Abstract
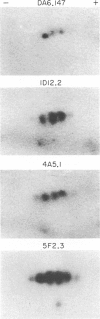
When mice were immunized with a mixture of human MHC class II alpha and beta glycoprotein chains, the predominant antibody response was anti-alpha, and from a subsequent fusion experiment over 60 hybridomas showing anti-alpha activity were generated, compared with 11 anti-beta secretors. These findings contrast with the relative paucity of anti-alpha monoclonals described previously. Use of a miniaturized Western blot screening protocol was a critical factor in the present study since the anti-alpha monoclonals do not bind to the surface of living B cells and would therefore be missed in conventional screening assays. After glutaraldehyde fixation of target B lymphocytes or B-cell lines, the majority of anti-alpha monoclonals do react in a radio-immunobinding assay, although none binds as strongly as pan-reactive anti-beta chain antibodies. This suggests that the immunogenic epitopes of alpha chains are normally concealed by the three-dimensional folding of the alpha beta dimer. The anti-alpha monoclonals were all monomorphic but varied in the extent of their reactivity with alpha chains separated on one-dimensional and two-dimensional IEF gels. The most reactive antibodies identified up to seven distinct components among mature class II antigens from solubilized cell membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. E., Bodmer J. G., Bodmer W. F. Production and characterization of monoclonal antibodies recognizing the alpha-chain subunits of human ia alloantigens. Immunology. 1983 Dec;50(4):613–624. [PMC free article] [PubMed] [Google Scholar]
- Cohen B. B., Deane D. L., Moxley M., Steel C. M. Methods of detecting mature human Ia-like antigen and their metabolic precursors. J Immunol Methods. 1983 Jun 24;61(1):91–97. doi: 10.1016/0022-1759(83)90012-1. [DOI] [PubMed] [Google Scholar]
- Cohen B. B., Deane D. L., Van Heyningen V., Guy K., Hutchins D., Moxley M., Steel C. M. Biochemical variation of human Ia like antigens detected with monoclonal antibodies. Clin Exp Immunol. 1983 Jul;53(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Cohen B. B., Moxley M., Crichton D., Deane D. L., Steel C. M. A mild procedure for separating polypeptide chains prior to immunoprecipitation and western blotting analysis. J Immunol Methods. 1984 Dec 14;75(1):99–105. doi: 10.1016/0022-1759(84)90229-1. [DOI] [PubMed] [Google Scholar]
- Crichton D. N., Cohen B. B. Analysis of the murine sperm surface with monoclonal antibodies. J Reprod Fertil. 1983 Jul;68(2):497–505. doi: 10.1530/jrf.0.0680497. [DOI] [PubMed] [Google Scholar]
- Crichton D., Grattage L., McDonald A., Corrie J. E., Steel C. M., Hubbard A. L., Al-Dujaili E. A., Edwards C. R. The production and assessment of monoclonal antibodies to cortisol. Steroids. 1985 Jun;45(6):503–517. doi: 10.1016/0039-128x(85)90016-9. [DOI] [PubMed] [Google Scholar]
- Deane D. L., Cohen B. B., Moxley M., Steel C. M. Detection of Ia epitopes by monoclonal antibody blotting. Immunol Lett. 1983 Feb;6(2):93–96. doi: 10.1016/0165-2478(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Fermand J. P., Chevalier A., Brouet J. C. Characterization of a monoclonal antibody recognizing a monomorphic determinant of the alpha chain of class II DQ antigens. Scand J Immunol. 1986 Sep;24(3):313–319. doi: 10.1111/j.1365-3083.1986.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Fermand J. P., Schmitt C., Brouet J. C. Distribution of class IIDQ antigens on normal and leukemic lymphoid cells. Eur J Immunol. 1985 Dec;15(12):1183–1187. doi: 10.1002/eji.1830151208. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Capra J. D. Structure, function, and genetics of human class II molecules. Adv Immunol. 1985;37:1–71. doi: 10.1016/s0065-2776(08)60337-5. [DOI] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hutchins D., Steel C. M. Phytohaemagglutinin-induced proliferation of human T lymphocytes: differences between neonate and adults in accessory cell requirements. Clin Exp Immunol. 1983 May;52(2):355–364. [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Strominger J. L. A monoclonal antibody that recognizes the alpha chain of HLA-DR antigens. Hum Immunol. 1986 Feb;15(2):150–163. doi: 10.1016/0198-8859(86)90023-6. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Boss J. M., Spies T., Sorrentino R., Okada K., Strominger J. L. Genetic complexity and expression of human class II histocompatibility antigens. Immunol Rev. 1985 Jul;85:45–86. doi: 10.1111/j.1600-065x.1985.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Knudsen P. J., Kaufman J. F., Strominger J. L. cDNA clones for the heavy chain of HLA-DR antigens obtained after immunopurification of polysomes by monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1844–1848. doi: 10.1073/pnas.79.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Cresswell P. Human B cell alloantigens; alpha subunit variability. J Immunol. 1982 May;128(5):1999–2003. [PubMed] [Google Scholar]
- Neefjes J. J., Doxiadis I., Stam N. J., Beckers C. J., Ploegh H. L. An analysis of class I antigens of man and other species by one-dimensional IEF and immunoblotting. Immunogenetics. 1986;23(3):164–171. doi: 10.1007/BF00373817. [DOI] [PubMed] [Google Scholar]
- Palacios R., Claesson L., Möller G., Peterson P. A., Möller E. The alpha chain, not the beta chain of HLA-DR antigens participates in activation of T cells in autologous mixed lymphocyte reaction. Immunogenetics. 1982;15(4):341–356. doi: 10.1007/BF00364258. [DOI] [PubMed] [Google Scholar]
- Sanderson A. R. HLA "help" for human B2-microglobulin across species barriers. Nature. 1977 Sep 29;269(5627):414–417. doi: 10.1038/269414a0. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Lee J., Bodmer W. F., Bodmer J. G., Trowsdale J. Six HLA-D region alpha-chain genes on human chromosome 6: polymorphisms and associations of DC alpha-related sequences with DR types. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3461–3465. doi: 10.1073/pnas.81.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel C. M., Van Heyningen V., Guy K., Cohen B. B., Deane D. L. Influence of monoclonal anti-Ia like antibodies on activation of human lymphocytes. Immunology. 1982 Dec;47(4):597–603. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco M. M., Stocker J. W., Caeppellini R. Monoclonal antibodies against human lymphocyte antigens. Nature. 1978 Jun 22;273(5664):666–668. doi: 10.1038/273666a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]