Abstract
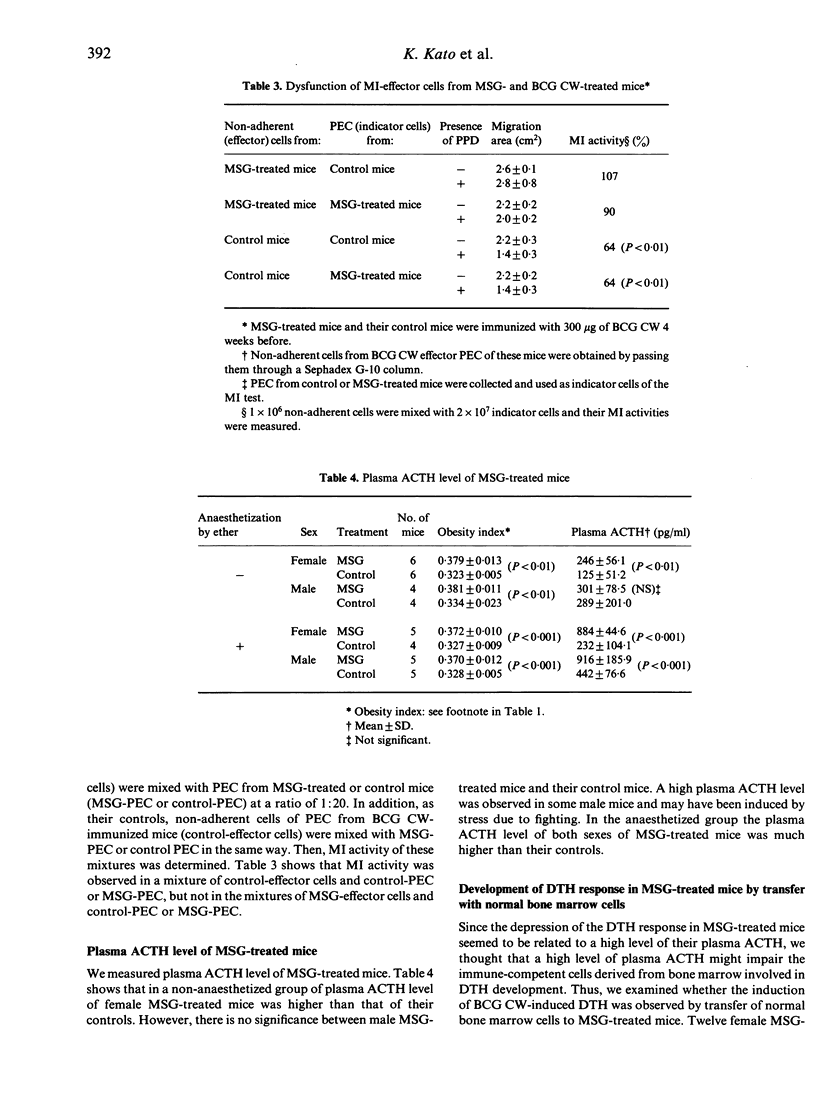
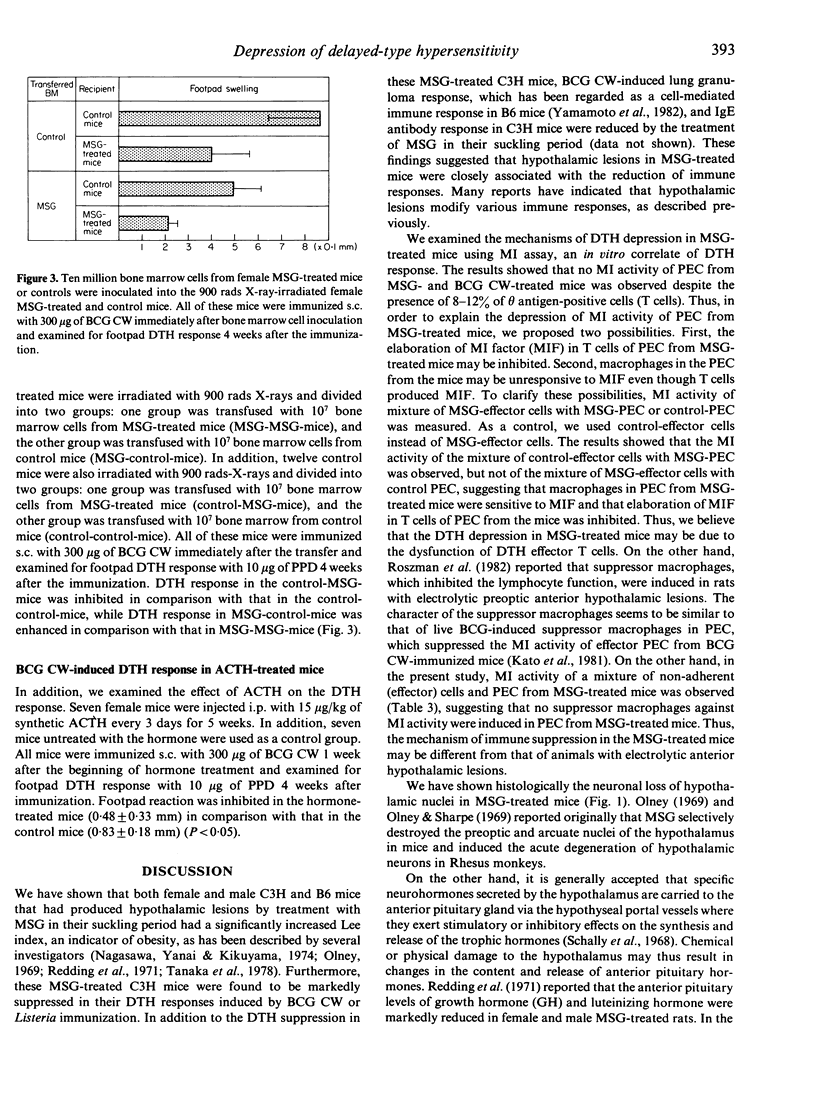
Delayed-type hypersensitivity (DTH) was depressed in mice that had been treated with monosodium glutamate (MSG) in their suckling period. Analysis of the DTH depression by use of the macrophage migration inhibition assay showed dysfunction of DTH effector T cells. The neuronal loss of nuclei in the hypothalamus, which elaborates the corticotropin-releasing factor and the hypersecretion of adrenocorticotrophic hormone, was observed in the MSG-treated mice. Therefore, DTH response may be modulated by the neuroendocrine system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBROSE C. T. THE REQUIREMENT FOR HYDROCORTISONE IN ANTIBODY-FORMING TISSUE CULTIVATED IN SERUM-FREE MEDIUM. J Exp Med. 1964 Jan 1;119:1027–1049. doi: 10.1084/jem.119.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy D., Janssens P. A., Leonard R. A. Sequential changes in rat thymus after the subcutaneous injection of cortisol. J Endocrinol. 1966 May;35(1):19–27. doi: 10.1677/joe.0.0350019. [DOI] [PubMed] [Google Scholar]
- Bernardis L. L., Patterson B. D. Correlation between 'Lee index' and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol. 1968 Apr;40(4):527–528. doi: 10.1677/joe.0.0400527. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E., Keller M., Müller J. Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med. 1975 Nov;150(2):466–470. doi: 10.3181/00379727-150-39057. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E. Network of immune-neuroendocrine interactions. Clin Exp Immunol. 1977 Jan;27(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Da Prada M., Burri R., Honegger C. The immune response evokes changes in brain noradrenergic neurons. Science. 1983 Aug 5;221(4610):564–566. doi: 10.1126/science.6867729. [DOI] [PubMed] [Google Scholar]
- Brehmer W., Anacker R. L., Ribi E. Immunogenicity of cell walls from various mycobacteria against airborne tuberculosis in mice. J Bacteriol. 1968 Jun;95(6):2000–2004. doi: 10.1128/jb.95.6.2000-2004.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie K. E., Kjosen B., Solberg C. O. Influence of hydrocortisone on granulocyte function and glucose metabolism. Acta Pathol Microbiol Scand C. 1977 Aug;85C(4):284–288. doi: 10.1111/j.1699-0463.1977.tb03643.x. [DOI] [PubMed] [Google Scholar]
- Duquesnoy R. J. Immunodeficiency of the thymus-dependent system of the Ames dwarf mouse. J Immunol. 1972 Jun;108(6):1578–1590. [PubMed] [Google Scholar]
- Fabris N., Pierpaoli W., Sorkin E. Hormones and the immunological capacity. IV. Restorative effects of developmental hormones or of lymphocytes on the immunodeficiency syndrome of the dwarf mouse. Clin Exp Immunol. 1971 Aug;9(2):227–240. [PMC free article] [PubMed] [Google Scholar]
- Greer M. A., Rockie C. Inhibition by pentobarbital of ether-induced ACTH secretion in the rat. Endocrinology. 1968 Dec;83(6):1247–1252. doi: 10.1210/endo-83-6-1247. [DOI] [PubMed] [Google Scholar]
- ISHIDATE M., METCALF D. THE PATTERN OF LYMPHOPOIESIS IN THE MOUSE THYMUS AFTER CORTISONE ADMINISTRATION OR ADRENALECTOMY. Aust J Exp Biol Med Sci. 1963 Dec;41:637–649. doi: 10.1038/icb.1963.53. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamamoto K. I., Kakinuma M., Ishihara C., Azuma I. Suppression of BCG cell wall induced delayed-type hypersensitivity by BCG pre-treatment. I. Induction of adherent suppressor cells by live BCG injection and their characterization. Immunology. 1981 Feb;42(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Kato K., Yamamoto K. Suppression of BCG cell wall-induced delayed-type hypersensitivity by BCG pre-treatment. II. Induction of suppressor T cells by heat-killed BCG injection. Immunology. 1982 Apr;45(4):655–661. [PMC free article] [PubMed] [Google Scholar]
- Keller S. E., Stein M., Camerino M. S., Schleifer S. J., Sherman J. Suppression of lymphocyte stimulation by anterior hypothalamic lesions in the guinea pig. Cell Immunol. 1980 Jul 1;52(2):334–340. doi: 10.1016/0008-8749(80)90354-8. [DOI] [PubMed] [Google Scholar]
- LUPARELLO T. J., STEIN M., PARK C. D. EFFECT OF HYPOTHALAMIC LESIONS ON RAT ANAPHYLAXIS. Am J Physiol. 1964 Oct;207:911–914. doi: 10.1152/ajplegacy.1964.207.4.911. [DOI] [PubMed] [Google Scholar]
- Macris N. T., Schiavi R. C., Camerino M. S., Stein M. Effect of hypothalamic lesions on immune processes in the guinea pig. Am J Physiol. 1970 Nov;219(5):1205–1209. doi: 10.1152/ajplegacy.1970.219.5.1205. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Yanai R., Kikuyama S. Irreversible inhibition of pituitary prolactin and growth hormone secretion and of mammary gland development in mice by monosodium glutamate administered neonatally. Acta Endocrinol (Copenh) 1974 Feb;75(2):249–259. doi: 10.1530/acta.0.0750249. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969 May 9;164(3880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Sharpe L. G. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969 Oct 17;166(3903):386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V., Arimura A., Wakabayashi I. Effect of monosodium glutamate on some endocrine functions. Neuroendocrinology. 1971;8(3):245–255. doi: 10.1159/000122011. [DOI] [PubMed] [Google Scholar]
- Ritter M. A. Embryonic mouse thymocyte development. Enhancing effect of corticosterone at physiological levels. Immunology. 1977 Aug;33(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Roszman T. L., Cross R. J., Brooks W. H., Markesbery W. R. Hypothalamic-immune interactions. II. The effect of hypothalamic lesions on the ability of adherent spleen cells to limit lymphocyte blastogenesis. Immunology. 1982 Apr;45(4):737–742. [PMC free article] [PubMed] [Google Scholar]
- SZENTIVANYI A., FILIPP G. Anaphylaxis and the nervous system. II. Ann Allergy. 1958 Mar-Apr;16(2):143–151. [PubMed] [Google Scholar]
- Schally A. V., Arimura A., Bowers C. Y., Kastin A. J., Sawano S., Reeding T. W. Hypothalamic neurohormones regulating anterior pituitary function. Recent Prog Horm Res. 1968;24:497–588. doi: 10.1016/b978-1-4831-9827-9.50016-2. [DOI] [PubMed] [Google Scholar]
- Schiavi R. C., Macris N. T., Camerino M. S., Stein M. Effect of hypothalamic lesions on immediate hypersensitivity. Am J Physiol. 1975 Feb;228(2):596–601. doi: 10.1152/ajplegacy.1975.228.2.596. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Sherman N. A., Middleton E., Jr Effect of hydrocortisone on immunoglobulin synthesis and secretion by human peripheral lymphocytes in vitro. Int Arch Allergy Appl Immunol. 1972;43(6):859–870. doi: 10.1159/000230903. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Shimada M., Nakao K., Kusunoki T. Hypothalamic lesion induced by injection of monosodium glutamate in suckling period and subsequent development of obesity. Exp Neurol. 1978 Oct;62(1):191–199. doi: 10.1016/0014-4886(78)90050-x. [DOI] [PubMed] [Google Scholar]
- Tyrey L., Nalbandov A. V. Influence of anterior hypothalamic lesions on circulating antibody titers in the rat. Am J Physiol. 1972 Jan;222(1):179–185. doi: 10.1152/ajplegacy.1972.222.1.179. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Altman L. C., Rosenstreich D. L. Inhibition of in vitro lymphokine synthesis by glucocorticosteroids. J Immunol. 1975 Aug;115(2):476–481. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H. Cyclic AMP-mediated stimulation of thymocyte proliferation by low concentrations of cortisol. Proc Soc Exp Biol Med. 1970 Sep;134(4):1170–1174. doi: 10.3181/00379727-134-34967. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Karinuma M. Genetic control of granuloma response to oil-associated BCG cell wall vaccine in mice. Microbiol Immunol. 1978;22(6):335–348. doi: 10.1111/j.1348-0421.1978.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kato K., Kakinuma M., Brehmer W. Cellular regulation of lung granuloma formation and delayed- type hypersensitivity induced with BCG cell walls. Further evidence of involvement of cellular immunity in lung granuloma formation. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Mar;251(3):357–368. [PubMed] [Google Scholar]
- Youdim S., Stutman O., Good R. A. Studies of delayed hypersensitivity to L. Monocytogenes in mice: nature of cells involved in passive transfers. Cell Immunol. 1973 Jan;6(1):98–109. doi: 10.1016/0008-8749(73)90010-5. [DOI] [PubMed] [Google Scholar]




