Abstract
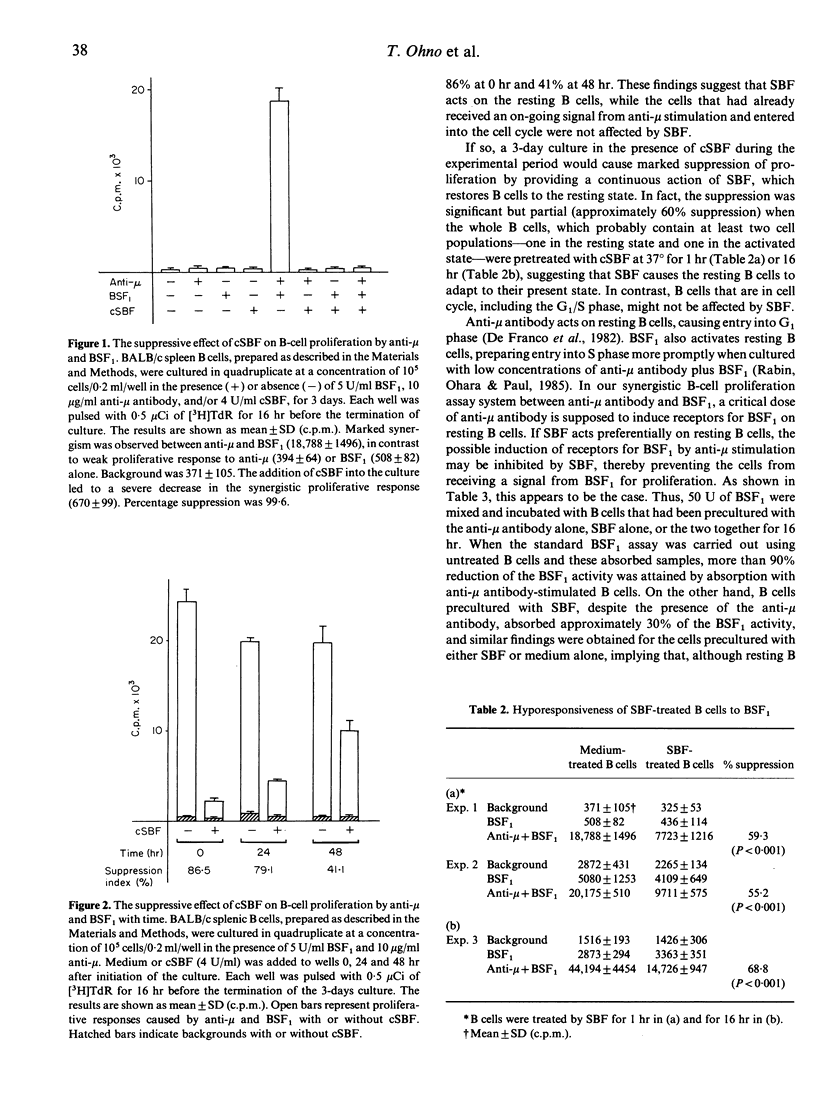
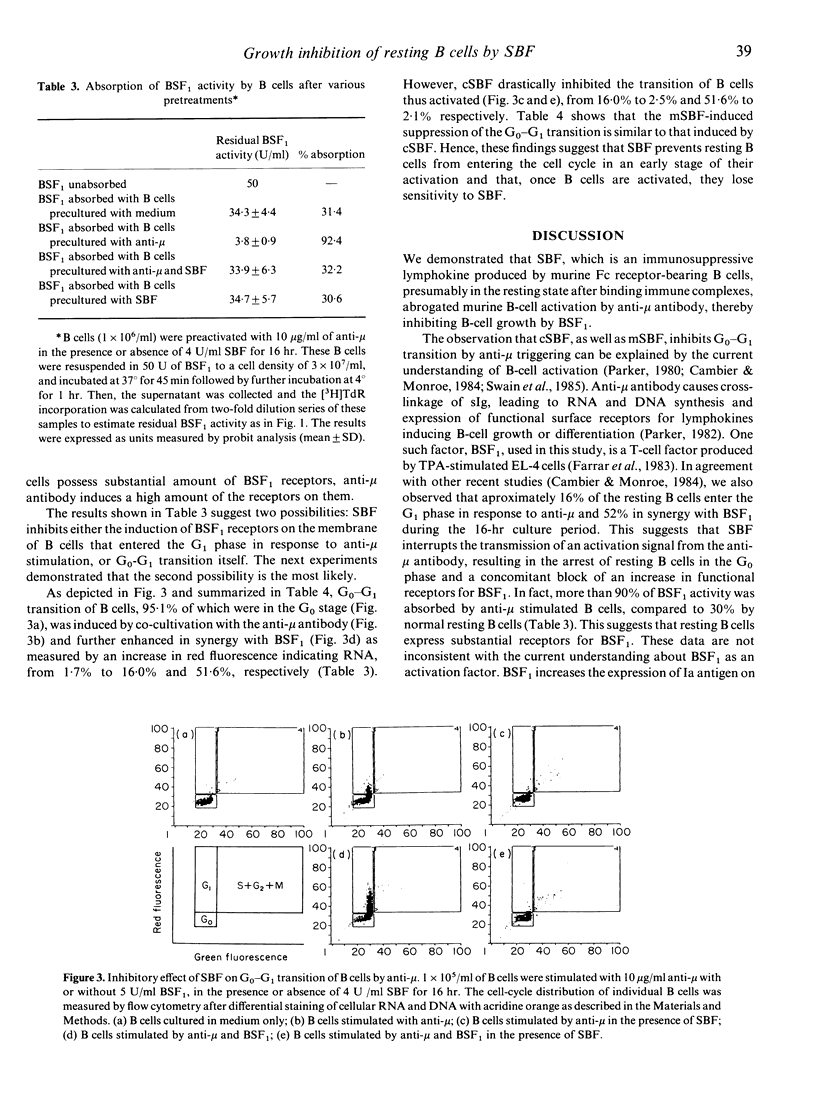
We investigated the effect of a lymphokine termed 'suppressive B-cell factor' (SBF), which is produced by FcR gamma (Fc receptor for IgG)-stimulated B cells or hybridoma TS4.44, and is known to suppress B-cell responses in vivo and in vitro by inhibiting their proliferation. Small B cells, fractionated by Percoll density gradient, from athymic nude mice (BALB/c) secreted SBF after binding EA (sheep erythrocytes sensitized with IgG mouse anti-sheep erythrocyte antibody), and the proliferation of small but not large B cells was preferentially suppressed by SBF in response to LPS in vitro. Proliferation of purified B cells from BALB/c nu/nu mice, induced by a synergistic interaction between F(ab')2 fragment of goat anti-mouse IgM antibody and B-cell stimulating factor (BSF1), was almost completely abrogated by the co-existence of SBF during the 72-hr culture period. However, the co-culture with SBF for the last 24 or 48 hr, as well as of B cells pretreated with SBF for 1 hr at 37 degrees, partially inhibited the growth response. These findings suggest that SBF operates on resting B cells and holds them in a resting state. This notion would be further supported by the fact that SBF inhibited G0-G1 transition. Taken together, we conclude that SBF acts on the early step of B-cell activation, thereby inhibiting B-cell growth. Arrest of resting B cells in the G0 phase and failure of an increase in functional receptors for BSF1 seem to be responsible for the suppression of B-cell responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cambier J. C., Monroe J. G. B cell activation. V. Differentiation signaling of B cell membrane depolarization, increased I-A expression, G0 to G1 transition, and thymidine uptake by anti-IgM and anti-IgD antibodies. J Immunol. 1984 Aug;133(2):576–581. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Howard M., Fuller-Farrar J., Paul W. E. Biochemical and physicochemical characterization of mouse B cell growth factor: a lymphokine distinct from interleukin 2. J Immunol. 1983 Oct;131(4):1838–1842. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gisler R. H., Fridman W. H. Suppression of in vitro antibody synthesis by immunoglobulin-binding factor. J Exp Med. 1975 Aug 1;142(2):507–517. doi: 10.1084/jem.142.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker M. L., Moore R. N., Farrar J. J. Biologic properties of chromatographically separated murine thymoma-derived Interleukin 2 and colony-stimulating factor. J Immunol. 1981 Nov;127(5):1983–1987. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Mizel S. B., Lachman L., Ansel J., Johnson B., Paul W. E. Role of interleukin 1 in anti-immunoglobulin-induced B cell proliferation. J Exp Med. 1983 May 1;157(5):1529–1543. doi: 10.1084/jem.157.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen C. H., Ambrus J. L., Jr, Fauci A. S. Production of B cell growth factor by normal human B cells. J Immunol. 1986 Jun 15;136(12):4542–4547. [PubMed] [Google Scholar]
- Masuda T., Miyama M., Kuribayashi K., Yodoi J., Takabayashi A., Kyoizumi S. Immunological properties of Fc receptor on lymphocytes. 5. Suppressive regulation of humoral immune response by Fc receptor bearing B lymphocytes. Cell Immunol. 1978 Sep;39(2):238–249. doi: 10.1016/0008-8749(78)90100-4. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M., Suzuki T., Paku Y. H., Masuda T. Feedback regulation of immune responses by immune complexes; possible involvement of a suppressive lymphokine by FcR gamma-bearing B cell. J Immunol. 1982 Feb;128(2):882–887. [PubMed] [Google Scholar]
- Miyama M., Kuribayashi K., Yodoi J., Takabayashi A., Masuda T. Immunological properties of Fc receptor on lymphocytes. 1. Functional differences between Fc receptor-positive and negative lymphocytes in humoral immune responses. Cell Immunol. 1978 Feb;35(2):253–265. doi: 10.1016/0008-8749(78)90147-8. [DOI] [PubMed] [Google Scholar]
- Miyama M., Yamada J., Masuda T. Immunological properties of Fc receptor on lymphocytes. 6. Characterization of suppressive B-cell factor (SBF) released from Fc receptor-bearing B cells. Cell Immunol. 1979 Apr;44(1):51–63. doi: 10.1016/0008-8749(79)90027-3. [DOI] [PubMed] [Google Scholar]
- Mizuguchi J., Beaven M. A., Ohara J., Paul W. E. BSF-1 action on resting B cells does not require elevation of inositol phospholipid metabolism or increased [Ca2+]i. J Immunol. 1986 Oct 1;137(7):2215–2219. [PubMed] [Google Scholar]
- Mond J. J., Finkelman F. D., Sarma C., Ohara J., Serrate S. Recombinant interferon-gamma inhibits the B cell proliferative response stimulated by soluble but not by Sepharose-bound anti-immunoglobulin antibody. J Immunol. 1985 Oct;135(4):2513–2517. [PubMed] [Google Scholar]
- Muraguchi A., Nishimoto H., Kawamura N., Hori A., Kishimoto T. B cell-derived BCGF functions as autocrine growth factor(s) in normal and transformed B lymphocytes. J Immunol. 1986 Jul 1;137(1):179–186. [PubMed] [Google Scholar]
- Nakagawa T., Hirano T., Nakagawa N., Yoshizaki K., Kishimoto T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J Immunol. 1985 Feb;134(2):959–966. [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. H., Suzuki T., Miyama-Inaba M., Masuda T., Yoshida Y., Uchino H. Feedback regulation of antibody formation by suppressive B cell factor: preferential suppression of high-affinity antibody production by memory B lymphocytes. Int Arch Allergy Appl Immunol. 1986;81(2):156–164. doi: 10.1159/000234125. [DOI] [PubMed] [Google Scholar]
- Parker D. C. Induction and suppression of polyclonal antibody responses by anti-Ig reagents and antigen-nonspecific helper factors: a comparison of the effects of anti-Fab, anti-IgM, and anti IgD on murine B cells. Immunol Rev. 1980;52:115–139. doi: 10.1111/j.1600-065x.1980.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Parker D. C. Separable helper factors support B cell proliferation and maturation to Ig secretion. J Immunol. 1982 Aug;129(2):469–474. [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Mingari C., Maggi E., Liang C. M., Moretta L. B cell growth factor activity of interferon-gamma. Recombinant human interferon-gamma promotes proliferation of anti-mu-activated human B lymphocytes. J Immunol. 1986 May 15;136(10):3513–3516. [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Miyama-Inaba M., Masuda T., Uchino H., Ishii K. Monoclonal SBF produced by a hybridoma: in-vitro and in-vivo suppression of B tumour-cell proliferation. Immunology. 1983 Dec;50(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Miyama-Inaba M., Masuda T., Uchino H., Murakami A., Tanaka H. Suppressive B-cell factor (SBF) produced by FcR-bearing B cells; suppression of B, but not non-B-cell proliferation. Immunology. 1983 Sep;50(1):149–157. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Park Y. H., Miyama-Inaba M., Masuda T., Saji H., Nagata I., Uchino H. A novel lymphokine derived from human IgG Fc receptor-bearing B cells: its suppressive effect on activated T and B lymphocytes including tumour cells in vitro. Immunology. 1985 Jul;55(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


