Abstract
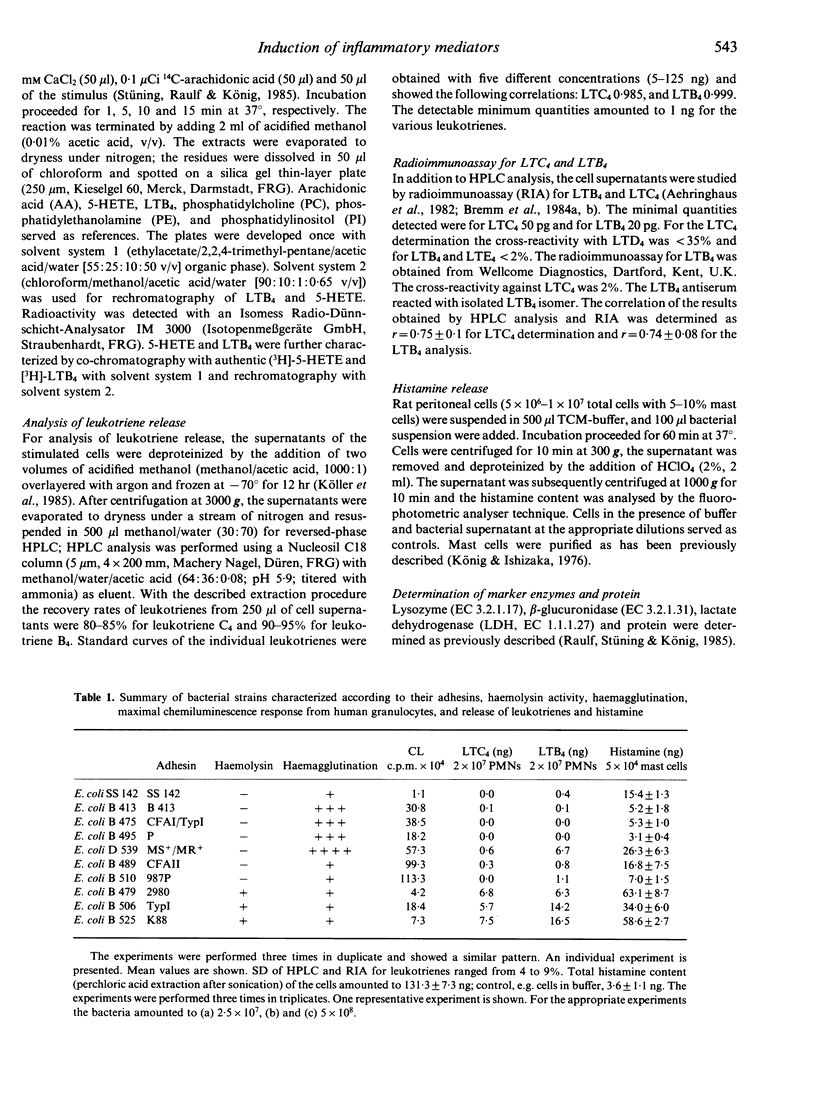
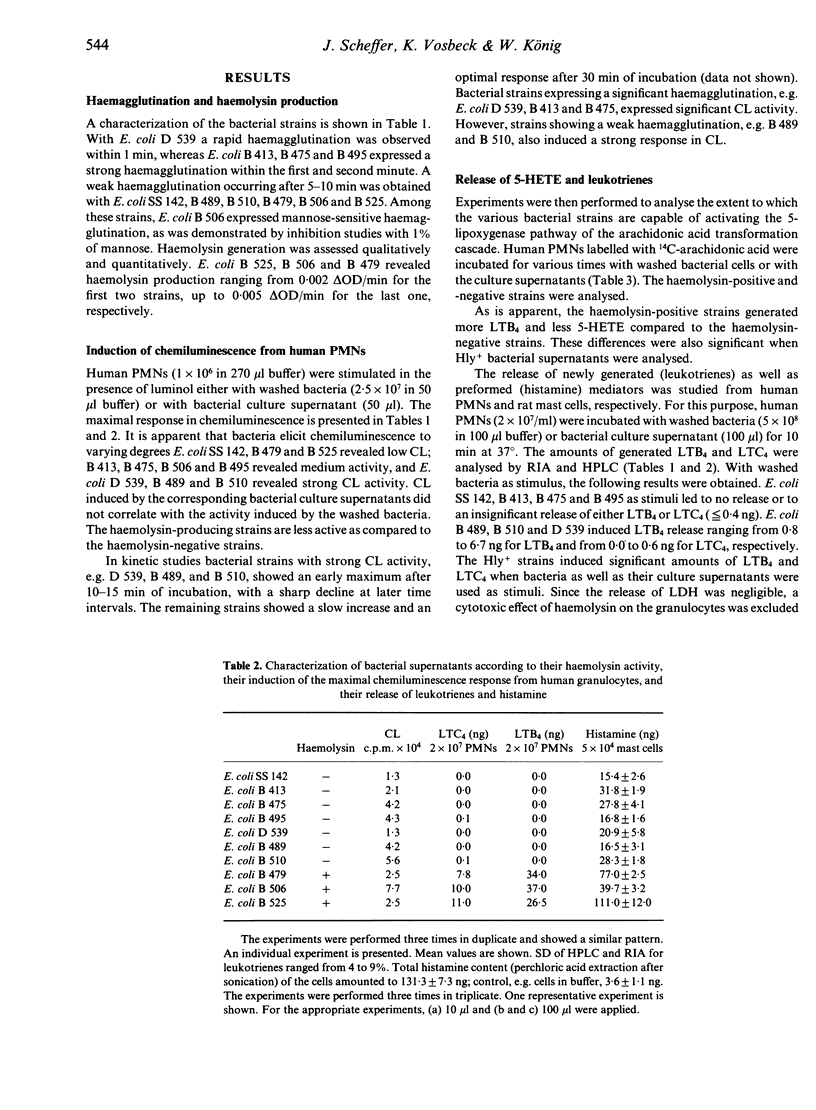
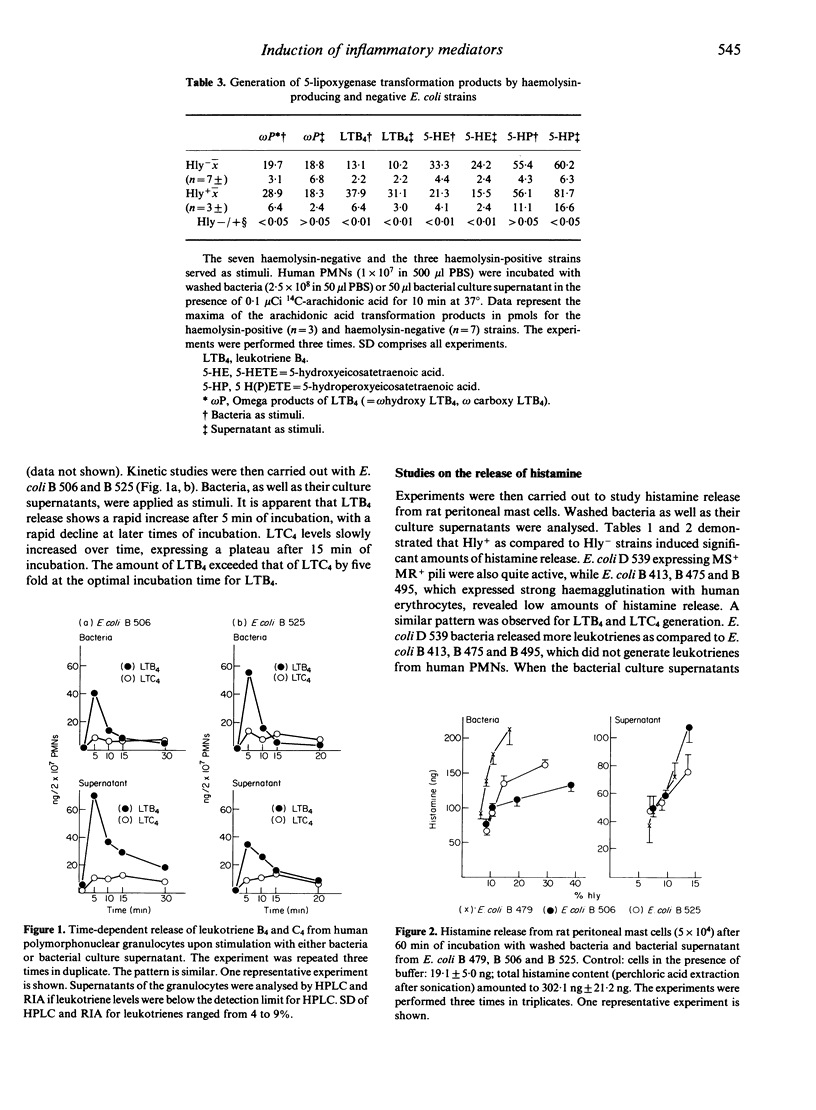
We investigated the role of various E. coli strains that expressed different adhesins and/or generated haemolysin with regard to the induction of inflammatory mediators, e.g. histamine release from rat mast cells as well as the chemiluminescence response and the release of lipoxygenase transformation products from human polymorphonuclear neutrophils. Our data show that the degree of haemagglutination did not parallel the induction of the chemiluminescence response. Haemolysin-negative bacteria with different adhesins induced more 5-hydroxyeicosatetraenoic acid as compared to haemolysin-positive bacteria, which generated more leukotriene B4 as compared to 5-hydroxyeicosatetraenoic acid. Among the leukotrienes, more leukotriene B4 as compared to leukotriene C4 was released from peripheral leucocytes. Studies with rat mast cells showed that histamine release was dependent on the haemolysin activity expressed by washed bacteria or present within the bacterial culture supernatant. Histamine release was markedly diminished when haemolysin activity decayed. Several haemolysin-negative bacteria with defined adhesins also released histamine, suggesting that, in addition to haemolysin, other factors contribute to mediator release. Thus, various properties of bacteria (e.g. adhesins, haemolysin) may participate to varying degrees in the induction of inflammatory mediators, e.g. oxygen radicals, lipoxygenase transformation products, leucotrienes and histamine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aehringhaus U., Wölbling R. H., König W., Patrono C., Peskar B. M., Peskar B. A. Release of leukotriene C4 from human polymorphonuclear leucocytes as determined by radioimmunoassay. FEBS Lett. 1982 Sep 6;146(1):111–114. doi: 10.1016/0014-5793(82)80715-1. [DOI] [PubMed] [Google Scholar]
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Snyder I. S. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J Bacteriol. 1985 Dec;164(3):1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Fruteau de Laclos B., Rabinovitch H., Picard S., Braquet P., Hébert J., Laviolette M. Eosinophil-rich human polymorphonuclear leukocyte preparations characteristically release leukotriene C4 on ionophore A23187 challenge. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):310–315. doi: 10.1016/0091-6749(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Cunningham F. M., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981 Mar;72(3):483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom H. J., Alouf J. E., König W., Spur B., Crea A., Peters W. Generation of leukotrienes from human granulocytes by alveolysin from Bacillus alvei. Infect Immun. 1984 Apr;44(1):188–193. doi: 10.1128/iai.44.1.188-193.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom J., König W., Spur B., Crea A., Bhakdi S., Lutz F., Fehrenbach F. J. Generation of leukotrienes and lipoxygenase factors from human polymorphonuclear granulocytes during bacterial phagocytosis and interaction with bacterial exotoxins. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Jul;254(4):500–514. [PubMed] [Google Scholar]
- Bremm K. D., König W., Pfeiffer P., Rauschen I., Theobald K., Thelestam M., Alouf J. E. Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun. 1985 Dec;50(3):844–851. doi: 10.1128/iai.50.3.844-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., König W., Spur B., Crea A., Galanos C. Generation of slow-reacting substance (leukotrienes) by endotoxin and lipid A from human polymorphonuclear granulocytes. Immunology. 1984 Oct;53(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köller M., Schönfeld W., Knöller J., Bremm K. D., König W., Spur B., Crea A., Peters W. The metabolism of leukotrienes in blood plasma studied by high-performance liquid chromatography. Biochim Biophys Acta. 1985 Jan 9;833(1):128–134. doi: 10.1016/0005-2760(85)90260-7. [DOI] [PubMed] [Google Scholar]
- König W., Ishizaka K. Binding of rat IgE with the subcellular components of normal rat mast cells. Immunochemistry. 1976 Apr;13(4):345–353. doi: 10.1016/0019-2791(76)90346-3. [DOI] [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985 May;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Robinson P., Wakefield D., Breit S. N., Easter J. F., Penny R. Chemiluminescent response to pathogenic organisms: normal human polymorphonuclear leukocytes. Infect Immun. 1984 Feb;43(2):744–752. doi: 10.1128/iai.43.2.744-752.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüning M., Raulf M., König W. Localization of 5-lipoxygenase within human polymorphonuclear leukocytes. Biochem Pharmacol. 1985 Nov 15;34(22):3943–3950. doi: 10.1016/0006-2952(85)90370-3. [DOI] [PubMed] [Google Scholar]
- Vosbeck K., Handschin H., Menge E. B., Zak O. Effects of subminimal inhibitory concentrations of antibiotics on adhesiveness of Escherichia coli in vitro. Rev Infect Dis. 1979 Sep-Oct;1(5):845–851. doi: 10.1093/clinids/1.5.845. [DOI] [PubMed] [Google Scholar]
- Vosbeck K., Mett H., Huber U., Bohn J., Petignat M. Effects of low concentrations of antibiotics on Escherichia coli adhesion. Antimicrob Agents Chemother. 1982 Jun;21(6):864–869. doi: 10.1128/aac.21.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers P., Picken R., Schmidt G., Jann B., Jann K., Golecki J. R., Kist M. Characterization of pili associated with Escherichia coli O18ac. Infect Immun. 1980 Aug;29(2):685–691. doi: 10.1128/iai.29.2.685-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


