by oxidative deamination to α-ketoglutarate, with subsequent oxidation to CO2 via the Krebs cycle. The addition of 100 μM CCCP to cells growing logarithmically at pH 10.0 completely arrested growth, suggesting that a proton gradient across the cell membrane was required for growth under these conditions (data not shown).
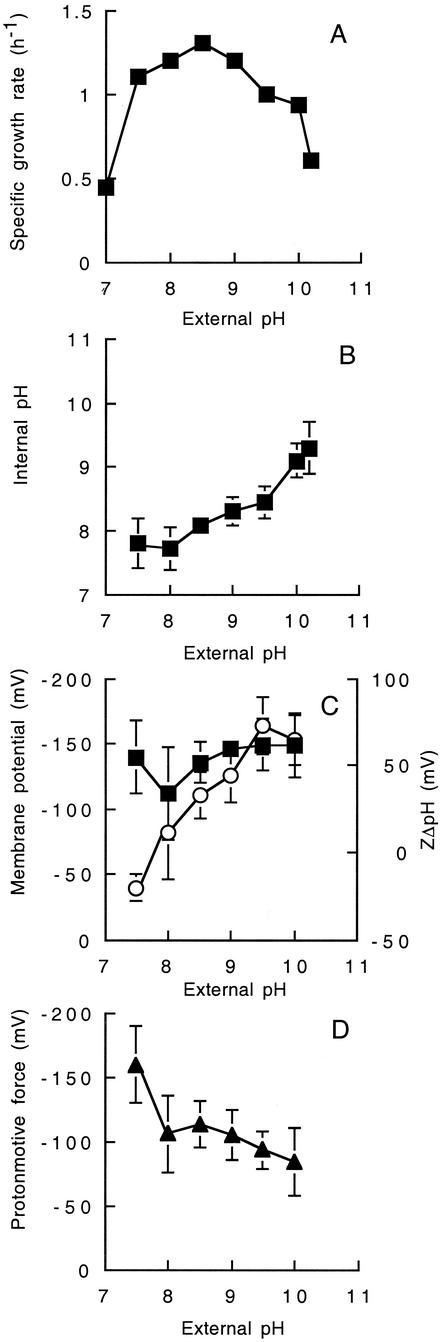
FIG. 1.
(A) Effect of external pH on specific growth rate of Bacillus sp. strain TA2.A1 grown in pH-controlled batch culture at the pH indicated. The growth rate (h−1) was determined in the exponential phase of growth. The effect of external pH on (B) internal pH, (C) Δψ (▪) and ZΔpH (○), and (D) proton motive force (▴) was determined. Exponentially growing cells were taken from pH-controlled batch culture at the pH indicated, and measurements were made as described in Materials and Methods. The values reported are the means of three independent experiments, and the standard error associated with these determinations is shown.
Regulation of intracellular pH in strain TA2.A1.
To study intracellular pH homeostasis and Δp generation by strain TA2.A1, exponentially growing cells from pH-controlled batch cultures were sampled (external pH 7.5 to 10.0), and the internal pH and membrane potential were determined with the radioactive probes [14C]methylamine (external pH 8 to 10), [7-14C]benzoic acid (pH 7.5), and [3H]TPP+, with corrections for nonspecific binding. As the external pH was increased from pH 7.5 to pH 10, the intracellular pH was maintained between pH 7.8 and 9.1 (Fig. 1B). The maximum ΔpH (1.1 units) was observed at an external pH of 9.5. The membrane potential (Δψ) was maintained between −114 mV and −150 mV, and little significant change was observed over the pH range for growth (Fig. 1C). However, the Δp declined from −164 mV at pH 7.5 to approximately −78 mV at pH 10.0 (Fig. 1D) due to the increase in ZΔpH from −21 mV at pH 7.5 to +74 mV at pH 10.0 (Fig. 1C).
To further substantiate the above pattern of pH regulation and Δp determinations, the internal pH and individual components of the Δp were determined independently on energized cell suspensions prepared from mid-log-phase cultures (grown at pH 9.5) and resuspended in 25 mM Na2CO3 buffer at pHs of 7.5 to 10.0. An identical pattern of pH regulation was observed, and the values of Δp and ΔpH were comparable to those cited above. For example, the Δψ ranged from −135 mV at pH 8.0 to a maximum value of −150 mV at pH 9.5. The Δp decreased from −130 mV at pH 8.0 to −90 mV at pH 10.0 (data not shown).
In comparison to other alkaliphilic bacilli, the magnitude of the ΔpH generated by strain TA2.A1 was low under a variety of conditions tested (e.g., glutamate-grown cells and washed cell suspensions resuspended in buffer). In addition, succinate-grown cells of strain TA2.A1 also generated a low ΔpH (data not shown). The cells used in these experiments were from actively growing cultures, and hence the low ΔpH values are not a reflection of cells that were deenergized. In contrast, the facultative alkaliphile B. pseudofirmus OF4 generates a ΔpH of 2.5 units at an external pH of 10.8 (28), and the obligate alkaliphile B. alcalophilus has a ΔpH of 1.9 at pH 10.8 (9).
It has been proposed that thermophilic bacteria may lack the ability to regulate internal pH due to increased proton permeability (26). However, several studies have shown that pH regulation is evident in these bacteria, but the magnitude of the ΔpH generated may be lower than that observed in mesophilic bacteria (3, 5). While an increase in proton permeability as observed for thermophilic bacilli (1) could provide a plausible explanation for the lowered ΔpH values, this remains to be experimentally proven with thermophilic alkaliphiles. It should be noted that the proton permeability of the alkaliphile B. alcalophilus is comparable to that of neutrophilic bacteria (23). Moreover, alkaliphilic bacilli have been shown to have appreciable amounts of squalene, squalene derivatives, and C40 isoprenoids among their neutral membrane lipids, which may prevent proton leakage (2).
Phosphorylation potential (ΔGp) and proton stoichiometry of ATP synthesis in strain TA2.A1.
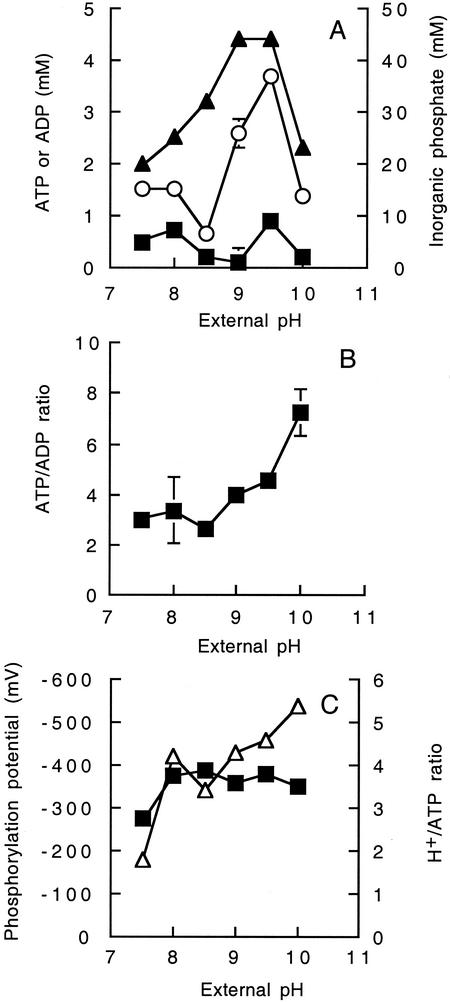
The ATP and ADP content, phosphorylation potential, and H+ stoichiometry of the ATP synthase (i.e., phosphorylation potential/total proton motive force) was determined in cells grown in pH-controlled batch culture. The ATP content of strain TA2.A1 increased from 1.5 mM at pH 7.5 to 3.8 mM at pH 9.5 (Fig. 2A). Under the same growth conditions, the ADP content of strain TA2.A1 cells was less than 1 mM (0.2 mM to 0.9 mM) (Fig. 2A). The inorganic phosphate content of strain TA2.A1 was approximately 20 mM at pH 7.5 and increased to a maximum value of 45 mM at an external pH of 9.5 (Fig. 2A). The ATP-to-ADP ratio increased steadily from 3.0 at an external pH of 7.5 to 7.3 at an external pH of 10.0 (Fig. 2B).
FIG. 2.
Effect of external pH on (A) internal ATP (○), ADP (▪), and inorganic phosphate (▴) concentrations of exponentially growing cells from pH-controlled batch culture. The values shown in A were used to calculate the ATP/ADP ratio shown in B. (C) Phosphorylation potential (▪) as a function of external pH, calculated with the data shown in A and a value for ΔG° at 65°C of 33.3 kJ/mol, or the equivalent of −347 mV. The number of H+ ions translocated per molecule of ATP synthesized (▵) was calculated from the phosphorylation potential/total proton motive force. The values for all experiments are the means of three independent experiments, and the standard error associated with these determinations is shown.
With the data in Fig. 2B and a value for ΔG° at 65°C of 33.3 kJ/mol, or the equivalent of −347 mV, the ΔGp of strain TA2.A1 was maintained between −300 mV and −418 mV over the pH range from 7.5 to 10.0 (Fig. 2C). The H+/ATP stoichiometry of the ATP synthase was then calculated from the ΔGp/Δp. As the external pH increased from 7.5 to 10.0, the H+/ATP stoichiometry increased from 2.0 to 5.7 (Fig. 2C).
The highest ATP-to-ADP ratio occurred at an external pH of 10.0, and the ΔGp was comparable to values reported for neutrophilic bacteria (4, 18, 29) and alkaliphiles (6, 9), indicating that ATP synthesis was not affected by the low Δp. Sturr et al. (28) reported that the stoichiometry of ATP synthesis via a chemiosmotic coupling mechanism increased from 3 H+/ATP at pH 7.5 to 13 H+/ATP at pH 11.2 in B. pseudofirmus OF4, but the significance of this finding has not been elucidated. A ratio of 2 to 4 H+/ATP is generally accepted in bacteria growing at neutral pHs (4, 14, 15, 18). The H+ stoichiometry of the ATP synthase from strain TA2.A1 also increased from 2 H+/ATP synthesized to 5.7 H+/ATP at pH 10.0. Furthermore, the highest ATP/ADP ratios were not observed at the highest Δp values, as would be expected in a chemiosmotic coupling situation.
The above observations are based on the premise that ΔGp/Δp = H+/ATP when ΔGp and Δp are in thermodynamic equilibrium and delocalized into bulk phases. However, if a localized microcircuit of protons existed between the respiratory chain and the ATP synthase, the ratio may in fact remain constant as the external pH increases. Several theories have been proposed as to how ATP synthesis remains coupled to the low Δp in alkaliphilic bacteria, but these remain to be proven experimentally (10).
Intracellular concentration of Na+ and K+ as a function of external pH.
Bacillus sp. strain TA2.A1 requires a ΔpNa+ for growth and solute transport (19). Cells from exponentially growing cultures at pH 9.5 had a sodium content of 1.90 μmol/mg of protein, and based on a ratio of cell volume to protein of 3.90 μl/mg, the apparent intracellular sodium concentration was 487 mM at an external Na+ concentration of 150 mM. These results suggested that the ΔpNa+ was poised in the reverse direction. Previous studies (9, 27) have shown that some bacterial cells bind large amounts of Na+ tightly to cellular components, and this can overestimate the apparent intracellular Na+ concentration. Tightly bound sodium can be removed by specific exchange with potassium (9, 27). After exchanging Na+ for K+ prior to cell lysis of strain TA2.A1, the internal Na+ concentration ranged from 5 to 10 mM Na+ over the pH range studied. With the equation ΔpNa+ = 67 × log([Na+]in/[Na+]out), strain TA2.A1 had a ΔpNa+ of −75 mV at pH 7.5 that increased to −100 mV at pH 10.0. Over the same external pH range, the intracellular levels of K+ increased from 123 mM at pH 7.5 to 210 mM at pH 10.0 (data not shown). These results indicated that sodium-driven solute transport in strain TA2.A1 (19, 20) would be unaffected by the low Δp.
To conclude, the data presented in this communication demonstrate that, like mesophilic alkaliphiles, the thermoalkaliphilic Bacillus sp. strain TA2.A1 maintains an inverted pH gradient at alkaline pH and must synthesize ATP via a proton-coupled F1F0-ATP synthase at suboptimal Δp values. Recent work from our laboratory has shown that strain TA2.A1 has a conventional F1F0-ATP synthase that is proton coupled (G. M. Cook, S. Keis, H. W. Morgan, C. von Ballmoos, U. Matthey, G. Kaim, and P. Dimroth, unpublished data). Despite the low Δp, the ATP/ADP ratio, ΔGp, and ΔpNa+ would all appear to be optimal for growth of strain TA2.A1 under these conditions. Future studies will be aimed at elucidating the mechanism of pH homeostasis in strain TA2.A1 and how this bacterium synthesizes ATP via the ATP synthase at suboptimal Δp values.
Acknowledgments
This work and S.K. were supported by a Marsden grant from the Royal Society of New Zealand. K.O. was supported by a University of Otago international postgraduate scholarship.
We thank Arthur Guffanti and Terry Krulwich for supplying filters and helpful discussion. The technical advice of David Jones and the assistance of Katherine Young in the use of gas chromatography are appreciated.
REFERENCES
- 1.Albers, S. V., J. L. C. M. Van de Vossenberg, A. J. M. Driessen, and W. N. Konings. 2001. Bioenergetics and solute uptake under extreme conditions. Extremophiles 5:285-294. [DOI] [PubMed] [Google Scholar]
- 2.Clejan, S., T. A. Krulwich, K. R. Mondrus, and D. Seto-Young. 1986. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J. Bacteriol. 168:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook, G. M. 2000. The intracellular pH of the thermophilic bacterium Thermoanaerobacter wiegelii during growth and production of fermentation acids. Extremophiles 4:279-284. [DOI] [PubMed] [Google Scholar]
- 4.Cook, G. M., and J. B. Russell. 1994. Energy-spilling reaction of Streptococcus bovis and resistance of its membrane to proton conductance. Appl. Environ. Microbiol. 60:1942-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook, G. M., J. B. Russell, A. Reichert, and J. Wiegel. 1996. The intracellular pH of Clostridium paradoxum, an anaerobic, alkaliphilic, and thermophilic bacterium. Appl. Environ. Microbiol. 62:4576-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guffanti, A. A., and D. B. Hicks. 1991. Molar growth yields and bioenergetic parameters of extremely alkaliphilic Bacillus species in batch cultures, and growth in a chemostat at pH 10.5. J. Gen. Microbiol. 137:2375-2379. [DOI] [PubMed] [Google Scholar]
- 7.Hicks, D. B., and T. A. Krulwich. 1990. Purification and reconstitution of the F1F0-ATP synthase from alkaliphilic Bacillus firmus OF4. Evidence that the enzyme translocates H+ but not Na+. J. Biol. Chem. 265:20547-20554. [PubMed] [Google Scholar]
- 8.Hoffmann, A., and P. Dimroth. 1991. The ATPase of Bacillus alcalophilus. Reconstitution of energy-transducing functions. Eur. J. Biochem. 196:493-497. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann, A., and P. Dimroth. 1991. The electrochemical proton potential of Bacillus alcalophilus. Eur. J. Biochem. 201:467-473. [DOI] [PubMed] [Google Scholar]
- 10.Krulwich, T. A. 1995. Alkaliphiles: “basic” molecular problems of pH tolerance and bioenergetics. Mol. Microbiol. 15:403-410. [DOI] [PubMed] [Google Scholar]
- 11.Krulwich, T. A., and A. A. Guffanti. 1992. Proton-coupled bioenergetic processes in extremely alkaliphilic bacteria. J. Bioenerg. Biomemb. 24:587-599. [DOI] [PubMed] [Google Scholar]
- 12.Krulwich, T. A., M. Ito, D. B. Hicks, R. Gilmour, and A. A. Guffanti. 1998. pH homeostasis and ATP synthesis: studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species. Extremophiles 2:217-222. [DOI] [PubMed] [Google Scholar]
- 13.Lundin, A., and A. Thore. 1975. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl. Microbiol. 30:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloney, P. C. 1977. Obligatory coupling between proton entry and the synthesis of adenosine 5′-triphosphate in Streptococcus lactis. J. Bacteriol. 132:564-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maloney, P. C. 1983. Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J. Bacteriol. 153:1461-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 17.Monk, B. C., M. B. Kurtz, J. A. Marrinan, and D. S. Perlin. 1991. Cloning and characterization of the plasma membrane H+-ATPase from Candida albicans. J. Bacteriol. 173:6826-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto, R., B. Klont, B. Ten Brink, and W. N. Konings. 1984. The phosphate potential, adenylate charge and proton motive force in growing cells of Streptococcus cremoris. Arch. Microbiol. 139:338-343. [Google Scholar]
- 19.Peddie, C. J., G. M. Cook, and H. W. Morgan. 1999. Sodium-dependent glutamate uptake by an alkaliphilic, thermophilic Bacillus strain, TA2.A1. J. Bacteriol. 181:3172-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peddie, C. J., G. M. Cook, and H. W. Morgan. 2000. Sucrose transport by the alkaliphilic, thermophilic Bacillus sp. strain TA2.A1 is dependent on a sodium gradient. Extremophiles 4:291-296. [DOI] [PubMed] [Google Scholar]
- 21.Pirt, S. J. 1975. Principles of microbe and cell cultivation. Blackwell, Oxford, United Kingdom.
- 22.Riebeling, V., R. K. Thauer, and K. Jungermann. 1975. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur. J. Biochem. 55:445-453. [DOI] [PubMed] [Google Scholar]
- 23.Rius, N., and J. G. Loren. 1998. Buffering capacity and membrane H+ conductance of neutrophilic and alkalophilic gram-positive bacteria. Appl. Environ. Microbiol. 64:1344-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosing, J., and E. C. Slater. 1972. The value of ΔG° for the hydrolysis of ATP. Biochim. Biophys. Acta 267:275-290. [DOI] [PubMed] [Google Scholar]
- 25.Sakai, T., Y. Kobayashi, K. Kawagoe, and T. Beppu. 1985. Dictyoglomus thermophilum gen. nov., a chemoorganotrophic, anaerobic thermophilic bacterium. Int. J. Syst. Bacteriol. 35:253-259. [Google Scholar]
- 26.Speelmans, G., B. Poolman, T. Abee, and W. N. Konings. 1993. Energy transduction in the thermophilic anaerobic bacterium Clostridium fervidus is exclusively coupled to sodium ions. Proc. Natl. Acad. Sci. USA 90:7975-7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel, H. J., and J. B. Russell. 1989. Non-proton-motive-force-dependent sodium efflux from the ruminal bacterium Streptococcus bovis: bound versus free pools. Appl. Environ. Microbiol. 55:2664-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturr, M. G., A. A. Guffanti, and T. A. Krulwich. 1994. Growth and bioenergetics of alkaliphilic Bacillus firmus OF4 in continuous culture at high pH. J. Bacteriol. 176:3111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiegel, J. 1998. Anaerobic alkalithermophiles, a novel group of extremophiles. Extremophiles 2:257-267. [DOI] [PubMed] [Google Scholar]
- 31.Zilberstein, D., V. Agmon, S. Schuldiner, and E. Padan. 1982. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J. Biol. Chem. 257:3687-3691. [PubMed] [Google Scholar]