Abstract
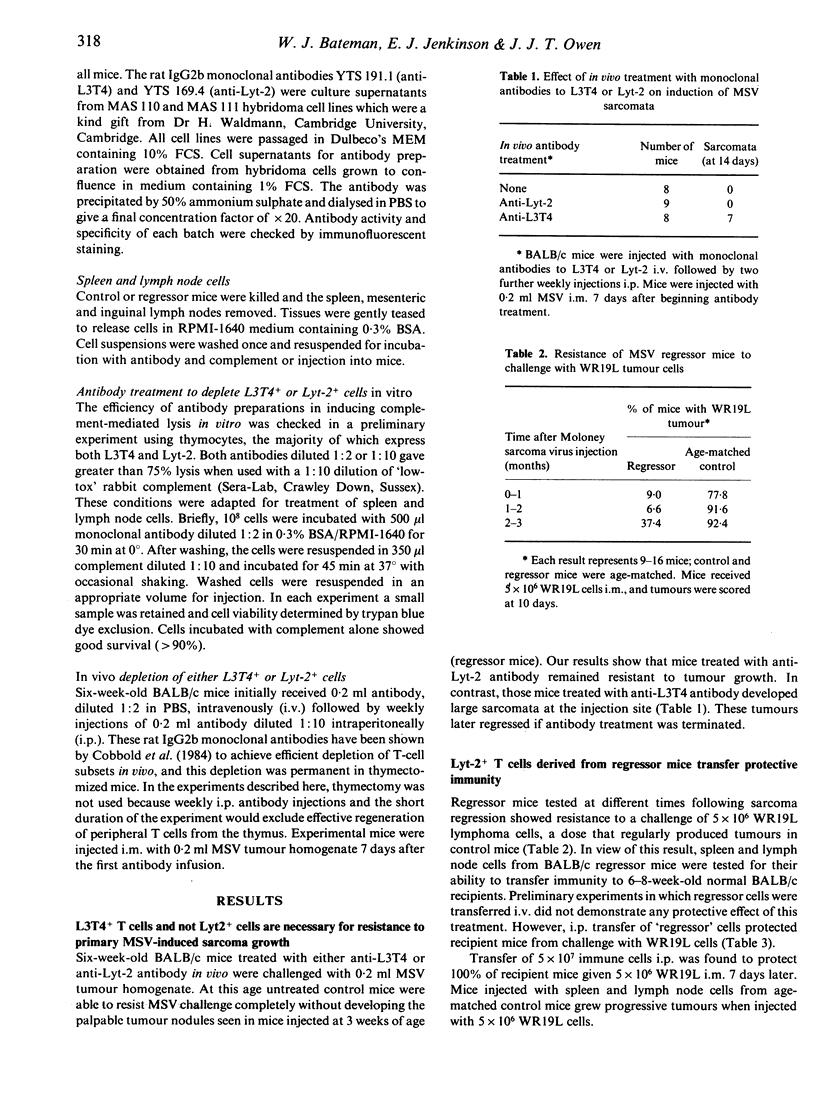
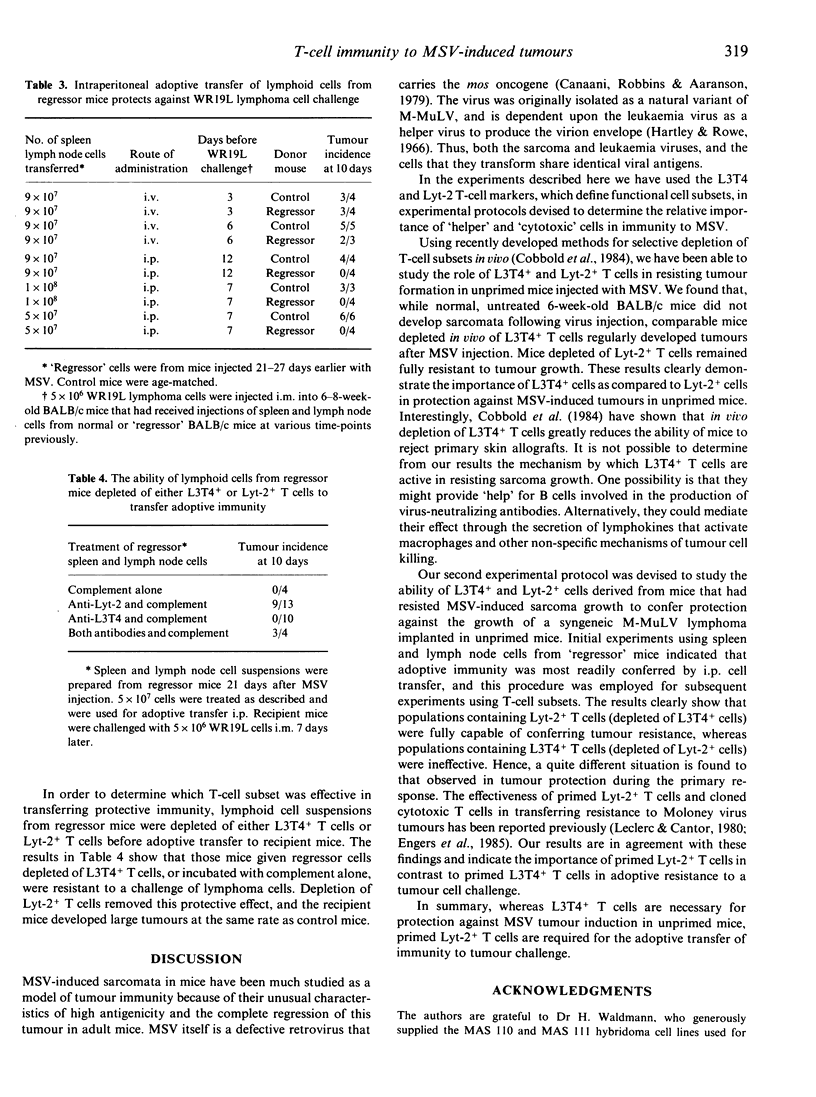
Experimental protocols have been devised to deliniate the importance of T-cell subsets in immunity to Moloney sarcoma virus-induced tumours using the surface antigens L3T4 and Lyt-2 as markers of helper and cytotoxic cells, respectively. Because the monoclonal antibodies used have been shown to deplete T-cell subsets in vivo, we have been able to study the role of L3T4+ and Lyt-2+ T cells in the primary response to MSV for the first time. The results clearly show that L3T4+ T cells are the most important in resistance to the viral challenge. Mice injected with monoclonal antibodies to L3T4 grew large tumours following injection of a viral innoculum that was resisted by untreated mice or mice injected with monoclonal antibodies to Lyt-2. The same monoclonal antibodies were used to remove primed L3T4+ or Lyt-2+ T cells in vitro in adoptive transfer experiments. Normal unirradiated mice were protected from a challenge of WR19L lymphoma cells when they were given primed spleen and lymph node cells intraperitoneally. Depletion of Lyt-2+ T cells before adoptive transfer abolished this protective effect. Depletion of L3T4+ cells had no effect on the ability of primed cells to transfer immunity. Thus, while L3T4+ T cells are required for the primary rejection of MSV, only primed Lyt-2+ T cells are able to transfer resistance to a secondary challenge of lymphoma cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner K. T., MacDonald H. R., Cerottini J. C. Quantitation and clonal isolation of cytolytic T lymphocyte precursors selectively infiltrating murine sarcoma virus-induced tumors. J Exp Med. 1981 Aug 1;154(2):362–373. doi: 10.1084/jem.154.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Robbins K. C., Aaronson S. A. The transforming gene of Moloney murine sarcoma virus. Nature. 1979 Nov 22;282(5737):378–383. doi: 10.1038/282378a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Duprez V., Burakoff S. J. Cytolytic T lymphocyte response to Moloney sarcoma virus. Surv Immunol Res. 1983;2(3):309–311. doi: 10.1007/BF02918440. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Lahaye T., Sorenson G. D., Glasebrook A. L., Horvath C., Brunner K. T. Functional activity in vivo of effector T cell populations. II. Anti-tumor activity exhibited by syngeneic anti-MoMULV-specific cytolytic T cell clones. J Immunol. 1984 Sep;133(3):1664–1670. [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. C., Cantor H. T cell-mediated immunity to oncornavirus-induced tumors. II. Ability of different T cell sets to prevent tumor growth in vivo. J Immunol. 1980 Feb;124(2):851–854. [PubMed] [Google Scholar]
- Levy J. P., Leclerc J. C. The murine sarcoma virus-induced tumor: exception or general model in tumor immunology? Adv Cancer Res. 1977;24:1–66. doi: 10.1016/s0065-230x(08)61012-x. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]


