Abstract
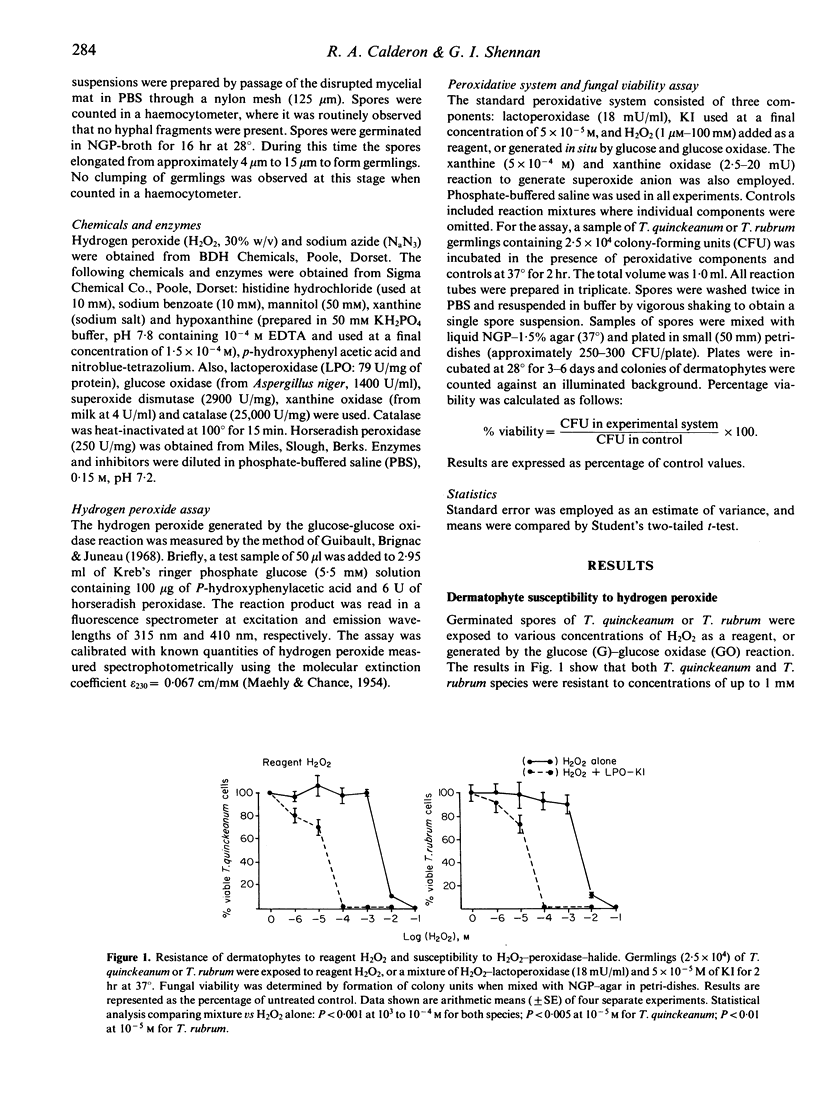
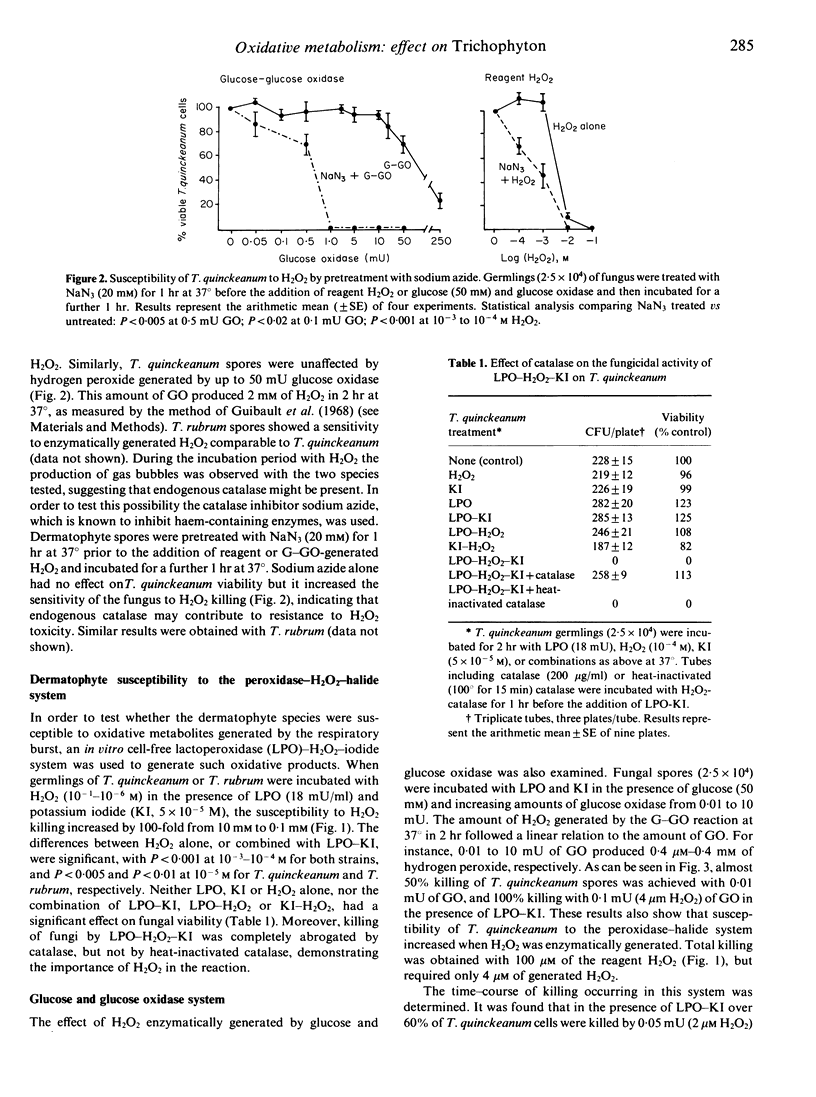
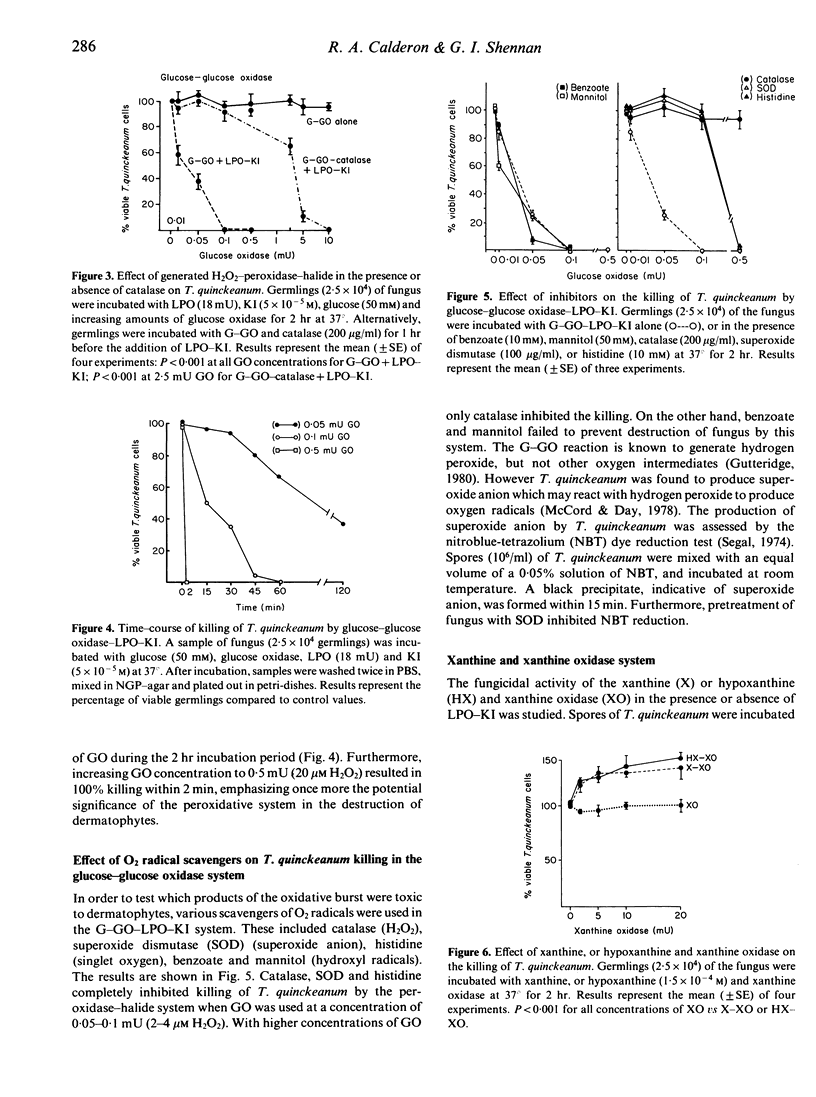
Two dermatophyte strains, Trichophyton quinckeanum and Trichophyton rubrum, were highly susceptible to in vitro killing by components of the H2O2-peroxidase-halide system. Both strains were, however, resistant to relatively high concentrations of reagent H2O2 or H2O2 enzymatically generated by glucose and glucose oxidase, KI, or lactoperoxidase (LPO) alone. Resistance to hydrogen peroxidase killing was found to be in part due to the presence of endogenous catalase in the fungi; susceptibility was increased by pretreatment of the fungi with a catalase inhibitor. Kinetic studies using small quantities of reagent or enzymatically generated H2O2 and LPO-KI showed that the system was lethal for both fungal strains within 1 min. Furthermore, using the glucose-glucose oxidase-LPO-KI system, it was shown that catalase, superoxide dismutase and histidine scavengers of H2O2, superoxide anion and singlet oxygen, respectively, prevented the killing of fungus, whereas scavengers of hydroxyl radicals such as benzoate and mannitol had no effect. T. quinckeanum was found to contain large quantities of superoxide anion, as judged by the nitroblue-tetrazolium test. Consequently, the xanthine (or hypoxanthine) and xanthine oxidase system in which the main product is superoxide anion had no toxic effect on the fungus. The high sensitivity of dermatophytes to killing by the H2O2-peroxidase-halide system active in polymorphonuclear neutrophils and macrophages may account in part for fungal toxicity in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982 Nov;38(2):487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of Plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984 Feb;43(2):451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault G. G., Brignac P. J., Jr, Juneau M. New substrates for the fluorometric determination of oxidative enzymes. Anal Chem. 1968 Jul;40(8):1256–1263. doi: 10.1021/ac60264a027. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron-oxygen reactions and their use in clinical chemistry. Med Lab Sci. 1980 Jun;37(3):267–273. [PubMed] [Google Scholar]
- Hay R. J., Calderon R. A., Collins M. J. Experimental dermatophytosis: the clinical and histopathologic features of a mouse model using Trichophyton quinckeanum (mouse favus). J Invest Dermatol. 1983 Sep;81(3):270–274. doi: 10.1111/1523-1747.ep12518292. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Comparative sensitivity of Histoplasma capsulatum conidiospores and blastospores to oxidative antifungal systems. Infect Immun. 1981 Apr;32(1):381–387. doi: 10.1128/iai.32.1.381-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Segal A. W. Nitroblue-tetrazolium tests. Lancet. 1974 Nov 23;2(7891):1248–1252. doi: 10.1016/s0140-6736(74)90758-2. [DOI] [PubMed] [Google Scholar]
- Sugar A. M., Chahal R. S., Brummer E., Stevens D. A. Susceptibility of Blastomyces dermatitidis strains to products of oxidative metabolism. Infect Immun. 1983 Sep;41(3):908–912. doi: 10.1128/iai.41.3.908-912.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


