Abstract
Antigen-specific T-helper cells for IgA responses arise in Peyer's patches (PP) following their immunization by subserosal injection of keyhole limpet haemocyanin (KLH). These are of the W3/25 phenotype and the W3/25 receptor is shown here to be involved in their helper function. These cells originate in PP and migrate via mesenteric lymph nodes (MLN) to thoracic duct lymph, although the MLN appear to be unnecessary for the induction or maturation of antigen-specific helper cells collected in thoracic duct lymph after intra-Peyer's patch (i.p.p.) immunization. KLH-specific helper cells can be detected subsequently in the intraepithelial lymphocyte population and also among lamina propria lymphocytes. The helper cells also relocate to PP distant to their site of origin where they are retained only when antigen is present. While i.p.p. immunization is an efficient route for the induction of IgA helper cells in gut-associated lymphoid tissue, it differs from oral immunization in that concomitant induction of antigen-specific splenic suppressor cells does not occur, indicating a role for epithelial antigen processing in this phenomenon.
Full text
PDF

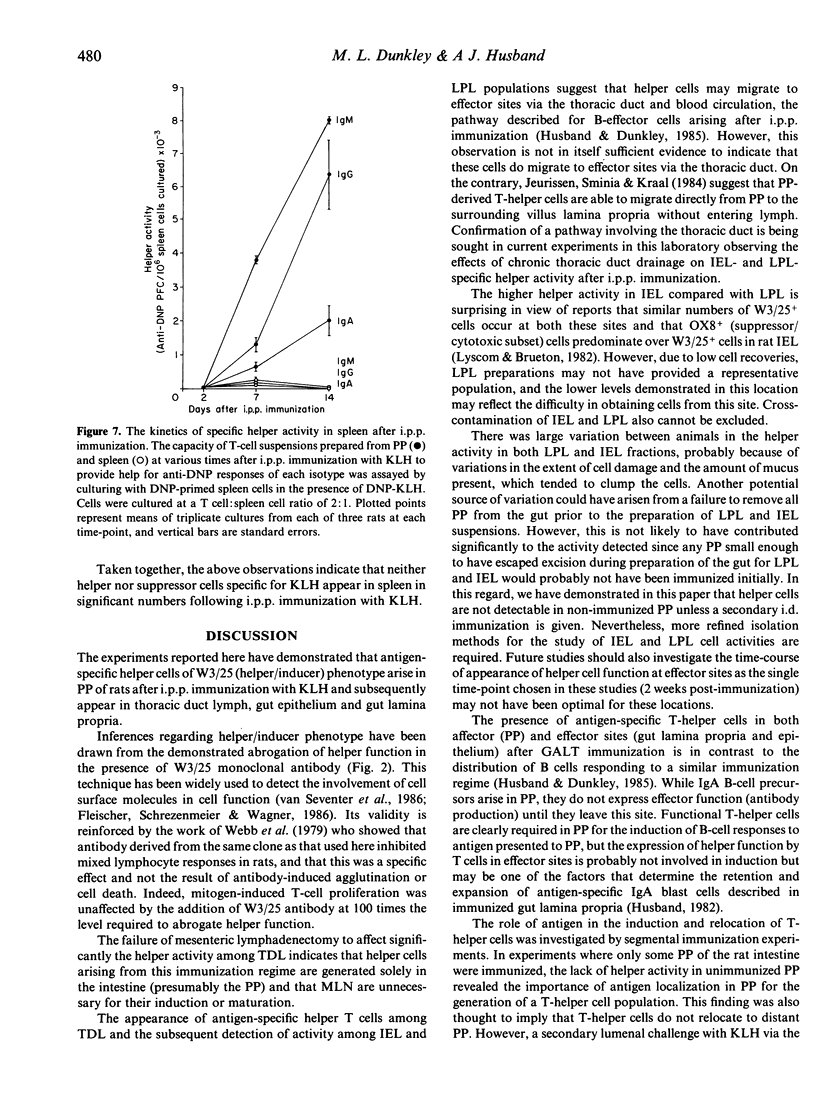
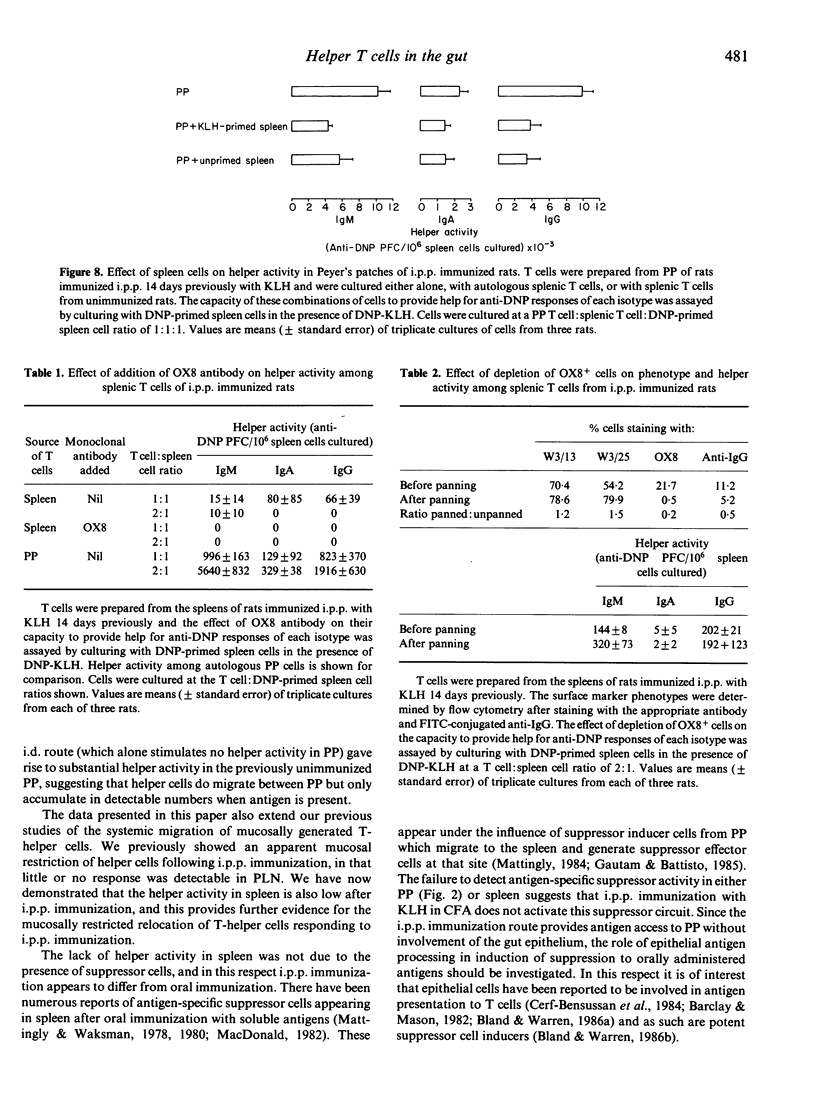

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Clough J. D., Mims L. H., Strober W. Deficient IgA antibody responses to arsanilic acid bovine serum albumin (BSA) in neonatally thymectomized rabbits. J Immunol. 1971 Jun;106(6):1624–1629. [PubMed] [Google Scholar]
- Crewther P., Warner N. L. Serum immunoglobulins and antibodies in congenitally athymic (nude) mice. Aust J Exp Biol Med Sci. 1972 Oct;50(5):625–635. doi: 10.1038/icb.1972.55. [DOI] [PubMed] [Google Scholar]
- Dailey M. O., Fathman C. G., Butcher E. C., Pillemer E., Weissman I. Abnormal migration of T lymphocyte clones. J Immunol. 1982 May;128(5):2134–2136. [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. The induction and migration of antigen-specific helper cells for IgA responses in the intestine. Immunology. 1986 Mar;57(3):379–385. [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Sakai H., Nomoto Y., Tomino Y., Kaneshige H. IgA-specific helper activity of T alpha cells in human peripheral blood. J Immunol. 1981 Dec;127(6):2612–2613. [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H., Wagner H. Function of the CD4 and CD8 molecules on human cytotoxic T lymphocytes: regulation of T cell triggering. J Immunol. 1986 Mar 1;136(5):1625–1628. [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gautam S. C., Battisto J. R. Orally induced tolerance generates an efferently acting suppressor T cell and an acceptor T cell that together down-regulate contact sensitivity. J Immunol. 1985 Nov;135(5):2975–2983. [PubMed] [Google Scholar]
- Husband A. J., Dunkley M. L. Lack of site of origin effects on distribution of IgA antibody-containing cells. Immunology. 1985 Feb;54(2):215–221. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J. Kinetics of extravasation and redistribution of IgA-specific antibody-containing cells in the intestine. J Immunol. 1982 Mar;128(3):1355–1359. [PubMed] [Google Scholar]
- Jeurissen S. H., Sminia T., Kraal G. Selective emigration of suppressor T cells from Peyer's patches. Cell Immunol. 1984 Apr 15;85(1):264–269. doi: 10.1016/0008-8749(84)90297-1. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Immunosuppression caused by antigen feeding. I. Evidence for the activation of a feedback suppressor pathway in the spleens of antigen-fed mice. Eur J Immunol. 1982 Sep;12(9):767–773. doi: 10.1002/eji.1830120912. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A. Immunologic suppression after oral administration of antigen. III. Activation of suppressor-inducer cells in the Peyer's patches. Cell Immunol. 1984 Jun;86(1):46–52. doi: 10.1016/0008-8749(84)90357-5. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. II. Antigen-specific helper and suppressor factors produced by spleen cells of rats fed sheep erythrocytes. J Immunol. 1980 Sep;125(3):1044–1047. [PubMed] [Google Scholar]
- McCaughan G. W., Adams E., Basten A. Human antigen-specific IgA responses in blood and secondary lymphoid tissue: an analysis of help and suppression. J Immunol. 1984 Mar;132(3):1190–1196. [PubMed] [Google Scholar]
- Pritchard H., Riddaway J., Micklem H. S. Immune responses in congenitally thymus-less mice. II. Quantitative studies of serum immunoglobulins, the antibody response to sheep erythrocytes, and the effect of thymus allografting. Clin Exp Immunol. 1973 Jan;13(1):125–138. [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Van Seventer G. A., Van Lier R. A., Spits H., Ivanyi P., Melief C. J. Evidence for a regulatory role of the T8 (CD8) antigen in antigen-specific and anti-T3-(CD3)-induced lytic activity of allospecific cytotoxic T lymphocyte clones. Eur J Immunol. 1986 Nov;16(11):1363–1371. doi: 10.1002/eji.1830161109. [DOI] [PubMed] [Google Scholar]
- Webb M., Mason D. W., Williams A. F. Inhibition of mixed lymphocyte response by monoclonal antibody specific for a rat T lymphocyte subset. Nature. 1979 Dec 20;282(5741):841–843. doi: 10.1038/282841a0. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]



