Abstract
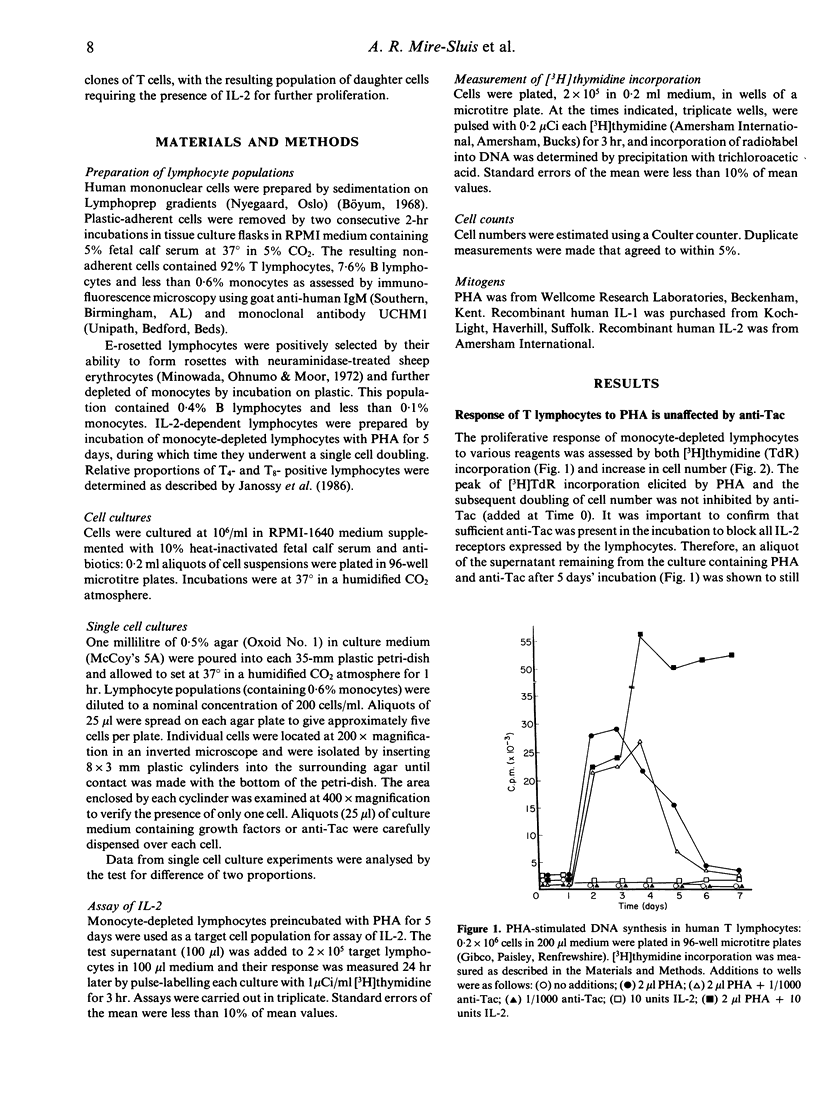
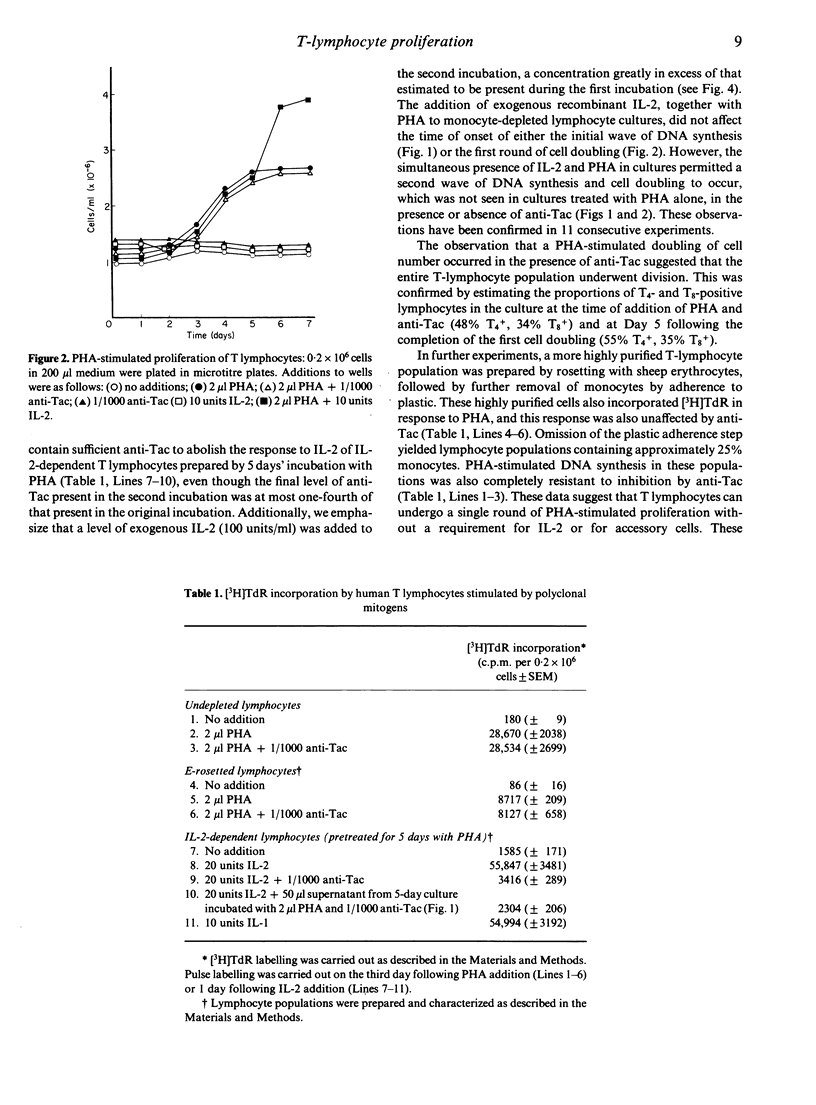
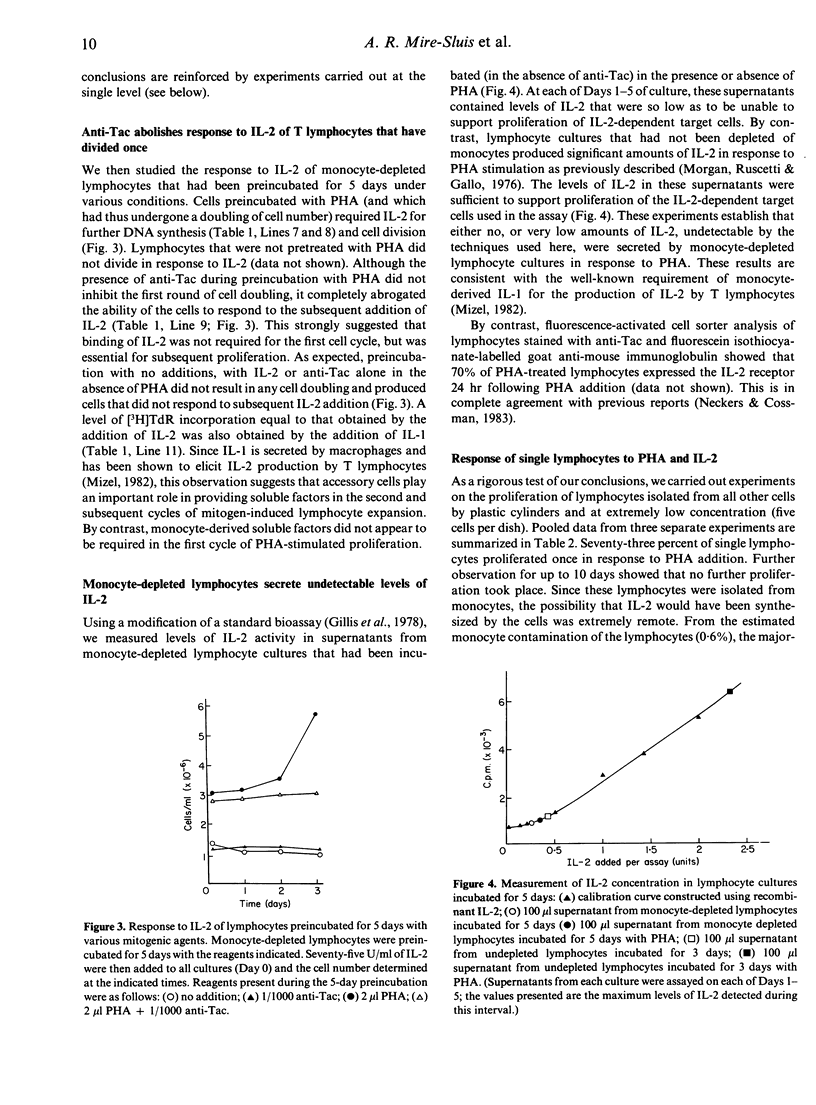
Monocyte-depleted human T lymphocytes completed a single round of DNA synthesis and cell division when treated with phytohaemagglutinin (PHA). Direct assay of culture supernatants showed that very low amounts of interleukin-2 (IL-2) were synthesized in these cultures. Furthermore, the first round of cell division was totally unaffected by the addition of saturating amounts of anti-Tac, a monoclonal antibody against the IL-2 receptor. These data strongly suggest that stimulation of IL-2 receptors by IL-2 was not required for the completion of the first round of mitosis. By contrast, proliferation of the daughter cells produced by the first cell doubling required the addition of exogenous IL-2 and was totally abolished by anti-Tac. These results were confirmed by experiments using single lymphocytes, which also showed that accessory cells were not required during the first cell doubling of PHA-stimulated lymphocytes. We therefore propose a modified model for in vitro T-lymphocyte activation, wherein PHA stimulate a single round of cell proliferation without a requirement for stimulation by IL-2. The resulting daughter cells now require IL-2 for further proliferation. By analogy, we suggest that triggering by antigen in vivo (in the context of products of the major histocompatibility locus) may also stimulate a single round of IL-2-independent division in appropriate clones of T lymphocytes, with the resulting population of daughter cells requiring IL-2 for further expansion of the population.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Depper J. M., Leonard W. J., Robb R. J., Waldmann T. A., Greene W. C. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol. 1983 Aug;131(2):690–696. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol. 1983 Jan;3(1):1–13. doi: 10.1007/BF00919133. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Bertoglio J., Quillet A., Marchiol C., Wakasugi H., Mishall Z., Fradelizi D. Interleukin 2 (IL 2) up-regulates its own receptor on a subset of human unprimed peripheral blood lymphocytes and triggers their proliferation. J Immunol. 1986 Apr 1;136(7):2463–2469. [PubMed] [Google Scholar]
- Janossy G., Prentice H. G., Grob J. P., Ivory K., Tidman N., Grundy J., Favrot M., Brenner M. K., Campana D., Blacklock H. A. T lymphocyte regeneration after transplantation of T cell depleted allogeneic bone marrow. Clin Exp Immunol. 1986 Mar;63(3):577–586. [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulos J. M., De Petris S., Leca G., Crumpton M. J. The mitogenic lectin from Phaseolus vulgaris does not recognize the T3 antigen of human T lymphocytes. Eur J Immunol. 1985 May;15(5):479–486. doi: 10.1002/eji.1830150512. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Jakway J. P., Chan C., Shevach E. M. The murine IL 2 receptor. II. Monoclonal anti-IL 2 receptor antibodies as specific inhibitors of T cell function in vitro. J Immunol. 1984 Oct;133(4):1976–1982. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mire A. R., Wickremasinghe R. G., Michalevicz R., Hoffbrand A. V. Interleukin-2 induces rapid phosphorylation of an 85 kilodalton protein in permeabilized lymphocytes. Biochim Biophys Acta. 1985 Oct 30;847(1):159–163. doi: 10.1016/0167-4889(85)90169-7. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- Palacios R. Mechanism of T cell activation: role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Direct demonstration of the identity of T cell growth factor binding protein and the Tac antigen. J Exp Med. 1983 Oct 1;158(4):1332–1337. doi: 10.1084/jem.158.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Valentine M. A., Tsoukas C. D., Rhodes G., Vaughan J. H., Carson D. A. Phytohemagglutinin binds to the 20-kDa molecule of the T3 complex. Eur J Immunol. 1985 Aug;15(8):851–854. doi: 10.1002/eji.1830150821. [DOI] [PubMed] [Google Scholar]



