Abstract
A soluble genus-specific chlamydial antigen has been isolated from the supernatants of cultures infected with Chlamydia trachomatis and from other sources. The antigen is a glycolipid that is secreted during the infective cycle. This exoglycolipid can be hydrolysed and fractionated into polysaccharide and lipid components. Both fractions retain antigenic activity. An immunodominant antigenic determinant of the lipid component contains fatty acids of C17 and C18:1. The polysaccharide immunodominant epitope gives rise to gulose when derivatives are formed. The secretion of the antigen into the media supernatant, the presence of gulose and the observed molecular weight are consistent with properties of alginate secreted by Gram-negative bacteria. Chemical analyses and SDS-PAGE indicate that the exoglycolipid is markedly different from LPS.
Full text
PDF

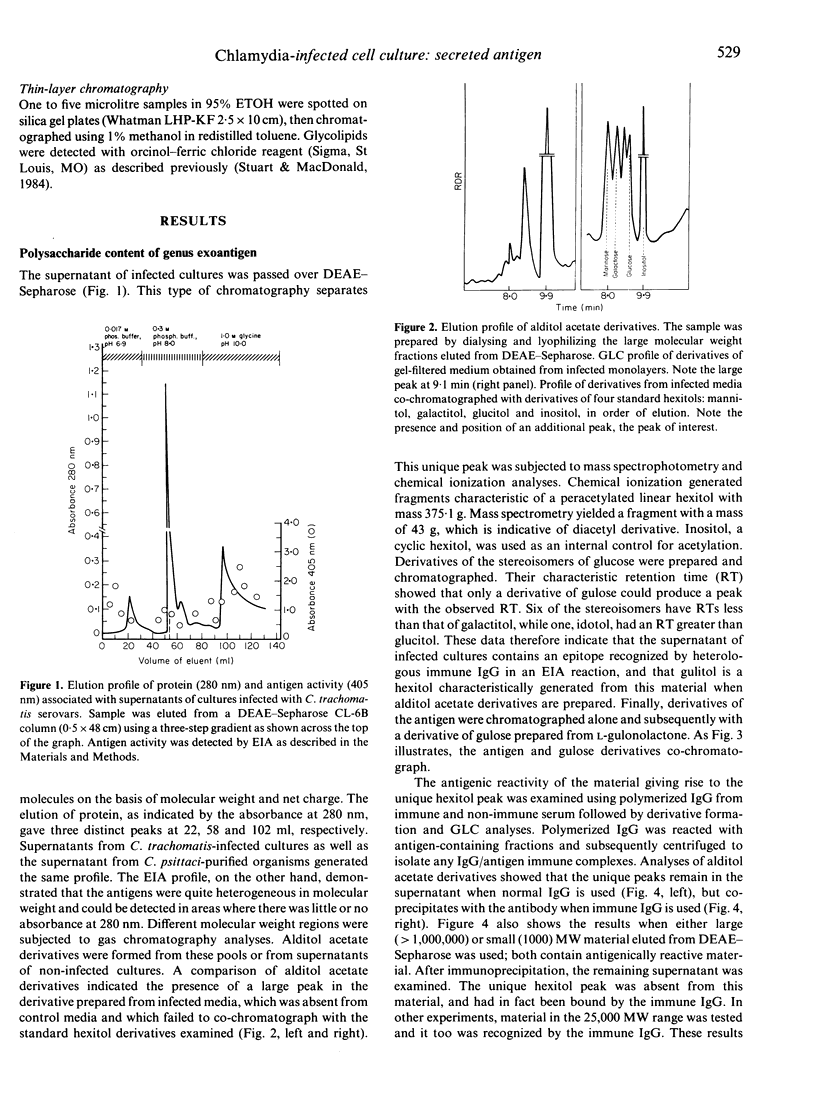
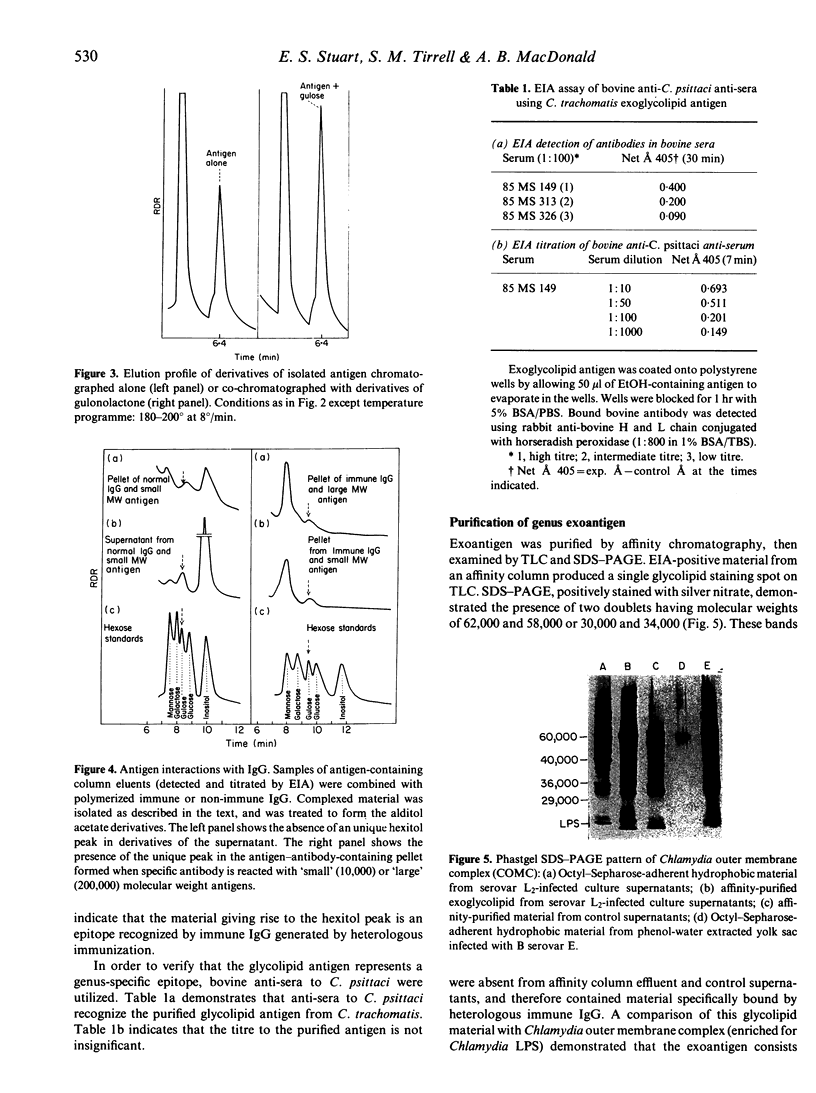
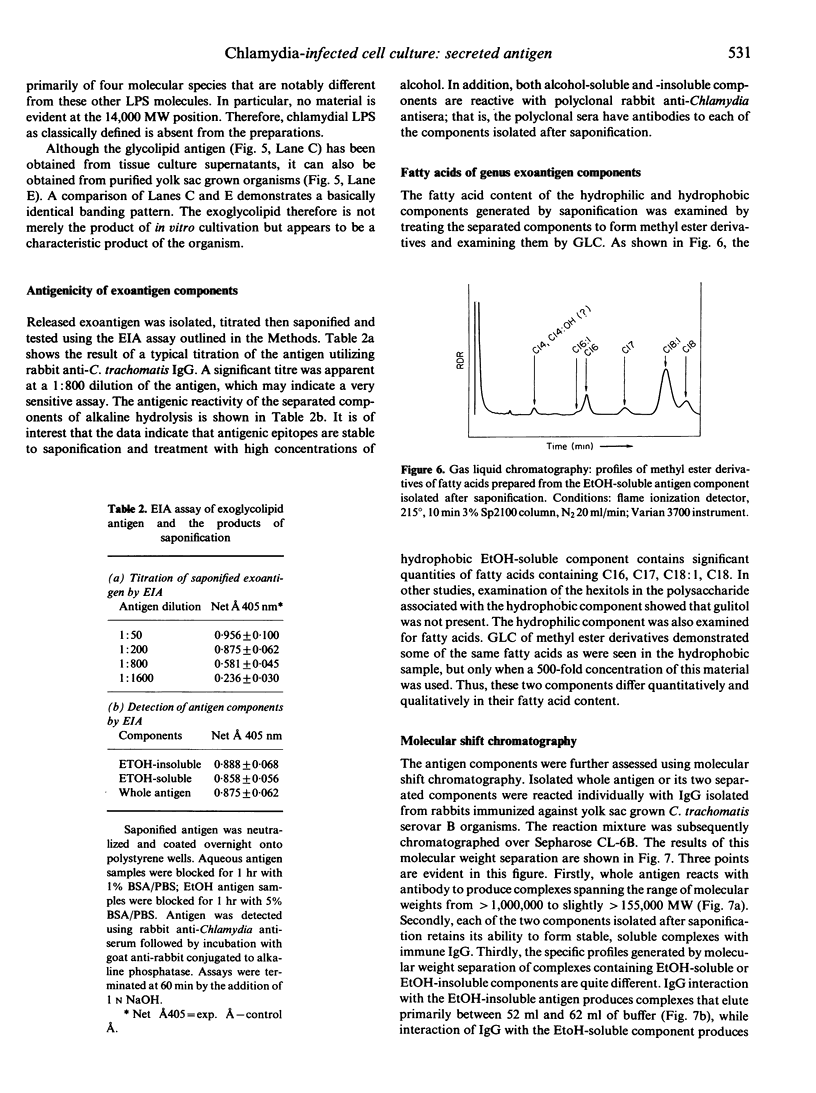

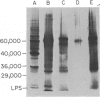
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs L. J., Post A., White E. R., Finkelstein J. A., Moeckel W. E., Holden K. G., Zarembo J. E., Weisbach J. A. Identification and quantitation of alditol acetates of neutral and amino sugars from mucins by automated gas-liquid chromatography. Anal Biochem. 1971 Oct;43(2):369–381. doi: 10.1016/0003-2697(71)90266-1. [DOI] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A., Larsen B. Biosynthesis of alginate. II. Polymannuronic acid C-5-epimerase from Azotobacter vinelandii (Lipman). Carbohydr Res. 1971 Apr;17(2):297–308. doi: 10.1016/s0008-6215(00)82537-9. [DOI] [PubMed] [Google Scholar]
- Hourihan J. T., Rota T. R., MacDonald A. B. Isolation and purification of a type-specific antigen from Chlamydia trachomatis propagated in cell culture utilizing molecular shift chromatography. J Immunol. 1980 May;124(5):2399–2404. [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald A. B. Antigens of Chlamydia trachomatis. Rev Infect Dis. 1985 Nov-Dec;7(6):731–736. doi: 10.1093/clinids/7.6.731. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Richmond S. J., Stirling P. Localization of chlamydial group Antigen in McCoy cell monolayers infected with Chlamydia trachomatis or Chlamydia psittaci. Infect Immun. 1981 Nov;34(2):561–570. doi: 10.1128/iai.34.2.561-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Todd W. J., Macdonald A. B. Cell-mediated immune responses in owl monkeys (Aotus trivirgatus) with trachoma to soluble antigens of Chlamydia trachomatis. Clin Exp Immunol. 1978 Jul;33(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Seid R. C., Jr, Sadoff J. C. Preparation and characterization of detoxified lipopolysaccharide-protein conjugates. J Biol Chem. 1981 Jul 25;256(14):7305–7310. [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]