Abstract
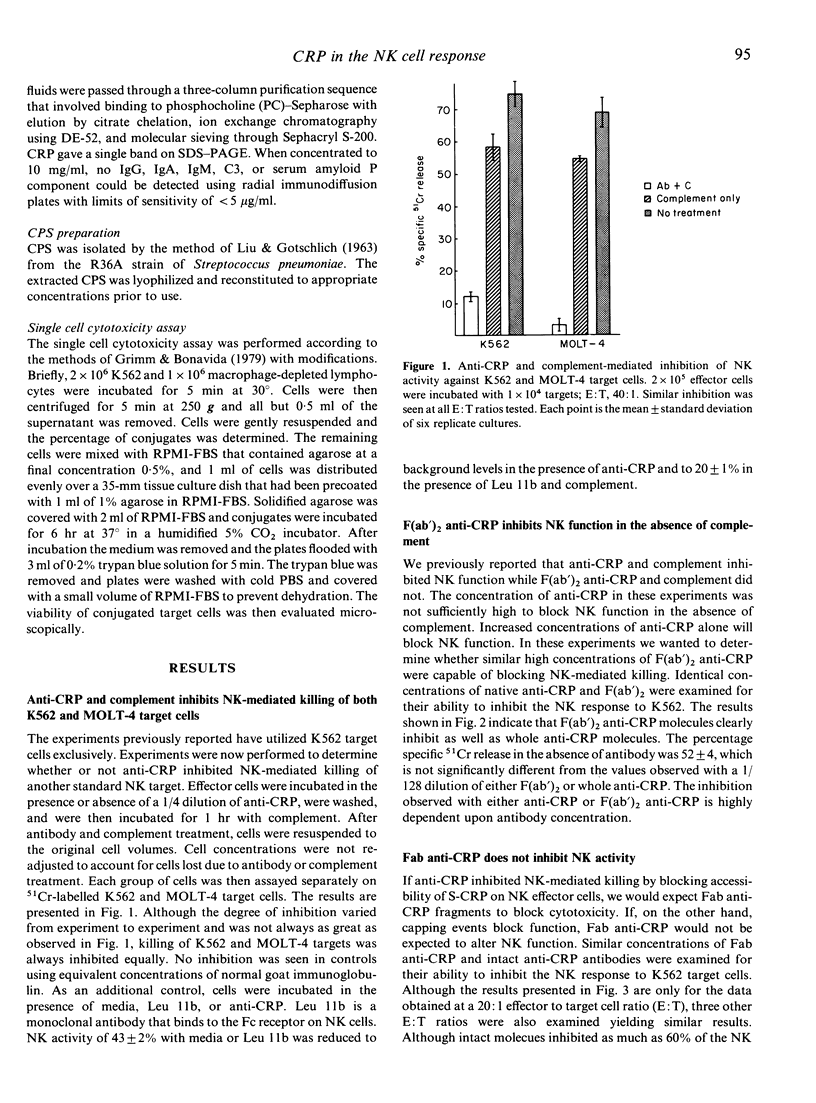
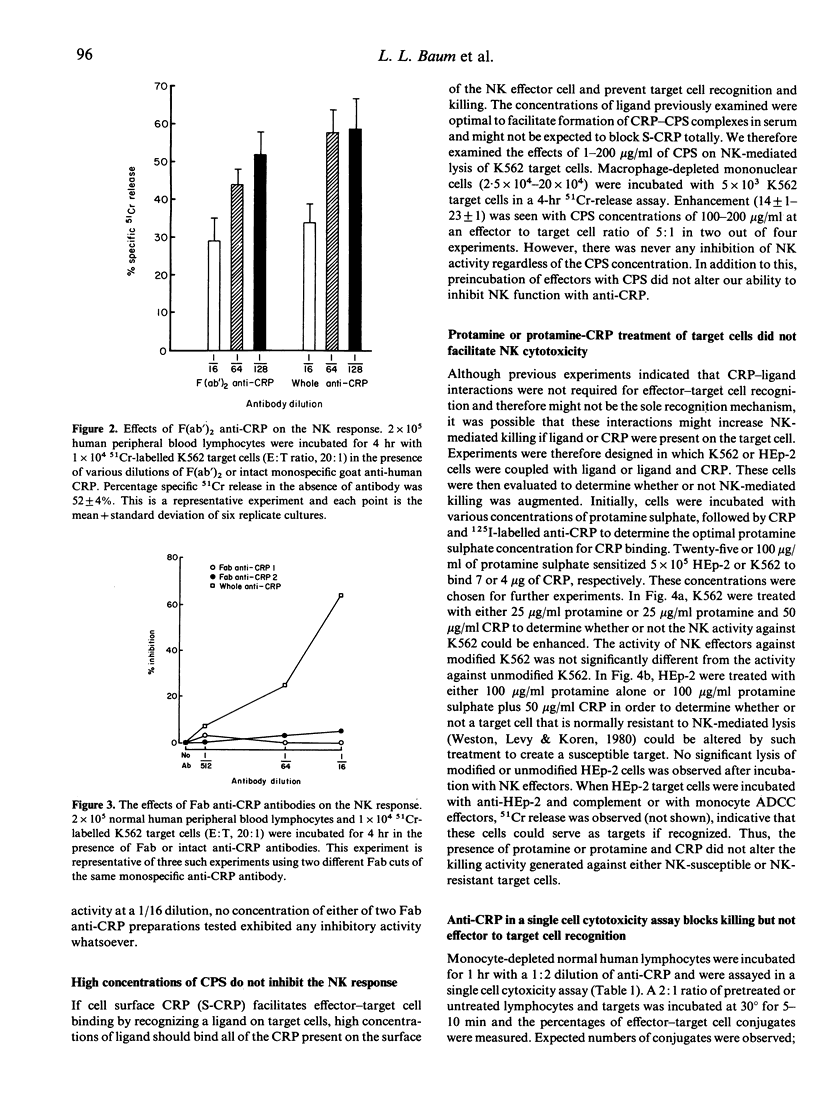
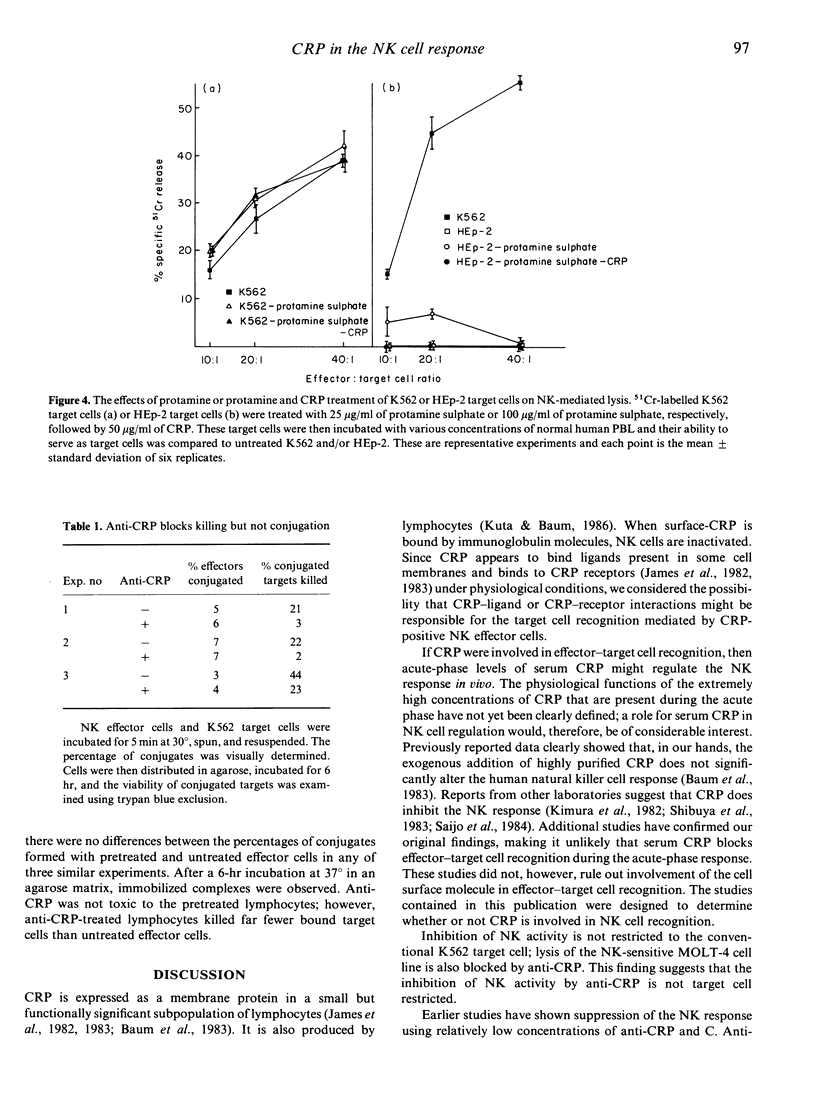
Anti-CRP and complement treatment of human peripheral blood lymphocytes significantly reduces natural killer (NK) cell-mediated cytotoxicity to K562 target cells as well as to MOLT-4 target cells. Although not all activity is eliminated by treatment of effector cells with antibody and complement, the reduction of NK function indicates that C-reactive protein (CRP) is present on a significant proportion of NK cells. Higher concentrations of anti-CRP or anti-CRP F(ab')2 fragments also reduce NK function; this suggests that CRP is not only present on these effector cells but may also play a role in NK-mediated killing. We initially suspected that CRP-ligand interactions might be involved in effector-target cell recognition. Several lines of evidence suggest that this is not the case. While F(ab')2 anti-CRP will block NK function, Fab anti-CRP will not, suggesting that the NK response is not impaired when surface CRP (S-CRP) is blocked but is only inhibited when the S-CRP is cross-linked and modulated. Neither CRP-C polysaccharide complexes (CRP-CPS) nor concentrations of CPS ranging from 0.1 microgram/ml to 200 micrograms/ml have any effect on NK cell-mediated killing. Treatment of target cells with a ligand for CRP or CRP prior to co-culture with NK effectors does not augment NK function. Single cell assays clearly demonstrate that high concentrations of anti-CRP have no effect on the formation of effector-target cell conjugates. Although these concentrations of anti-CRP do not block effector-target cell conjugation in the single cell assay, they do block the killing of conjugated target cells. In total, this evidence strongly suggests that although CRP appears to be involved in NK-mediated killing, it is not involved in effector-target cell-mediated recognition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum L. L., James K. K., Glaviano R. R., Gewurz H. Possible role for C-reactive protein in the human natural killer cell response. J Exp Med. 1983 Jan 1;157(1):301–311. doi: 10.1084/jem.157.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCamelli R., Potempa L. A., Siegel J., Suyehira L., Petras K., Gewurz H. Binding reactivity of C-reactive protein for polycations. J Immunol. 1980 Nov;125(5):1933–1938. [PubMed] [Google Scholar]
- Durdik J. M., Beck B. N., Clark E. A., Henney C. S. Characterization of a lymphoma cell variant selectively resistant to natural killer cells. J Immunol. 1980 Aug;125(2):683–688. [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Hercend T., Meuer S., Brennan A., Edson M. A., Acuto O., Reinherz E. L., Schlossman S. F., Ritz J. Identification of a clonally restricted 90 kD heterodimer on two human cloned natural killer cell lines. Its role in cytotoxic effector function. J Exp Med. 1983 Nov 1;158(5):1547–1560. doi: 10.1084/jem.158.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham J. D., Heidelberger M., Gotschlich E. C. Degradation of a pneumococcal type-specific polysaccharide with exposure of group-specificity. Proc Natl Acad Sci U S A. 1970 Sep;67(1):138–142. doi: 10.1073/pnas.67.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiserodt J. C., Laybourn K. A., Varani J. Expression of a laminin-like substance on the surface of murine natural killer (NK) lymphocytes and its role in NK recognition of tumor target cells. J Immunol. 1985 Aug;135(2):1484–1487. [PubMed] [Google Scholar]
- James K., Baum L. L., Vetter M. L., Gewurz H. Interactions of C-reactive protein with lymphoid cells. Ann N Y Acad Sci. 1982;389:274–285. doi: 10.1111/j.1749-6632.1982.tb22143.x. [DOI] [PubMed] [Google Scholar]
- James K., Baum L., Adamowski C., Gewurz H. C-reactive protein antigenicity on the surface of human lymphocytes. J Immunol. 1983 Dec;131(6):2930–2934. [PubMed] [Google Scholar]
- James K., Hansen B., Gewurz H. Binding of C-reactive protein to human lymphocytes. I. Requirement for a binding specificity. J Immunol. 1981 Dec;127(6):2539–2544. [PubMed] [Google Scholar]
- James K., Hansen B., Gewurz H. Binding of C-reactive protein to human lymphocytes. II. Interaction with a subset of cells bearing the Fc receptor. J Immunol. 1981 Dec;127(6):2545–2550. [PubMed] [Google Scholar]
- Kuta A. E., Baum L. L. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med. 1986 Jul 1;164(1):321–326. doi: 10.1084/jem.164.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., GOTSCHLICH E. C. The chemical composition of pneumococcal C-polysaccharide. J Biol Chem. 1963 Jun;238:1928–1934. [PubMed] [Google Scholar]
- Moingeon P., Nowill A., Courtois G., Azzarone B., Motte P., Ythier A., Bohuon C., Hercend T. A target structure for a series of human cloned natural killer cell lines is recognized by both anti-TNKtar and 4F2 monoclonal antibodies. J Immunol. 1985 May;134(5):2930–2934. [PubMed] [Google Scholar]
- Newman R. A., Warner J. F., Dennert G. NK recognition of target structures: is the transferrin receptor the NK target structure? J Immunol. 1984 Oct;133(4):1841–1845. [PubMed] [Google Scholar]
- Robey F. A., Jones K. D., Tanaka T., Liu T. Y. Binding of C-reactive protein to chromatin and nucleosome core particles. A possible physiological role of C-reactive protein. J Biol Chem. 1984 Jun 10;259(11):7311–7316. [PubMed] [Google Scholar]
- Shibuya M., Saijo N., Shimizu E., Taniguchi T., Takizawa T., Fujioka H., Hoshi A. [Effect of C-reactive protein on natural killer activity]. Gan To Kagaku Ryoho. 1983 Nov;10(11):2341–2346. [PubMed] [Google Scholar]
- Siegel J., Osmand A. P., Wilson M. F., Gewurz H. Interactions of C-reactive protein with the complement system. II. C-reactive protein-mediated consumption of complement by poly-L-lysine polymers and other polycations. J Exp Med. 1975 Sep 1;142(3):709–721. doi: 10.1084/jem.142.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J., Rent R., Gewurz H. Interactions of C-reactive protein with the complement system. I. Protamine-induced consumption of complement in acute phase sera. J Exp Med. 1974 Sep 1;140(3):631–647. doi: 10.1084/jem.140.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbruck G., Karduck D., Haupt H., Schwick H. G. C-reactive protein (CRP), 9.5 salpha1-glycoprotein and C1q: serum proteins with lectin properties? Z Immunitatsforsch Immunobiol. 1979 Feb;155(3):262–266. [PubMed] [Google Scholar]
- Vetter M. L., Gewurz H., Baum L. L. The effects of C-reactive protein on human cell-mediated cytotoxicity. J Leukoc Biol. 1986 Jan;39(1):13–25. doi: 10.1002/jlb.39.1.13. [DOI] [PubMed] [Google Scholar]
- Vetter M. L., Gewurz H., Hansen B., James K., Baum L. L. Effects of C-reactive protein on human lymphocyte responsiveness. J Immunol. 1983 May;130(5):2121–2126. [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston P. A., Levy N. L., Koren H. S. Spontaneous cytoxicity against virus-infected cells: cellular immunoadsorption on infected cell monolayers. J Immunol. 1980 Sep;125(3):1387–1394. [PubMed] [Google Scholar]
- Young W. W., Jr, Durdik J. M., Urdal D., Hakomori S., Henney C. S. Glycolipid expression in lymphoma cell variants: chemical quantity, immunologic reactivity, and correlations with susceptibility to NK cells. J Immunol. 1981 Jan;126(1):1–6. [PubMed] [Google Scholar]


