Abstract
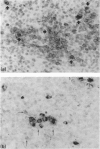
The adoptive transfer of immunity by means of spleen and lymph node cells has been analysed in rabbits of defined major histocompatibility (RLA) types and immunoglobulin (Ig) allotypes. Previous results had shown that chimaeras formed by transplanting adult cells into RLA-matched newborn hosts become stable B-lymphocyte chimaeras, as determined by their continuous production of donor type Ig. However, specific antibodies made by adult chimaeras were demonstrably only of recipient origin, unless the donor had been primed. The present report describes early events after the transfer of cells from donors primed with trinitrophenylated keyhole limpet haemocyanin (TNP-KLH). Double-staining with enzyme-labelled antigen or antibody conjugates was applied to identify lymphocytes of donor or recipient origin in spleen sections of transplanted rabbits and also to identify cells producing anti-TNP. Lymphocytes with membrane-bound Ig (mIg+ cells) of donor allotype were readily identified scattered singly or in small clusters throughout the recipients' B-lymphocyte areas within 5 days after transfer. Groups of donor-derived cells producing anti-TNP were demonstrable only in animals that had been specifically stimulated. Serological analysis and lymphocyte marker and function studies correlated well with results obtained by the histocytochemical approach. These results show that a systematic study of the role and importance of mature B lymphocytes in transplantation is now feasible.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler F. L., Adler L. T. Immunocompetence of chimeric rabbits. II. Persistence of memory and antigen-driven responses of donor cells. Transplantation. 1984 Oct;38(4):387–391. [PubMed] [Google Scholar]
- Adler F. L., Adler L. T. Immunocompetence of chimeric rabbits. III. Serial passage and persistence of B-lymphocyte memory. Cell Immunol. 1985 Apr 1;91(2):385–396. doi: 10.1016/0008-8749(85)90236-9. [DOI] [PubMed] [Google Scholar]
- Adler F. L., Adler L. T. Passive hemagglutination and hemolysis for estimation of antigens and antibodies. Methods Enzymol. 1980;70(A):455–466. doi: 10.1016/s0076-6879(80)70069-1. [DOI] [PubMed] [Google Scholar]
- Adler F. L., Noelle R. J. Enduring antibody responses in "normal" rabbits to maternal immunoglobulin allotypes. J Immunol. 1975 Sep;115(3):620–625. [PubMed] [Google Scholar]
- Adler L. T., Adler F. L., Cohen C., Tissot R. G. Induction of lymphoid cell chimerism in noninbred, histocompatible rabbits. A new model for studying allotype suppression in the rabbit. J Exp Med. 1981 Oct 1;154(4):1085–1099. doi: 10.1084/jem.154.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L. T., Adler F. L. Immunocompetence of chimeric rabbits. I. Participation of donor-derived B lymphocytes in immunoglobulin, but not in antibody, synthesis. Transplantation. 1984 Oct;38(4):382–386. doi: 10.1097/00007890-198410000-00013. [DOI] [PubMed] [Google Scholar]
- Adler L. T., Rehg J. E., Cohen C., LeBeau M. M. Graft-versus-host reactions and abrogation of allotype suppression following histoincompatible lymphoid cell transfers in rabbits. Transplantation. 1984 Jun;37(6):606–611. doi: 10.1097/00007890-198406000-00016. [DOI] [PubMed] [Google Scholar]
- BLAESE R. M., MARTINEZ C., GOOD R. A. IMMUNOLOGIC INCOMPETENCE OF IMMUNOLOGICALLY RUNTED ANIMALS. J Exp Med. 1964 Feb 1;119:211–224. doi: 10.1084/jem.119.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J. J. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981 Oct;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Boorsma D. M., Kalsbeek G. L. A comparative study of horseradish peroxidase conjugates prepared with a one-step and a two-step method. J Histochem Cytochem. 1975 Mar;23(3):200–207. doi: 10.1177/23.3.47869. [DOI] [PubMed] [Google Scholar]
- Brown A. R., DeWitt C. L., Bosma M. J., Nisonoff A. Dominance of an immune response by secondary cells: quantitation by allotype analysis. J Immunol. 1980 Jan;124(1):250–254. [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. II. Linear transmission of cellular memory. J Exp Med. 1967 Feb 1;125(2):199–211. doi: 10.1084/jem.125.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Adler L. T., Adler F. L. Double immunocytochemical staining for the in situ study of allotype distribution during an anti-trinitrophenyl immune response in chimeric rabbits. J Histochem Cytochem. 1986 Aug;34(8):989–994. doi: 10.1177/34.8.2426338. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-trinitrophenyl antibody-forming cells in the murine spleen. Eur J Immunol. 1986 Mar;16(3):271–276. doi: 10.1002/eji.1830160311. [DOI] [PubMed] [Google Scholar]
- Claassen E., Van Rooijen N. A trinitrophenyl(TNP)-poly-L-lysine-horseradish peroxidase conjugate for the detection of anti-TNP antibodies in vivo. J Histochem Cytochem. 1985 Aug;33(8):840–844. doi: 10.1177/33.8.3894501. [DOI] [PubMed] [Google Scholar]
- Claassen E., Van Rooijen N. TNP-enzyme conjugates for the detection of anti-TNP antibody producing cells in vivo. J Immunol Methods. 1984 Dec 14;75(1):181–188. doi: 10.1016/0022-1759(84)90237-0. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R. P. Graft-versus-host disease. Immunol Rev. 1985 Dec;88:193–214. doi: 10.1111/j.1600-065x.1985.tb01159.x. [DOI] [PubMed] [Google Scholar]
- KARRER H., MEYER K. F., EDDIE B. The complement fixation inhibition test and its application to the diagnosis of ornithosis in chickens and in ducks; confirmation of the specificity and epidemiological application of the test. J Infect Dis. 1950 Jul-Aug;87(1):24–36. doi: 10.1093/infdis/87.1.24. [DOI] [PubMed] [Google Scholar]
- Lum L. G., Munn N. A., Schanfield M. S., Storb R. The detection of specific antibody formation to recall antigens after human bone marrow transplantation. Blood. 1986 Mar;67(3):582–587. [PubMed] [Google Scholar]
- Möller G. Regulation of cellular antibody synthesis. Cellular 7S production and longevity of 7S antigen-sensitive cells in the absence of antibody feedback. J Exp Med. 1968 Feb 1;127(2):291–306. doi: 10.1084/jem.127.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Ohama Y., Adler L. T. Suppression of rabbit lymphocyte functions by antibodies specific for allotypic membrane determinants. Cell Immunol. 1984 Jul;86(2):429–438. doi: 10.1016/0008-8749(84)90398-8. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N. Double immunocytochemical staining in the study of antibody-producing cells in vivo. Combined detection of antigen specificity (anti-TNP) and (sub)class of intracellular antibodies. J Histochem Cytochem. 1985 Mar;33(3):175–178. doi: 10.1177/33.3.2579119. [DOI] [PubMed] [Google Scholar]
- Wimperis J. Z., Brenner M. K., Prentice H. G., Reittie J. E., Karayiannis P., Griffiths P. D., Hoffbrand A. V. Transfer of a functioning humoral immune system in transplantation of T-lymphocyte-depleted bone marrow. Lancet. 1986 Feb 15;1(8477):339–343. doi: 10.1016/s0140-6736(86)92315-9. [DOI] [PubMed] [Google Scholar]