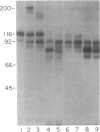
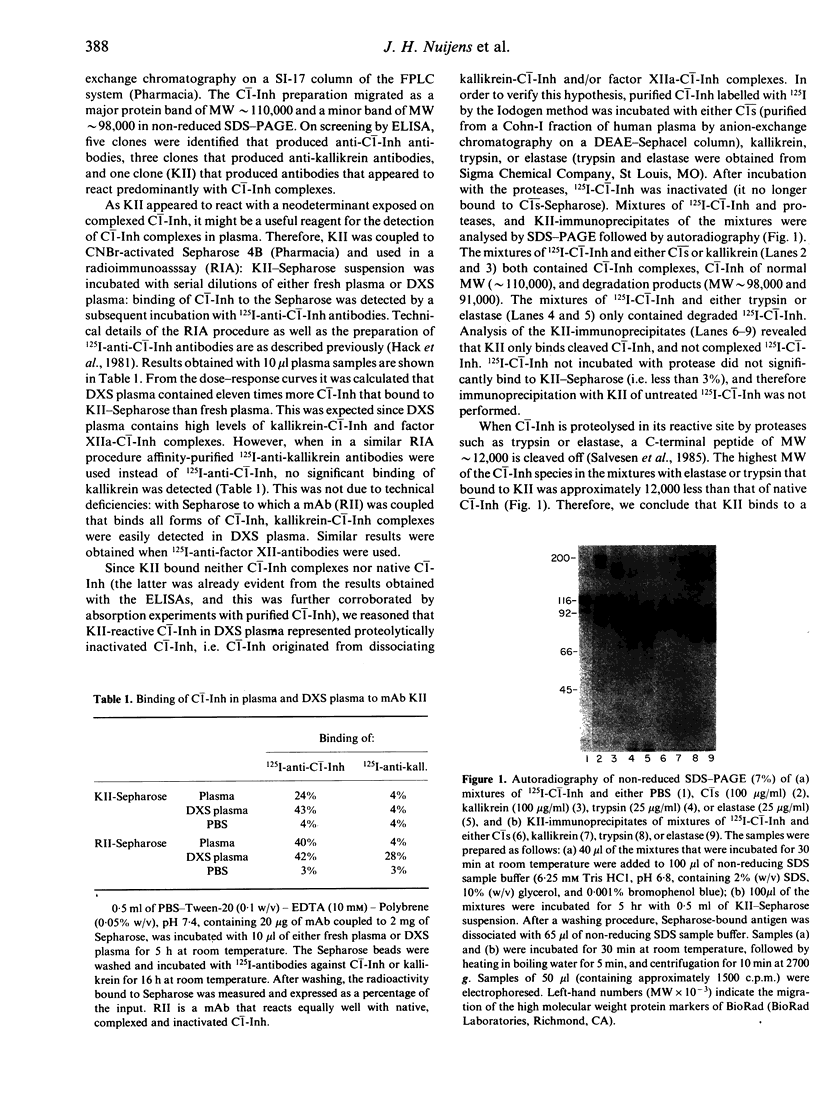
Abstract
Monoclonal antibodies were raised against kallikrein-C-1 inhibitor and factor XIIa-C-1 inhibitor complexes. One of the monoclonal antibodies (KII) appeared to react predominantly with C-1 inhibitor complexes in an ELISA. However, the apparent binding of KII to C-1 inhibitor complexes was probably due to the presence of proteolytically inactivated C-1 inhibitor in the complex mixture used for the coating:KII did not bind either kallikrein-C-1 inhibitor or factor XIIa-C-1 inhibitor complexes generated in plasma by dextran sulphate. SDS-PAGE analysis of C-1 inhibitor incubated with proteases revealed that KII-reactive C-1 inhibitor has a lower molecular weight than native C-1 inhibitor. We propose that the determinant that reacts with KII is exposed after cleavage of C-1 inhibitor in its reactive site. The monoclonal antibody KII will enable us to study the inactivation of C-1 inhibitor in human inflammatory disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hack C. E., Hannema A. J., Eerenberg-Belmer A. J., Out T. A., Aalberse R. C. A C1-inhibitor-complex assay (INCA): a method to detect C1 activation in vitro and in vivo. J Immunol. 1981 Oct;127(4):1450–1453. [PubMed] [Google Scholar]
- Nagase H., Barrett A. J. Human plasma kallikrein. A rapid purification method with high yield. Biochem J. 1981 Jan 1;193(1):187–192. doi: 10.1042/bj1930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Catanese J. J., Kress L. F., Travis J. Primary structure of the reactive site of human C1-inhibitor. J Biol Chem. 1985 Feb 25;260(4):2432–2436. [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- de Agostini A., Lijnen H. R., Pixley R. A., Colman R. W., Schapira M. Inactivation of factor XII active fragment in normal plasma. Predominant role of C-1-inhibitor. J Clin Invest. 1984 Jun;73(6):1542–1549. doi: 10.1172/JCI111360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Agostini A., Schapira M., Wachtfogel Y. T., Colman R. W., Carrel S. Human plasma kallikrein and C1 inhibitor form a complex possessing an epitope that is not detectable on the parent molecules: demonstration using a monoclonal antibody. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5190–5193. doi: 10.1073/pnas.82.15.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf F., Koedam J. A., Bouma B. N. Inactivation of kallikrein in human plasma. J Clin Invest. 1983 Jan;71(1):149–158. doi: 10.1172/JCI110743. [DOI] [PMC free article] [PubMed] [Google Scholar]