Abstract
In this study, the in vivo function and properties of two cytochrome c maturation proteins, CcmF and CcmH from Rhodobacter sphaeroides, were analyzed. Strains lacking CcmH or both CcmF and CcmH are unable to grow under anaerobic conditions where c-type cytochromes are required, demonstrating their critical role in the assembly of these electron carriers. Consistent with this observation, strains lacking both CcmF and CcmH are deficient in c-type cytochromes when assayed under permissive growth conditions. In contrast, under permissive growth conditions, strains lacking only CcmH contain several soluble and membrane-bound c-type cytochromes, albeit at reduced levels, suggesting that this bacterium has a CcmH-independent route for their maturation. In addition, the function of CcmH that is needed to support anaerobic growth can be replaced by adding cysteine or cystine to growth media. The ability of exogenous thiol compounds to replace CcmH provides the first physiological evidence for a role of this protein in thiol chemistry during c-type cytochrome maturation. The properties of R. sphaeroides cells containing translational fusions between CcmF and CcmH and either Escherichia coli alkaline phosphatase or β-galactosidase suggest that they are each integral cytoplasmic membrane proteins with their presumed catalytic domains facing the periplasm. Analysis of CcmH shows that it is synthesized as a higher-molecular-weight precursor protein with an N-terminal signal sequence.
c cytochromes are hemoproteins that play a critical role in electron transport by prokaryotes and eukaryotes. In bacteria, c-type cytochromes typically function on the external side of the cytoplasmic membrane, or in the periplasm of gram-negative species (24, 55). Thus, the maturation of c-type cytochromes requires export of a cytochrome c precursor protein out of the cytoplasm and subsequent covalent heme attachment to two cysteine side chains of the polypeptide chain. A number of studies (24, 55, 57) have implicated a group of conserved proteins, called the cytochrome c maturation proteins (Ccm), in this process, although the role of each protein is still not well understood. In this study, we analyzed the properties of Rhodobacter sphaeroides CcmF and CcmH, two proteins suggested to function in the later steps of c-type cytochrome maturation.
In the early steps of this maturation pathway, a c-type cytochrome precursor protein and heme are separately translocated across the cytoplasmic membrane by the general bacterial export pathway and some combination of CcmABCD, respectively (24, 49, 55). Once present in the oxidizing environment of the periplasm (41), thiol chemistry is critical to c-type cytochrome maturation. For example, Dsb proteins are thought to form an intramolecular disulfide bond at the cysteine thiols of the heme attachment site within the c-type cytochrome precursor protein (24, 55). In addition, thiol reductants are needed in vitro for heme to covalently bind to the periplasmic heme chaperone CcmE (6). In the latter part of the pathway, CcmH is proposed to reduce the intramolecular disulfide bond in the c-type cytochrome precursor protein because it contains a CX2C motif that is similar to those found in disulfide oxidoreductases (13, 49). Next, a bimolecular complex of CcmF and CcmH is proposed to catalyze thioether bond formation between two vinyl side chains on the tetrapyrrole ring and the reduced cysteine thiols on the polypeptide backbone of the c-type cytochrome apoprotein (40). The suggestion that thiols might be needed both for reduction of the cytochrome c apoprotein (13, 49) and for covalent binding of heme to CcmE (6) underscores the importance of characterizing the functions of Ccm proteins, especially ones like CcmH that contain redox-active thiols (49).
This study focuses on two c-type cytochrome maturation genes, ccmFH, from the α-proteobacterium R. sphaeroides. This facultative phototroph uses a number of well-characterized c-type cytochromes to generate energy when growing by aerobic respiration, by photosynthesis, and by anaerobic respiration (10, 19, 35). Previous work has described the R. sphaeroides ccmABCDG locus (5), and the genome sequence (www.rhodobacter.org and reference 29) predicts the presence of a ccmE gene in this α-proteobacterium. In addition to reporting on the characterization of R. sphaeroides mutants lacking CcmF and CcmH, we provide the first physiological evidence that CcmH participates in thiol chemistry during c-type cytochrome maturation. In addition, our data suggest that another activity can partly substitute for CcmH function in R. sphaeroides, adding another new level of complexity to the c-type cytochrome assembly pathway of this bacterium. We also found that CcmF and CcmH were each localized to the cytoplasmic membrane with proposed catalytic domains oriented towards the periplasm.
(A report of this work was made at the annual meeting of the American Society for Microbiology in May 1999.)
MATERIALS AND METHODS
Growth conditions.
Escherichia coli strains (Table 1) were grown at 37°C in Luria-Bertani medium (30) supplemented with 100 μg of ampicillin/ml, 10 μg of tetracycline/ml, 25 μg of spectinomycin/ml, or 25 μg of kanamycin/ml, as needed. R. sphaeroides strains were grown in Sistrom's medium A (51) at 30°C, supplemented as necessary with 1 μg of tetracycline/ml, 25 μg of kanamycin/ml, or 25 μg of spectinomycin/ml. E. coli DH5α (30) was used to maintain plasmids, while S17-1 (50) was used as a conjugal donor. When necessary, plates contained 5-bromo-4-chloro-3-indolyl-d-galactoside (IPTG; 40 μg/ml), isopropyl-β-d-thiogalactopyranoside (X-Gal; 40 μg/ml), or 5-bromo-4-chloro-3-indolyl-phosphate-p-toluidine (X-P; 40 μg/ml).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description (reference) |
|---|---|
| Strains | |
| E. coli DH5α | Plasmid maintenance |
| E. coli S17-1 | Donor for plasmid transfer into R. sphaeroides (50) |
| E. coli CC118 | Tester strain for PhoA and LacZ fusions, lacks phoA and lacZ genes |
| R. sphaeroides 2.4.1 | Wild type |
| R. sphaeroides C2NS3 | cycA2(ΔNruI-StuI)::kan (28) |
| R. sphaeroides CcmFH1 | ccmF1(ΔNot)I::spc |
| R. sphaeroides CcmH1 | ccmH1::spc |
| Plasmids | |
| pU18618 | Cosmid containing ccmFH |
| pRK415 | Broad-host-range cloning vector; Tcr (23) |
| pUCR1 | pUC19 with ∼9.0-kb EcoRI fragment of pU18618 |
| pUCR2 | pUC18 with ∼3.486-kb SmaI fragment of pUCR1 |
| pRKFH | pRK415 with ∼3.8-kb SmaI fragment of pUCR1 |
| pRP80 | pRK415 containing CcmF::PhoA fusion at residue 80 of CcmF; generated by TnphoA |
| pRH302 | pRK415 containing CcmF::PhoA fusion at residue 302 of CcmF; generated by TnphoA |
| pRL442 | pRK415 containing CcmF::PhoA fusion at amino acid residue 442, generated by cloning into pUI320 |
| pRA528 | pRK415 containing CcmF::PhoA fusion at residue 528 of CcmF; generated by TnphoA |
| pRL630 | pRK415 containing CcmF::PhoA fusion at residue 630 of CcmF; generated by TnphoA |
| pRV25 | pRK415 containing CcmF::LacZ fusion at residue 25 of CcmF; generated by TnlacZ |
| pRV113 | pRK415 containing CcmF::LacZ fusion at residue 113 of CcmF; generated by TnlacZ |
| pRL383 | pRK415 containing CcmF::LacZ fusion at residue 383 of CcmF; generated by TnlacZ |
| pRF494 | pRK415 containing CcmF::PhoA fusion at residue 494; generated by cloning into pUI320 |
| pRLN34 | pRK415 containing CcmF::PhoA fusion at residue 654; generated by cloning into pUI523 |
| pRL218 | pRK415 containing CcmF::PhoA fusion at residue 218; generated by cloning into pUI320 |
| pRV341 | pRK415 containing CcmF::PhoA fusion at residue 341; generated by cloning into pUI320 |
| pRC46 | pRK415 containing CcmH::PhoA fusion at residue 46 of CcmH; generated by TnphoA |
| pRL64 | pRK415 containing CcmH::PhoA fusion at residue 64 of CcmH; generated by TnphoA |
| pRC118 | pRK415 containing CcmH::PhoA fusion at residue 118 of CcmH; generated by TnphoA |
| pRD151 | pRK415 containing CcmH::LacZ fusion at residue 151 of CcmH; generated by cloning into pUI523 |
| pHRC | HindIII/XbaI-amplified ccmH gene from pUCR2 cloned into pRK415 |
| pF4 | pRK415-1 derivative containing a 1,452-bp in-frame deletion of codons 98 to 581 of ccmF |
Identification of ccmFH.
To identify R. sphaeroides ccmFH, a genomic library (11) was probed with an internal PstI-BamHI restriction fragment of Rhodobacter capsulatus ccmF (2). To determine if ccmH was also present on this cosmid, an R. capsulatus ccmH probe (MscI-SacII fragment from pL2917) was used as a probe (2). The R. sphaeroides ccmFH locus was cloned as an ∼9-kb EcoRI fragment (pUCR1) or as an ∼3.5-kb SmaI fragment (pUCR2) from cosmid pUI8618. The ccmF and ccmH genes were individually amplified from pUCR2 to create flanking KpnI-HindIII and BamHI-HindIII restriction sites before cloning them individually into pUC18 (pCRF1 and pCRH1, respectively). To map R. sphaeroides ccmFH, these genes were hybridized to genomic DNA that was digested singly or with different combinations of AseI, SpeI, DraI, or SnaBI and separated by pulsed-field electrophoresis (52).
DNA and protein sequence analysis.
DNA sequencing was performed with Taq DNA polymerase (Promega, Inc., Madison, Wis.) and deazanucleoside triphosphate reagent kits or at the University of Wisconsin—Madison Biotechnology Center (accession number AF321136). DNA and protein sequences were analyzed using Genetics Computer Group software (8). Databases were searched using Blitz (http://www2.ebi.ac.uk/bic_sw/) or FASTA/TFASTA (http://www2.ebi.ac.uk/fasta3/). Hydrophilicity plots were generated with Tmpred (http://ulrec3.unil.ch/software/tmpred_form.html), using a transmembrane helix length of 21 amino acids.
Construction of CcmF or CcmH hybrid proteins.
CcmF and CcmH hybrid proteins to alkaline phosphatase (PhoA) or β-galactosidase (LacZ) were generated using TnphoA/TnlacZ (32) or pUI523 and pUI320 (54, 56). When using Tn5 delivery systems, E. coli cells were plated on solid medium containing ampicillin, kanamycin, and X-Gal or X-P to identify strains that contained translational fusions (4). The position of the gene fusion junction was mapped by DNA sequencing with phoA-specific (4) or lacZ-specific (5′-CGTTTTACAACGTCGTGACTGGG-3′) primers.
Cell fractionation.
Soluble and membrane fractions were obtained from cell lysates prepared by sonication of mid-exponential-phase aerobic cultures of R. sphaeroides (∼5 × 108 cells/ml) (45). Periplasmic, cytoplasmic, inner, and outer membrane fractions were prepared by osmotic shock (53), using compartment-specific enzymes to estimate purity (1, 4, 42).
Construction of hexahistidine-tagged CcmH proteins.
A full-length N-terminal His6-tagged version of CcmH (CcmHSP) and one lacking the N-terminal 21 amino acids (CcmHNSP) was generated using the QIAExpress system (Qiagen, Valencia, Calif.). In each case, the ccmH coding region was amplified from pUCR2 using primers that contain a BamHI and HindIII site at their 5′ and 3′ ends to facilitate cloning these PCR products into pQE30.
CcmHSP and CcmHNSP were individually purified after induction of E. coli cultures containing pQHSP or pQHNSP. When the A600 of the culture was ∼0.8, IPTG was added (2 mM final concentration). After 4 h of incubation at 37°C with shaking, the cells were harvested by centrifugation (5,000 × g for 15 min at 4°C). Proteins were batch purified on nitrilotriacetic acid-resin under denaturing conditions using 0.5 M imidazole to elute the protein.
Immunological techniques.
Rabbit antiserum against CcmHNSP was produced at the University of Wisconsin Animal Care Center. Western blotting (3) used antiserum against R. sphaeroides CcmH (this study), E. coli PhoA (4), or E. coli LacZ (Promega, Inc., Fitchburg, Wis.). The indicated amount of protein from aerobically grown cells was solubilized with dithiothreitol (DTT; 100 mM) at 90°C and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentrations were determined using bovine serum albumin as a standard (27).
β-Galactosidase and alkaline phosphatase assays.
β-Galactosidase assays (48) used mid-exponential-phase cultures (∼8 × 108 cells/ml) that were harvested and suspended in 0.85% NaCl prior to enzyme assays. One unit of β-galactosidase activity is the amount of enzyme needed to cleave 1 nmol of o-nitrophenyl-β-d-galactopyranoside per min (54). To measure PhoA activity, extracts from mid-exponential-phase cultures were prepared in 10 mM Tris-HCl (pH 8.0), 0.1 mM EDTA buffer. One hundred microliters of cell extract was added to prewarmed tubes (30°C) containing 900 μl of a 5.57-mg/ml solution of para-nitrophenyl phosphate in diethanolamine buffer (pH 9.4). Assays were terminated by adding 1.5 ml of 2 N NaOH, and the amount of product formed was determined by measuring the optical density at 420 nm (4). One unit of alkaline phosphatase activity is the amount of enzyme needed to convert 1 nmol of substrate to product per min (34).
Mutant construction.
To generate the ccmF1 allele (Fig. 1), a spectinomycin resistance gene (spc) from pHP45Ω (39) was inserted as a SmaI restriction fragment into a unique NotI site within ccmF that had been made blunt by treatment with Klenow fragment DNA polymerase. The resultant plasmid (pCR2ΩF) was digested with SmaI and an ∼5.5-kb restriction fragment containing ccmF1 was cloned into a filled-in EcoRI site within the mobilizable suicide plasmid pSUP202 (50) to generate pSUPCR2ΩF. To generate the ccmH1 allele (Fig. 1), PCR was used to generate a 291-bp deletion that removed 97 codons (including those that encode the 43CX2C46 motif of CcmH) and created a unique EcoRV restriction enzyme site within ccmH for inserting an spc gene. To generate a suicide plasmid containing ccmH1, a restriction fragment containing this mutation was amplified (using ClaI upstream and SmaI downstream restriction sites in the primers) and cloned into a filled-in EcoRI site of pSup202 (pSup202ΔHΩSp).
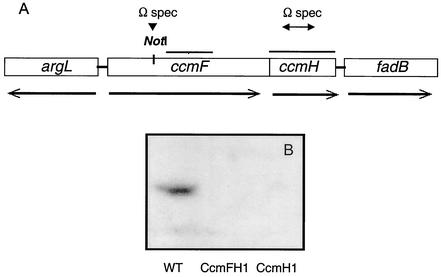
FIG. 1.
Organization of the R. sphaeroides ccmFH locus. (A) Location and transcription of genes at the R. sphaeroides ccmFH locus. The bars above ccmFH indicate regions used as probes in Northern blotting. The arrows indicate where Ωspc cassettes were placed within ccmF or ccmH to generate the ccmF1 and ccmH1 alleles, respectively. The genes that flank ccmFH encode proteins with significant amino acid sequence identity to arginase (argL) and to enoyl-CoA hydratase (fadB). (B) Western blot analysis using CcmH antibody and ∼400 μg of crude extract protein from wild-type cells, CcmFH1, or CcmH1.
After each suicide plasmid was mobilized into R. sphaeroides (9), Spr cells were screened for Tcs to distinguish ones in which ccmF1 or ccmH1 had integrated in the genome (Tcr Spr) or recombined by an even number of crossover events (Tcs Spr). Southern blotting with ccmF, pSUP202, and spc-specific probes was used to confirm the lack of plasmid DNA and the replacement of a wild-type gene with the mutant allele (data not shown).
Heme peroxidase assays.
To assay for heme peroxidase activity under denaturing conditions, ∼400 μg of soluble or membrane protein was heated for 10 min at 90°C in solubilization buffer containing 0.2 M DTT, prior to separation by SDS-15% PAGE. Following electrophoresis, heme peroxidase activity was tested (17).
Cytochrome spectra.
Reduced (1 mM sodium ascorbate) minus-oxidized (800 μM potassium ferricyanide) difference spectra were performed at room temperature in an SLM DW2000 spectrophotometer. The c-type cytochrome content was estimated by subtracting the absorbance at 540 nm from that at the α-peak maximum, assuming an ɛ of 20 mM (22).
RESULTS
Identification of R. sphaeroides ccmFH.
Hybridization of specific R. capsulatus probes (2) to R. sphaeroides genomic DNA that was digested with different combinations of AseI, SpeI, DraI, or SnaBI identified related sequences at approximately coordinate 2500 (± 100 kb) of chromosome 1 (data not shown), some 1,000 kb away from the ccmABCDG locus (5). The same probes identified related sequences on an ∼9.0-kb EcoRI fragment within an R. sphaeroides cosmid library (Fig. 1A). The DNA sequence of an ∼3.8-kb region from this EcoRI fragment located open reading frames encoding proteins that have considerable amino acid identity to CcmF and CcmH homologs from α- or γ-proteobacteria (∼50 to 70% for CcmF; 30 to 54% for CcmH), including stretches of primary sequence identity as high as 90%. It seems likely that ccmF is cotranscribed with ccmH, since the predicted ccmF termination codon overlaps the ccmH initiation codon. The genes that flank ccmFH encode proteins with significant amino acid sequence identity to arginase (argL) and to enoyl-coenzyme A (CoA) hydratase (fadB) (Fig. 1A), suggesting that these gene products are not involved in c-type cytochrome maturation.
R. sphaeroides cells lacking CcmH, or CcmF and CcmH are unable to grow under conditions that require c-type cytochrome function.
To determine the function of these gene products, a spectinomycin resistance gene was placed within ccmF (the ccmF1 allele) or ccmH (ccmH1) by marker exchange. Cells containing either the ccmF1 or the ccmH1 mutation lacked proteins that reacted with polyclonal antibodies to CcmH (Fig. 1B), demonstrating the loss of CcmH in both strains and suggesting that the ccmF1 allele was polar to expression of ccmH, as would be expected if ccmFH were cotranscribed (see above).
To test if these mutations prevented growth under conditions that require c-type cytochrome function (10), we analyzed the ability of these strains to grow via photosynthesis or anaerobic respiration. Strains lacking both CcmF and CcmH (CcmFH1) or CcmH alone (CcmH1) were unable to grow by either photosynthesis or anaerobic respiration (Table 2), consistent with a defect in c-type cytochrome maturation. Complementation assays indicated that both ccmF and ccmH are required to restore photosynthetic growth to cells lacking both of these proteins, since ccmH alone did not restore photosynthetic growth (Table 2). In addition, complementation of cells lacking CcmH alone was restored when either ccmFH or a ccmH gene whose transcription is under control of ∼750 bp of DNA upstream of ccmF (pF4) was placed in trans, but not when a ccmH gene that lacked this presumed promoter region was present on a comparable plasmid (Table 2). This complementation analysis provides further support of the notion that ccmFH are cotranscribed.
TABLE 2.
Phenotypes of strains lacking different combinations of CcmFH
| Strain | Relevant genotype | Plasmid | Growtha
|
||
|---|---|---|---|---|---|
| A | AR | PS | |||
| 2.4.1 | Wild type | + | + | + | |
| CcmFH1 | ccmF1 | + | − | − | |
| CcmFH1 | ccmF1 | pRKFH | + | + | + |
| CcmFH1 | ccmF1 | pRKF | + | − | − |
| CcmFH1 | ccmF1 | pRKH | + | − | − |
| CcmH1 | ccmH1 | + | − | − | |
| CcmH1 | ccmH1 | pRKFH | + | + | + |
| CcmH1 | ccmH1 | pF4b | + | + | NDc |
| CcmH1 | ccmH1 | pRKH | + | − | − |
A = aerobic respiration, AR = anaerobic respiration using DMSO as external electron acceptor, PS = photosynthesis.
pF4 contains an in-frame deletion in ccmF, a possible promoter within 750 bp upstream of ccmF (see text).
ND = not determined.
Loss of CcmF or CcmH alters the production of c-type cytochromes.
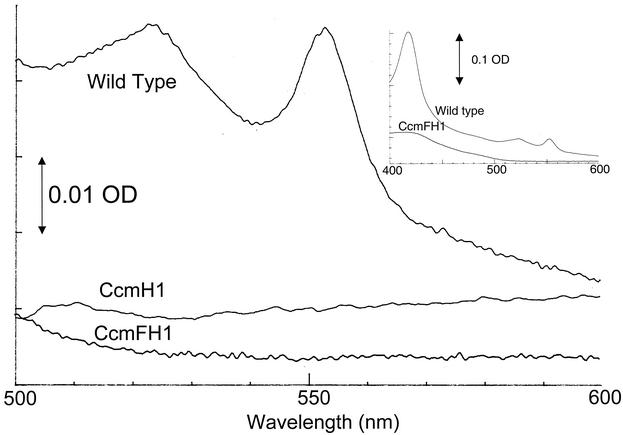
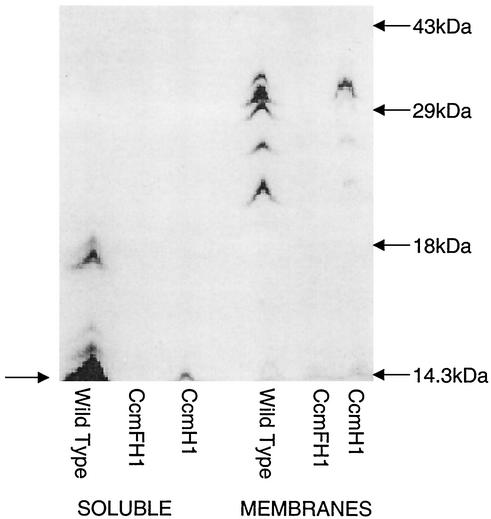
To test if these mutations caused a defect in c-type cytochrome accumulation, the levels of these electron carriers were measured in cells that were grown by aerobic respiration, a condition under which R. sphaeroides contains detectable soluble and membrane-bound c-type cytochromes (10, 19). Reduced minus-oxidized spectra revealed that soluble (Fig. 2) or membrane (data not shown) fractions from wild-type aerobically grown cells contained the 550-nm absorbance feature that is characteristic of c-type cytochromes (38). Membrane (data not shown) or soluble (Fig. 2) fractions from aerobically grown strains lacking both CcmF and CcmH did not contain a detectable absorbance feature at ∼550 nm, suggesting that c-type cytochrome production was blocked. The presence of proteins that contain covalently bound heme (i.e., c-type cytochromes) was also assayed by monitoring the heme peroxidase activity of samples that were solubilized and electrophoresed under denaturing conditions. While it is possible to detect c-type cytochromes in this assay with as little as ∼100 μg of soluble or membrane protein from aerobically grown wild-type cells (45), c-type cytochromes were not detected, even when as much as 400 μg of soluble or membrane protein from cells lacking CcmF and CcmH was analyzed (Fig. 3), providing a second line of evidence that the ccmF1 allele has a negative effect on the assembly of these electron carriers. In contrast, the 560-nm spectral feature that is indicative of b-type cytochromes is retained in membrane samples from CcmFH1 (data not shown), suggesting that the ccmF1 allele does not abolish assembly of all membrane-bound hemoproteins under aerobic conditions.
FIG. 2.
Absorption spectra of soluble extracts. Reduced minus-oxidized absorption spectra of ∼2.5 mg of soluble protein from wild-type, CcmFH1, or CcmH1 cells grown via aerobic respiration. The insert shows spectra from soluble extracts of wild-type or CcmFH1 cells when scanned from 400 to 600 nm.
FIG. 3.
Heme peroxidase staining of c-type cytochromes. Shown are the results obtained when ∼400 μg of soluble or membrane protein from wild-type, CcmFH1, or CcmH1 cells was solubilized at 90°C in the presence of 0.2 mM DTT, separated by SDS-PAGE, and stained for heme peroxidase activity. The arrow on the left highlights known soluble c-type cytochromes (cytochrome c2 and cytochrome c554) that are detectable in cells lacking CcmH (see text).
In contrast, aerobically grown cells lacking only CcmH contained low, but detectable, levels of the major soluble or membrane-bound c-type cytochromes that are found in wild-type cells (Fig. 3). In the membrane samples, this includes proteins of a size expected for cytochrome c1 of the cytochrome bc1 complex (20), plus others that could represent c-type cytochromes in the cytochrome cbb3 oxidase (37). Using published extinction coefficients to estimate the concentration of ascorbate-reducible soluble c-type cytochromes (Fig. 2), it appears that strains lacking only CcmH had ∼30-fold less of these proteins (∼0.01 nmol/mg of soluble protein) than aerobically grown wild-type cells (∼0.36 nmol/mg). Consistent with this finding, Western blotting using antibodies to either cytochrome c2 (3) or cytochrome c554 (16) showed that each protein was at least 10-fold less abundant in cells lacking only CcmH compared to wild-type cells (data not shown).
Evidence for the role of CcmH in thiol chemistry.
It has been proposed that CcmH reduces an intramolecular disulfide bond within the c-type cytochrome precursor protein (24, 55), but this protein could also serve as a thiol reductant to facilitate covalent heme attachment to CcmE (6). To test the role of CcmH as a thiol reductant during c-type cytochrome maturation, we asked if the addition of thiols or reducing agents repairs the growth defects of cells lacking CcmH.
These experiments showed that addition of cysteine (0.1 to 2 mM) or cystine (2 to 5 mM) to solid media was able to support photosynthetic growth of cells lacking CcmH. In contrast, DTT, β-mercaptoethanol, or the reductant tris(carboxyethyl)phosphine, at concentrations up to 2 mM, did not restore photosynthetic growth to cells lacking CcmH. Cysteine or cystine (at concentrations up to 5 mM) failed to restore photosynthetic growth to cells lacking both CcmF and CcmH (data not shown), suggesting that these thiols could not replace the putative heme lyase enzyme, CcmF.
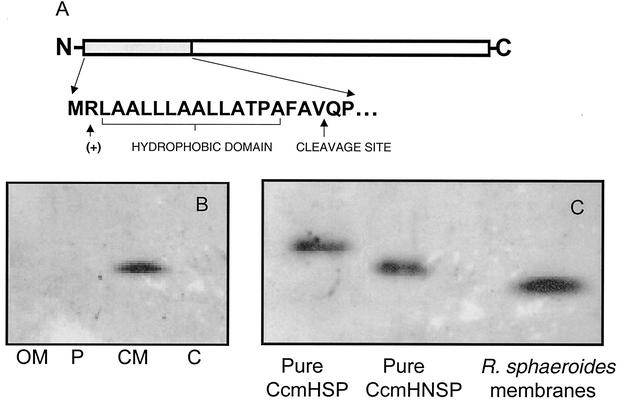
R. sphaeroides CcmH is synthesized as a precursor protein.
CcmH has been proposed to be an integral cytoplasmic membrane protein (33) with N- and C-terminal hydrophobic domains crossing the bilayer (44). R. sphaeroides CcmH was localized to the cytoplasmic membrane (Fig. 4B) when Western blotting with antibody against this protein was performed with cell fractions whose purity was tested by assaying known marker enzyme activities (data not shown). However, the deduced amino acid sequence of R. sphaeroides CcmH indicates that its N-terminal 21 amino acids contain features typical of procaryotic signal peptides (Fig. 4A). To test if this N-terminal region of CcmH was present in vivo, the SDS-PAGE mobility of the membrane-bound form of the wild-type protein was compared to that of purified N-terminal His6-tagged recombinant proteins that either contain (CcmHSP) or lack (CcmHNSP) these 21 N-terminal residues. Western blot analysis with CcmH antisera indicated that CcmHSP and CcmHNSP have mobilities by SDS-PAGE that are consistent with the ∼2-kDa difference that is associated with the presence of the N-terminal 21 amino acids (Fig. 4C). In addition, the apparent molecular weight of the CcmH protein that is present in the R. sphaeroides cytoplasmic membrane was less than that of a purified recombinant protein in which the 21 N-terminal residues were replaced with an N-terminal His6 tag (CcmHNSP) (Fig. 4C). If one assumes that the His6 tag does not cause changes in the SDS-PAGE mobility of CcmH, these results suggest that the N-terminal region is missing from the protein that is present in the R. sphaeroides cytoplasmic membrane.
FIG. 4.
CcmH has a cleavable N-terminal signal sequence. (A) Amino acid similarity of the N-terminal region of the putative CcmH precursor protein to bacterial signal peptides, including a positively charged residue at position 2, a hydrophobic core of 16 to 17 residues, and a potential signal peptidase cleavage site. (B) Western blot analysis using CcmH antibody with outer membrane (OM), periplasm (P), cytoplasmic membrane (CM), and cytoplasmic (C) fractions from aerobically grown cells (25 μg of protein in each sample). (C) Results of a Western blot analysis using CcmH antibody to compare the relative mobility of a full-length His6-CcmH (CcmHSP), a CcmH protein which lacks the 19 N-terminal amino acids (CcmHNSP), and CcmH within the R. sphaeroides cytoplasmic membrane.
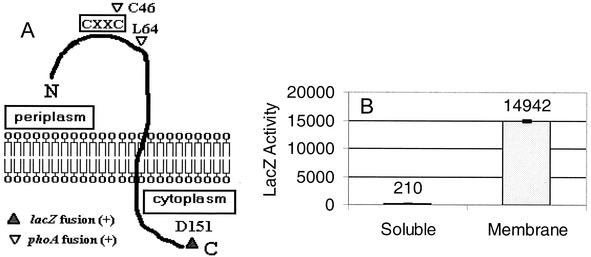
To further analyze the properties of R. sphaeroides CcmH, translational fusions were generated between ccmH and genes whose products can act as reporters of cytoplasmic (LacZ) and periplasmic (PhoA) domains (31). The fusions to CcmH were designed based on its cytoplasmic membrane localization, the proposed cleavage of an N-terminal signal peptide, and its presumed periplasmic catalytic domain (14). By analyzing cells containing individual hybrid proteins, the C terminus of CcmH appears cytoplasmic (Fig. 5A), since either R. sphaeroides (Fig. 5B) or E. coli (data not shown) cells containing a CcmH::LacZ hybrid at residue D151 exhibited β-galactosidase activity significantly higher than that present in cells which lack a functional lacZ gene. In contrast, the N terminus of CcmH appears periplasmic, since membrane samples from either R. sphaeroides (Fig. 6A) or E. coli (data not shown) cells containing CcmH::PhoA fusions at residues C46 or L64 contained high PhoA activity. To further test the location of a presumed “catalytic” cysteine, C46, of CcmH (43CX2C46), PhoA activity was measured in subcellular fractions whose purities were assessed by monitoring known enzyme markers (53). In cells harboring the C46 CcmH::PhoA fusion, >95% of the PhoA activity (Fig. 6B) and all the detectable C46 CcmH::PhoA protein that reacted with antibodies to either CcmH or PhoA (data not shown) were periplasmic, presumably because a hybrid that lacks the hydrophobic C terminus of CcmH is released from the membrane after cleavage of its predicted N-terminal signal peptide (see above).
FIG. 5.
CcmH is a transbilayer cytoplasmic membrane protein. (A) Model for the organization of CcmH in the cytoplasmic membrane. The box in the periplasm shows the predicted location of the proposed catalytic 43CX2C46 motif. Indicated in the model are amino acids within CcmH where translational fusions to either PhoA (C46 and L64) or LacZ (D151) produced active hybrid proteins. (B) Results of β-galactosidase enzyme assays on soluble (open bars) and membrane (hatched bars) fractions of R. sphaeroides containing a CcmH::LacZ hybrid protein at CcmH residue D151. This wild-type R. sphaeroides strain is laco (54) and it contains little detectable β-galactosidase activity (see Fig. 7).
FIG. 6.
The N-terminal domain of CcmH is periplasmic. (A) PhoA activity in soluble (open bars) and membrane fractions (hatched bars) of cells containing CcmH::PhoA hybrids at residue C46 or L64. (B) PhoA activity in outer membrane (OM), periplasm (P), cytoplasmic membrane (CM), and cytoplasmic (C) fractions from R. sphaeroides cells containing a CcmH::PhoA hybrid at position C46. This wild-type R. sphaeroides strain is phoAo (56) and it contains little detectable PhoA activity (see Fig. 7).
R. sphaeroides CcmF is a polytopic cytoplasmic membrane protein.
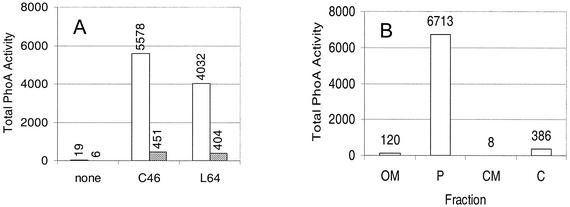
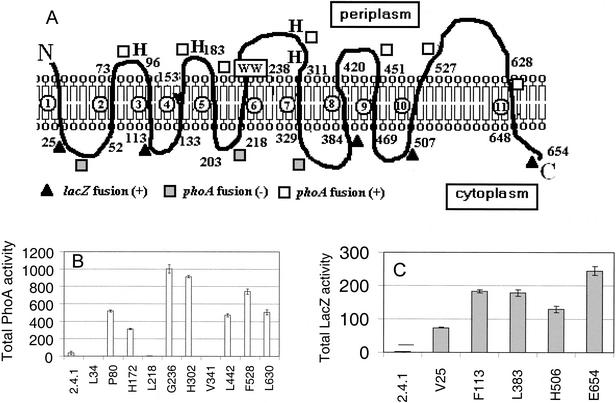
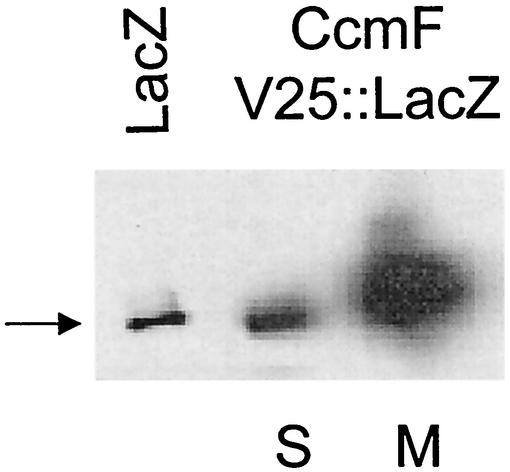
Given that CcmF and CcmH form a presumed heme lyase complex (40), we were also interested in investigating how CcmF was oriented in the membrane. Hydrophilicity profiles have generated models which predict that CcmF homologues contain anywhere from 11 to 15 membrane-spanning domains (24, 55). The properties of CcmF::PhoA hybrid proteins suggest that this protein crosses the bilayer 11 times and contains six periplasmic domains (Fig. 7A). R. sphaeroides (Fig. 7B) or E. coli (data not shown) cells containing CcmF::PhoA hybrids at residues P80, G236, H302, L442, F528, or L630 exhibit PhoA activity well above background. Western blot analysis using PhoA antibodies showed that cells containing CcmF::PhoA fusions at residues P80, G236, H302, L442, F528, or L630 contain a membrane-bound protein with an apparent molecular weight close to that predicted by the position of the gene fusion (data not shown). The properties of cells containing CcmF hybrid proteins suggest that this protein also contains six cytoplasmic domains (Fig. 7A). R. sphaeroides (Fig. 7C) or E. coli (data not shown) cells containing CcmF::LacZ hybrids at residues V25, F113, L383, H506, or E654 contain β-galactosidase activity levels higher than cells which lack a functional lacZ gene. Western blot analysis using LacZ antibodies showed that CcmF::LacZ hybrid proteins at CcmF residues V25 (Fig. 8), V113, L383, or E654 (data not shown) are membrane associated. The location of the V25::LacZ hybrid protein in the membrane provides one piece of experimental support for the notion that CcmF crosses the membrane in the first 25 residues. To further test this topology model, PhoA was fused to several regions of CcmF that are predicted to be cytoplasmic (Fig. 7A). R. sphaeroides (Fig. 7B) or E. coli (data not shown) cells containing CcmF::PhoA fusions at residues predicted to be cytoplasmic (L34, L218, V341, or K494) lacked detectable alkaline phosphatase activity, suggesting that these domains are not outside the cytoplasm. In summary, R. sphaeroides CcmF is predicted to be a polytopic cytoplasmic membrane protein with 11 membrane-spanning regions, a periplasmic N terminus, and a cytoplasmic C terminus (Fig. 7A). This model places residues of CcmF that have been proposed to be critical to its function (21, 25, 55) in the same subcellular compartment, the periplasm, as the CcmH cysteines that are proposed to perform thiol chemistry.
FIG. 7.
CcmF is a polytopic cytoplasmic membrane protein. (A) Model for CcmF in the cytoplasmic membrane. This model was generated by analyzing the behavior of CcmF::LacZ (filled triangles if active) and CcmF::PhoA (open squares if active, filled squares if inactive) fusion proteins (see text). The predicted membrane-spanning regions of CcmF are numbered as open circles. The letters H and the boxed WW indicate the predicted location of conserved histidinyl residues that are required for CcmE activity (40) and the tryptophan-rich motif of CcmF, respectively. (B and C) Results of PhoA or LacZ assays on soluble or membrane fractions, respectively, from R. sphaeroides cells that carried hybrid proteins generated at the indicated residue within CcmF.
FIG. 8.
Membrane localization of the CcmF V25::LacZ protein. Shown are the results of Western blotting using antiserum to β-galactosidase with 200 μg of soluble (S) or crude membrane (M) protein from R. sphaeroides cells containing the CcmF V25::LacZ fusion. The arrow indicates the position of migration of pure β-galactosidase (LacZ) in the leftmost lane.
DISCUSSION
In this work, a locus, ccmFH, which encodes homologues of R. sphaeroides c-type cytochrome maturation proteins was analyzed. The analyses of these and other R. sphaeroides Ccm mutants (5) have important implications for the process of bacterial c-type cytochrome maturation and the roles of Ccm proteins in this and other important pathways.
The contributions of R. sphaeroides CcmF and CcmH to c-type cytochrome maturation.
CcmF and CcmH are believed to act late in the c-type cytochrome biosynthetic pathway after the apocytochrome c precursor protein and heme are each exported across the cytoplasmic membrane (24, 55). CcmH forms a bimolecular complex with CcmF that has been proposed to form the active heme lyase enzyme (40). Thus, the fact that mutants lacking both CcmF and CcmH lack detectable c-type cytochromes and are unable to grow under anaerobic conditions where these electron carriers are required (10) suggests that their function in R. sphaeroides is probably similar to that in other bacteria (24, 55, 57).
However, the finding that low levels of several soluble and membrane-bound c-type cytochromes were present in an R. sphaeroides CcmH mutant was unexpected since, in other bacteria, the loss of CcmH alone is also sufficient to abolish the accumulation of all detectable c-type cytochromes (24, 55). One exception is in Bradyrhizobium japonicum, where reduced levels of a single membrane-bound c-type cytochrome (cytochrome c1) were observed in strains lacking its CcmH homolog (43). Mutations that remove the periplasmic domain of R. capsulatus CycH/CcmI have the selective ability to assemble cytochrome c1 (26). Whether the abilities of R. sphaeroides CcmH mutants to bypass the requirement for CcmH and assemble several soluble and membrane-bound c-type cytochromes, albeit at a reduced efficiency, occur as a consequence of another activity that is redundant with CcmH or as a spurious reaction of another thiol donor remains to be determined. In this regard, it is worthy to note that no other ccmH homologs have been identified in the R. sphaeroides genome sequence, whereas several other potential disulfide oxidoreductases are predicted to be present (29). In any event, the presence of a CcmH-independent pathway may have made it easier for exogenous thiols to rescue the defect of R. sphaeroides CcmH mutants, since the existing level of cytochromes may have lowered the threshold needed to support growth (see below).
What is the role of exogenous thiols in replacing CcmH?
To our knowledge, this is the first report that thiols can repair the growth defects of a bacterial CcmH protein. Exogenous thiols can repair the defect in c-type cytochrome assembly in cells lacking disulfide oxidoreductases like DipZ (47) or DsbAB (46). In these cases, it has been suggested that exogenous thiols directly provide the reducing power for c-type cytochrome biosynthesis. By analogy, the ability of cysteine or cystine to restore growth to R. sphaeroides CcmH mutants provides physiological evidence that this protein participates in thiol chemistry during c-type cytochrome maturation. The ability of exogenous thiols to support growth of cells lacking CcmH could reflect their capacity either to substitute for a presumed disulfide oxidoreductase (24, 55, 57) or to supply the thiol reductant that is needed for heme to covalently bind CcmE (6). The midpoint potential of the cystine-cysteine redox couple (−340 mV) could allow them to substitute for either function, given what is known about CcmH (thiol midpoint potential, −240 mV) (49) or the ability of cysteine to promote covalent attachment of heme to CcmE in vitro (6).
It is also interesting to find that both cystine and cysteine can rescue the growth of R. sphaeroides CcmH mutants. Because c-type cytochromes are only required for growth of R. sphaeroides under anaerobic conditions (10), it is difficult to assess whether cystine is responsible for restoring growth or if some fraction of this compound is reduced to cysteine when cells are grown in the absence of O2. In any event, this ability of thiols to repair the growth defects of R. sphaeroides CcmH mutants may allow for screening for different classes of mutations in ccmH that help identify the role of this protein in c-type cytochrome maturation.
Properties of R. sphaeroides CcmH and CcmF.
It has been suggested that bacterial CcmH homologs contain either a single C-terminal transmembrane domain (33) or distinct N- and C-terminal transmembrane domains (55). R. sphaeroides CcmH contains only a single C-terminal transmembrane domain, since its hydrophobic N terminus is apparently cleaved when the protein is present in cytoplasmic membrane. Our results suggest that R. sphaeroides CcmH contains a periplasmic N terminus, a cytoplasmic C terminus, and a periplasmic domain that contains the presumed redox-active cysteine residues (49). If the N terminus of CcmH is normally cleaved in vivo, this suggests that the conserved hydrophobic domain of CcmH plays a critical role in anchoring this protein in the cytoplasmic membrane so that its presumed active site can face the periplasm.
Different computer models have predicted that CcmF homologs contain anywhere from 11 to 15 transmembrane segments (24, 55). Our results predict that R. sphaeroides CcmF has a periplasmic N terminus, a cytoplasmic C terminus, and 11 transmembrane domains. In many respects, our model for the topology of CcmF is similar to one generated by analyzing the organization of a recombinant R. capsulatus CcmF protein in E. coli (21). Both models place the regions that are proposed to be critical for heme lyase activity in the periplasm, where covalent heme attachment is proposed to occur (24, 55). The R. capsulatus CcmF model places the first 100 residues in the periplasm, based on analysis of only two active PhoA fusion proteins (21), but the properties of several CcmF hybrid proteins predict that the R. sphaeroides protein crosses the bilayer once in the first 25 amino acids. In addition, residues 400 to 450 of R. capsulatus CcmF are predicted to be cytoplasmic (21), while the alkaline phosphatase activity found when PhoA is fused to residue 442 of R. sphaeroides CcmF suggests that this domain is periplasmic. Another important aspect of this model is that it places all the conserved histidine residues of CcmF that are required for activity (40) in the periplasm (Fig. 7). It also supports the notion that one other conserved histidine of E. coli CcmE (histidine 491), which is not required for activity (40), is cytoplasmic. Despite this, all topology models for CcmF should be considered preliminary, since cases are known where the use of hybrid proteins alone generates different predictions of the number and relative position of transmembrane domains (15).
Other important features of R. sphaeroides Ccm mutants.
It has been suggested that homologs of the putative disulfide oxidoreductase, CcmG, can participate in both c-type cytochrome maturation and other important pathways in both R. sphaeroides (5) and other bacteria (7, 18, 44, 58). R. sphaeroides CcmH does not appear to have another metabolic function, since cells lacking this protein only exhibit a defect in c-type cytochrome maturation.
Another important observation is that membranes from R. sphaeroides cells lacking CcmF and CcmH have a significant ∼600-nm-absorbing feature (data not shown) that is not found in either wild-type cells or those lacking CcmA (5). This 600-nm-absorbing feature is characteristic of cytochromes that contain a-type heme (19), suggesting that synthesis of such a metalloenzyme is induced in cells that lack CcmF and CcmH. The R. sphaeroides genome sequence predicts the presence of several oxidases with a heme a feature (29), so another unexpected outcome of studying Ccm mutants may be the characterization of additional R. sphaeroides respiratory enzymes.
There is also evidence that alterations in the oxidation-reduction state of the R. sphaeroides aerobic electron transport chain plays a critical role in controlling the expression of genes regulated by the PrrABC signal transduction chain (12, 36). It appears that potential differences in the cytochrome oxidase content (see above) alter the activity of the PrrABC signaling pathway, since loss of CcmA (5), CcmF and CcmH, or CcmH alone (data not shown) leads to changes in colony pigmentation similar to that seen when signaling through the PrrABC pathway is interrupted (37). Thus, analysis of R. sphaeroides Ccm mutants could also provide important insights into how the oxidation-reduction state of the aerobic respiratory chain acts as a global regulator of gene expression.
Acknowledgments
This work was supported by NIH grant GM37590 to T.J.D. and NIGMS NRSA fellowship GM19382 to C.R.V. R.C. received funding from the UW—Madison Hilldale Foundation and the ASM Undergraduate Fellowship Program. We also acknowledge the NSF Research Experience for Undergraduates program (DBI-9820163) for support of K. Jackson.
We thank Gregory J. Horwitz and Kurt Jackson for help in the topology analysis and the generation of mutants and Patricia Kiley for helpful comments on the manuscript.
REFERENCES
- 1.Barber, R. D., and T. J. Donohue. 1998. Function of a glutathione-dependent formaldehyde dehydrogenase in Rhodobacter sphaeroides formaldehyde oxidation and assimilation. Biochemistry 37:530-537. [DOI] [PubMed] [Google Scholar]
- 2.Beckman, D. L., D. R. Trawick, and R. G. Kranz. 1992. Bacterial cytochromes c biogenesis. Genes Dev. 6:268-283. [DOI] [PubMed] [Google Scholar]
- 3.Brandner, J. P., and T. J. Donohue. 1994. The Rhodobacter sphaeroides cytochrome c2 signal peptide is not necessary for export and heme attachment. J. Bacteriol. 176:602-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandner, J. P., E. V. Stabb, R. Temme, and T. J. Donohue. 1991. Regions of Rhodobacter sphaeroides cytochrome c2 required for export, heme attachment, and function. J. Bacteriol. 173:3958-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, R. L., C. Patterson, and T. J. Donohue. 2001. Roles for the Rhodobacter sphaeroides CcmA and CcmG proteins. J. Bacteriol. 183:4643-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daltrop, O., J. M. Stevens, C. W. Higham, and S. J. Ferguson. 2002. The CcmE protein of the c-type cytochrome biogenesis system: unusual in vitro heme incorporation into apo-CcmE and transfer from holo-CcmE to apocytochrome. Proc. Natl. Acad. Sci. USA 99:9703-9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado, M. J., K. H. Yeoman, G. Wu, C. Vargas, A. E. Davies, R. K. Poole, A. W. B. Johnston, and A. Downie. 1995. Characterization of the cycHJKL genes involved in cytochrome c biogenesis and symbiotic nitrogen fixation in Rhizobium leguminosarum. J. Bacteriol. 177:4927-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue, T. J., and S. Kaplan. 1991. Genetic techniques in Rhodospirillaceae. Methods Enzymol. 204:459-485. [DOI] [PubMed] [Google Scholar]
- 10.Donohue, T. J., A. G. McEwan, S. Van Doren, A. R. Crofts, and S. Kaplan. 1988. Phenotypic and genetic characterization of cytochrome c2 deficient mutants of Rhodobacter sphaeroides. Biochemistry 27:1918-1925. [DOI] [PubMed] [Google Scholar]
- 11.Dryden, S. C., and S. Kaplan. 1990. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 18:7267-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eraso, J. M., and S. Kaplan. 1995. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J. Bacteriol. 177:2695-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabianek, R. A., H. Hennecke, and L. Thony-Meyer. 2000. Periplasmic protein thiol:disulfide oxidoreductase of Escherichia coli. FEMS Microbiol. Rev. 24:303-316. [DOI] [PubMed] [Google Scholar]
- 14.Fabianek, R. A., T. Hofer, and L. Thony-Meyer. 1999. Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch. Microbiol. 171:92-100. [DOI] [PubMed] [Google Scholar]
- 15.Fillingame, R. H., P. C. Jones, W. Jiang, F. I. Valiyaveetil, and O. Y. Dmitriez. 1998. Subunit organization and structure in the F0 sector of Escherichia coli F1F0 ATP synthase. Biochim. Biophys. Acta 13:65135-65142. [DOI] [PubMed] [Google Scholar]
- 16.Flory, J. E., and T. J. Donohue. 1995. Organization and expression of the Rhodobacter sphaeroides cycFG operon. J. Bacteriol. 177:4311-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis, R. T. J., and R. R. Becker. 1984. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal. Biochem. 136:509-514. [DOI] [PubMed] [Google Scholar]
- 18.Gaballa, A., N. Koedam, and P. Cornelis. 1996. A cytochrome c biogenesis gene involved in pyoverdine production in Pseudomonas fluorescens ATCC 17400. Mol. Microbiol. 21:777-785. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Horsman, J. A., E. Berry, J. P. Shapleigh, J. O. Alben, and R. B. Gennis. 1994. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry 33:3113-3119. [DOI] [PubMed] [Google Scholar]
- 20.Gennis, R. B., B. Barquera, B. Hacker, S. R. Van Doren, S. Arnaud, A. R. Crofts, E. Davidson, K. A. Gray, and F. Daldal. 1993. The bc1 complexes of Rhodobacter sphaeroides and Rhodobacter capsulatus. J. Bioenerg. Biomembr. 25:195-209. [DOI] [PubMed] [Google Scholar]
- 21.Goldman, B. S., D. L. Beck, E. M. Monika, and R. G. Kranz. 1998. Transmembrane heme delivery systems. Proc. Natl. Acad. Sci. USA 95:5003-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendry, G. A. F., J. D. Houghton, and O. T. G. Jones. 1991. The cytochromes in microsomes of germinating mung beans. J. Biochem. 194:743-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 24.Kranz, R., R. Lill, B. Goldman, G. Bonnard, and S. Merchant. 1998. Molecular mechanism of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29:383-396. [DOI] [PubMed] [Google Scholar]
- 25.Kranz, R. G., and D. L. Beckman. 1995. Cytochrome biogenesis, p. 709-723. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordecht, The Netherlands.
- 26.Lang, S. E., F. E. Jenney, Jr., and F. Daldal. 1996. Rhodobacter capsulatus CycH: a bipartite gene product with pleiotropic effects on the biogenesis of structurally different c-type cytochromes. J. Bacteriol. 178:5279-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 28.MacGregor, B. J., and T. J. Donohue. 1991. Evidence for two promoters for the cytochrome c2 gene (cycA) of Rhodobacter sphaeroides. J. Bacteriol. 173:3949-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenzie, C., M. Choudhary, F. W. Larimer, P. F. Predki, S. Stilwagen, J. P. Armitage, R. D. Barber, T. J. Donohue, J. P. Hosler, J. Newman, J. P. Shapliegh, R. E. Sockett, J. Zeilstra-Ryalls, and S. Kaplan. 2001. The home stretch, a preliminary analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynthesis Res. 70:19-41. [DOI] [PubMed] [Google Scholar]
- 30.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Manoil, C., J. J. Mekalanos, and J. Beckwith. 1990. Alkaline phosphatase fusions: sensors of subcellular location. J. Bacteriol. 172:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manoil, C. 1991. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusions. Methods Cell. Biol. 34:61-75. [DOI] [PubMed] [Google Scholar]
- 33.Monika, E. M., B. S. Goldman, D. L. Beckman, and R. G. Kranz. 1997. A thioreduction pathway tethered to the membrane for periplasmic cytochromes c biogenesis: in vitro and in vivo studies. J. Mol. Biol. 271:679-692. [DOI] [PubMed] [Google Scholar]
- 34.Moore, M. D., and S. Kaplan. 1989. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J. Bacteriol. 171:4385-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouncey, N. J., M. Choudhary, and S. Kaplan. 1997. Characterization of genes encoding dimethyl sulfoxide reductase of Rhodobacter sphaeroides 2.4.1T: an essential metabolic gene function encoded on chromosome II. J. Bacteriol. 179:7617-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh, J.-I., and S. Kaplan. 2000. Redox signaling: globalization of gene expression. EMBO J. 19:4237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh, J. I., and S. Kaplan. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688-2696. [DOI] [PubMed] [Google Scholar]
- 38.Pettigrew, G. W., and G. R. Moore. 1987. Cytochromes c: biological aspects. Springer-Verlag, Berlin, Germany.
- 39.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 40.Ren, Q., U. Ahuja, and L. Thony-Meyer. 2002. A bacterial cytochrome c heme lyase: CcmF forms a complex with the heme chaperone CcmE and CcmH but not with apocytochrome c. J. Biol. Chem. 277:7657-7663. [DOI] [PubMed] [Google Scholar]
- 41.Rietsch, A., and J. Beckwith. 1998. The genetics of disulfide bond metabolism. Annu. Rev. Genet. 32:163-184. [DOI] [PubMed] [Google Scholar]
- 42.Rios-Velazquez, C., R. L. Cox, and T. J. Donohue. 2001. Characterization of Rhodobacter sphaeroides cytochrome c2 proteins with altered heme attachment sites. Arch. Biochem. Biophys. 389:234-244. [DOI] [PubMed] [Google Scholar]
- 43.Ritz, D., M. Bott, and H. Hennecke. 1993. Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol. Microbiol. 9:729-740. [DOI] [PubMed] [Google Scholar]
- 44.Ritz, D., L. Thony-Meyer, and H. Hennecke. 1995. The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum. Mol. Gen. Genet. 247:27-38. [DOI] [PubMed] [Google Scholar]
- 45.Rott, M. A., and T. J. Donohue. 1990. Rhodobacter sphaeroides spd mutations allow cytochrome c2-independent photosynthetic growth. J. Bacteriol. 172:1954-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambogni, Y., and S. J. Ferguson. 1996. Mutants of Escherichia coli lacking disulphide oxidoreductases DsbA and DsbB cannot synthesize monoheam c-type cytochromes except in the presence of disulphide compounds. FEBS Lett. 398:265-268. [DOI] [PubMed] [Google Scholar]
- 47.Sambogni, Y., and S. J. Ferguson. 1994. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase-like protein. FEBS Lett. 353:235-258. [DOI] [PubMed] [Google Scholar]
- 48.Schilke, B. A., and T. J. Donohue. 1992. δ-Aminolevulinate couples cycA transcription to changes in heme availability in Rhodobacter sphaeroides. J. Mol. Biol. 226:101-115. [DOI] [PubMed] [Google Scholar]
- 49.Setterdahl, A. T., B. S. Goldman, M. Hirasawa, P. Jacquot, A. J. Smith, R. G. Kranz, and D. B. Knaff. 2000. Oxidation-reduction properties of disulfide containing properties of the Rhodobacter capsulatus cytochrome c biogenesis system. Biochemistry 39:10172-10176. [DOI] [PubMed] [Google Scholar]
- 50.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:748-791. [Google Scholar]
- 51.Sistrom, W. R. 1960. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. Gen. Microbiol. 22:778-785. [DOI] [PubMed] [Google Scholar]
- 52.Suwanto, A., and S. Kaplan. 1989. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J. Bacteriol. 171:5850-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tai, S. P., and S. Kaplan. 1985. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J. Bacteriol. 164:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tai, T. N., W. A. Havelka, and S. Kaplan. 1988. A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid 19:175-188. [DOI] [PubMed] [Google Scholar]
- 55.Thony-Meyer, L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61:337-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varga, A. R., and S. Kaplan. 1989. Construction, expression, and localization of a CycA::PhoA fusion protein in Rhodobacter sphaeroides and Escherichia coli. J. Bacteriol. 171:5830-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie, Z., and S. Merchant. 1998. A novel pathway for cytochromes c biogenesis in chloroplasts. Biochim. Biophys. Acta 1365:309-318. [DOI] [PubMed] [Google Scholar]
- 58.Yang, C. H., H. R. Azad, and D. A. Cooksey. 1996. A chromosomal locus required for copper resistance, competitive fitness, and cytochrome c biogenesis in Pseudomonas fluorescens. Proc. Natl. Acad. Sci. USA 93:7315-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]