Abstract
Naturally acquired antibodies to Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1), the variant surface antigens expressed on the surface of infected erythrocytes, are thought to play a role in protection against P. falciparum malaria. Here, we have studied the development of antibodies to PfEMP-1 in adult malaria patients living in Rourkela, India, an area with a low malaria transmission rate, and prevalence of antibodies to PfEMP-1 in residents of San Dulakudar, India, a village in which P. falciparum malaria is hyperendemic. Convalescent-phase sera from adult malaria patients from Rourkela agglutinate homologous P. falciparum isolates as well as some heterologous isolates, suggesting that they develop partially cross-reactive antibodies to PfEMP-1 following infection. Adult sera from San Dulakudar agglutinate diverse P. falciparum isolates, suggesting that they have antibodies with wide recognition of diverse PfEMP-1. Mixed-agglutination assays using pairs of P. falciparum isolates confirm the presence of both variant-specific and partially cross-reactive antibodies in convalescent-phase sera from Rourkela and adult sera from San Dulakudar. Analysis of PfEMP-1 sequences suggests a molecular basis for the observed cross-reactivity.
Following repeated exposure to Plasmodium falciparum infection, individuals residing in areas of malaria endemicity acquire immunity (14). As a result it is primarily nonimmune children in areas of endemicity who suffer from severe malaria and are at risk of death from malaria. Adults in areas of endemicity suffer fewer clinical malaria episodes than do children and are protected from severe malaria syndromes such as cerebral malaria and severe anemia.
Naturally acquired immunity to malaria is not completely understood. Antibodies directed against variant surface antigens referred to as P. falciparum erythrocyte membrane protein-1 (PfEMP-1) that are expressed on the surface of infected erythrocytes are thought to be an important component of naturally acquired immunity (3, 13). Some members of the PfEMP-1 family bind to endothelial receptors and mediate adhesion of P. falciparum trophozoites and schizonts to vascular endothelium, a phenomenon referred to as cytoadherence (6). Cytoadherence is implicated in a number of pathological outcomes. Adhesion of P. falciparum-infected erythrocytes in brain capillaries is implicated in cerebral malaria, and cytoadherence in the placenta often leads to complications in pregnancy (11, 17, 24-25).
Agglutination assays with P. falciparum trophozoites and schizonts have been used to study antibody responses to PfEMP-1 (3-4, 7-9, 12-13, 15). A large prospective study conducted on the eastern coast of Kenya demonstrated that, when children suffer clinical malaria episodes, they are likelier to be infected with P. falciparum isolates against which they lack agglutinating antibodies than with isolates against which they have agglutinating antibodies (3). Sera from children in areas of endemicity agglutinate a limited number of P. falciparum isolates, indicating limited recognition of PfEMP-1 variants (4, 7, 9, 12). In contrast, immune-adult sera from areas of endemicity agglutinate a wide range of P. falciparum isolates, suggesting that they recognize diverse PfEMP-1 variants (4, 7, 9, 12). Do immune adults possess a repertoire of variant-specific antibodies directed against diverse PfEMP-1, or do they have cross-reactive antibodies directed against conserved epitopes shared by diverse PfEMP-1? Initial studies suggested that immune adults might have cross-reactive antibodies that agglutinate diverse P. falciparum isolates (12). However, later studies suggested that antibodies directed against PfEMP-1 are predominantly variant specific (8, 15).
In this study, we have used agglutination assays to study naturally acquired antibodies to PfEMP-1 in two areas with distinct malaria transmission patterns in Sundergarh District in the state of Orissa in eastern India. We have studied the development of antibodies directed against PfEMP-1 in adult malaria patients residing in the town of Rourkela, which is an area with a low malaria transmission rate. We have also studied the prevalence of antibodies directed against PfEMP-1 in children and adults residing in a village, San Dulakudar, located in a forest area in Sundergarh District, in which P. falciparum malaria is hyperendemic. Mixed-agglutination assays using parasite isolate pairs stained with distinguishable DNA intercalating dyes were used to investigate the presence of cross-reactive antibodies directed against PfEMP-1. Data from our field studies and comparative statistical analysis of PfEMP-1 sequences suggest that both variant-specific as well as partially cross-reactive antibodies against PfEMP-1 may be elicited during natural infection with P. falciparum.
MATERIALS AND METHODS
Study area.
The study was conducted with sera collected from two areas with distinct malaria transmission patterns in Sundergarh District, Orissa, in eastern India. Sundergarh District lies between 21°35′N and 22°35′N latitudes and between 83°32′E and 85°22′E longitudes. The area presents ideal ecological conditions for malaria transmission with undulating uplands intersected by forested hills, rocky streams, paddy fields, and springs with a tropical, humid, savanna-type climate and annual rainfall between 160 and 200 cm. The monsoon rains begin in mid-June and last till September, the cold season occurs in December and January, and the hot, dry summer extends from April to mid-June. Focal epidemiological variations are observed in malaria transmission patterns over short geographical distances in Sundergarh District. For example, the township of Rourkela (population 150,000), which was constructed for the employees of a local steel plant, has excellent urban infrastructure with around 20,000 houses of different types, roads, electricity, safe drinking water, underground sewage system, effective malaria control programs, and an excellent health care system. As a result, malaria transmission in Rourkela is extremely low. In contrast, villages situated in forests with perennial flowing streams surrounded by paddy fields just 25 to 30 km outside Rourkela have high malaria endemicity and perennial transmission. We have collected data over the past 3 years on the epidemiology of malaria in one such village, San Dulakudar, which has a population of 265. Cross-sectional and longitudinal studies involving periodic mass surveys as well as active case detection by trained village-based community workers were used to collect data on malaria prevalence and malaria-related morbidity. A detailed report describing the epidemiology of malaria in San Dulakudar will be published separately. Briefly, malaria is hyperendemic in San Dulakudar, with P. falciparum malaria accounting for more than 90% of malaria cases and Plasmodium vivax and Plasmodium malariae accounting for the rest. Malaria transmission is perennial, although transmission intensity varies during the year. The high transmission season extends from October to December following the monsoon rains, with parasite rates as high as 20 to 50% in this period. Parasite rates in the low transmission season during the hot dry summer months of April and May are between 5 and 15%. Other months have intermediate levels of malaria transmission. Based on active case detection over a 3-year period, it is estimated that on average there are 2.1 malaria episodes per person per year in the 1- to 5-year age group and 0.5 malaria episodes per person per year in adults. These data suggest that San Dulakudar residents develop natural immunity to malaria following repeated exposure.
Parasites and sera.
Adult residents of Rourkela reporting to Ispat General Hospital (IGH), Rourkela, and diagnosed with nonsevere P. falciparum malaria based on clinical symptoms and detection of parasites in blood smears were enrolled in the study. Ten milliliters of blood was collected at the time of admission prior to initiating antimalarial drug therapy. Five milliliters of blood was collected in 10% citrate-phosphate-dextrose, washed, and cryopreserved in aliquots in liquid nitrogen. The remaining 5 ml was used for preparation of acute-phase sera by standard methods. Five milliliters of blood was collected from the same group of volunteers 3 to 4 weeks after recovery and used for preparation of convalescent-phase sera. All patients had parasite-free blood smears at time of discharge and no malaria symptoms during the period between discharge and collection of convalescent-phase sera. All individuals enrolled in the study at IGH, Rourkela, had lived in Rourkela for more than 10 years. The majority of the volunteers had no history of malaria in the past 10 years. One individual reported having malaria twice, and three individuals reported having malaria once in the past 10 years.
Standard blood group typing procedures were used to determine the blood groups of donors. Parasite isolates collected from donors with the O blood group (R1, R13, R15, R28, R30, R35, R39, and R40) were used for agglutination assays with homologous as well as heterologous sera. Parasite isolates (R4, R7, R9, R11, R16, and R17) collected from donors with other blood groups were only used for agglutination assays with homologous sera.
Sera were also collected from asymptomatic adults and children (1 to 3 years) residing in San Dulakudar during periodic mass surveys. One child (DK557) was 18 months old. All other children were between 24 and 36 months old. This study received ethical clearance from the Institutional Review Boards of the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India, and IGH, Rourkela. Individuals were enrolled in the study after obtaining informed consent. In case of children, informed consent was obtained from parents.
Agglutination assay.
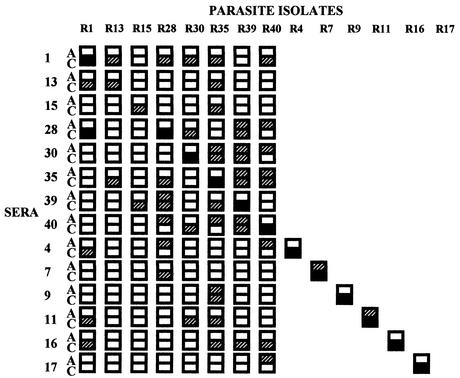
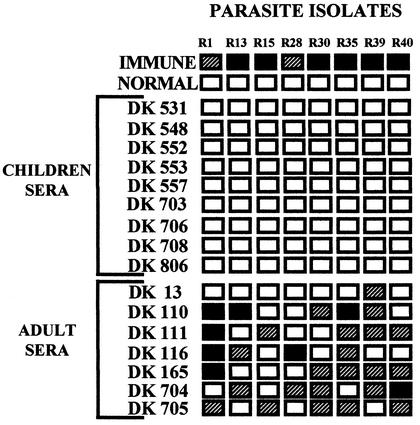
Cryopreserved P. falciparum isolates collected from malaria patients reporting to IGH, Rourkela, were thawed and cultured to the trophozoite-schizont stages in RPMI 1640 medium (Gibco BRL-Life Technologies) containing 10% heat-inactivated fetal calf serum (Gibco BRL-Life Technologies). In each case, parasitemia was ∼1%. A hematocrit of the culture was adjusted to ∼5% using RPMI 1640 containing ethidium bromide (Sigma) at a concentration of 10 μg/ml. Agglutination assays were performed as described previously (3). Briefly, 2.5 μl of each serum sample was spotted in wells of U-bottomed 96-well microtiter plates (Costar). Ten microliters of parasite culture in RPMI 1640 with ethidium bromide was added to each well. The microtiter plate was rotated at 30 rounds per min on a vertical rotator for 60 min at room temperature. The entire 12.5-μl reaction from each well was placed on a glass slide and covered with a coverslip. Slides were blinded and examined microscopically under visual and UV light. P. falciparum laboratory isolate A4 and field isolate R1 were initially used to standardize the assay and ensure reproducibility. Agglutinates were scored in 25 fields at ×40 magnification. Blank boxes indicate that no agglutinates containing three or more infected erythrocytes were seen (Fig. 1 and 2). Hatched boxes indicate that on average five or less agglutinates containing five or more infected erythrocytes were seen per field (Fig. 1 and 2). Black boxes indicate that on average greater than five agglutinates containing five or more infected erythrocytes were seen per field (Fig. 1 and 2).
FIG. 1.
Agglutination of P. falciparum field isolates with acute- and convalescent-phase sera from adult malaria patients residing in Rourkela, Orissa, India, a town with low malaria transmission rates. Acute-phase (A) and convalescent-phase (C) sera collected from adult malaria patients residing in Rourkela, a town with low malaria transmission, were used for agglutination assays with homologous and heterologous P. falciparum isolates. Blank boxes indicate that no agglutinates of three or more infected erythrocytes were seen. Hatched boxes indicate that on average five or less agglutinates containing five or more P. falciparum-infected red cells per agglutinate were seen per field. Black boxes indicate that on average greater than five agglutinates containing five or more infected red cells were seen per field. Twenty-five fields were examined at ×40 magnification for each agglutination assay.
FIG. 2.
Agglutination of P. falciparum field isolates with sera from children and adults from San Dulakudar, Orissa, India, a village where P. falciparum malaria is hyperendemic. Sera from children and adults residing in San Dulakudar, where P. falciparum malaria is hyperendemic, were used for agglutination assays with diverse P. falciparum isolates. Pooled sera from adults living in another area in Orissa in which P. falciparum was endemic were used as a positive control (Immune). Pooled sera from adults residing in regions of India where malaria was not endemic were used as a negative control (Normal). Twenty-five fields were examined at ×40 magnification for each agglutination assay. Blank, hatched, and black boxes indicate the frequency of agglutination as described for Fig. 1.
Mixed-agglutination assay.
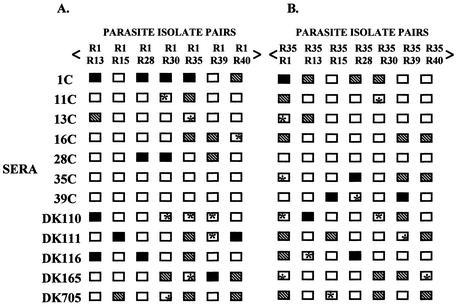
Mixed-agglutination assays were performed with pairs of P. falciparum isolates stained with distinguishable DNA intercalating dyes (15). The method used for the mixed-agglutination assay was similar to that described above for a single isolate except that two isolates were used, one labeled with ethidium bromide (10 μg/ml) and the other with acridine orange (10 μg/ml). Mixed-color agglutinates are defined as agglutinates with at least three infected erythrocytes with at least one infected erythrocyte of each color. Blank boxes indicate that no mixed-color agglutinates were seen (Fig. 3 and 4). The number of mixed-color agglutinates containing up to 20 infected erythrocytes with at least two infected erythrocytes of each color was scored in 25 fields at ×40 magnification. Hatched boxes indicate that on average five or less mixed-color agglutinates were seen per field, and black boxes indicate that on average greater than five mixed color agglutinates were seen per field (Fig. 3 and 4).
FIG. 3.
Mixed-agglutination assays with P. falciparum field isolates. (A and B) P. falciparum isolates were tested in mixed-agglutination assays in combination with parasite isolates R1 and R35 and convalescent-phase sera from adult malaria patients (1C, 11C, 13C, 16C, 28C, 35C, and 39C) residing in Rourkela, a town with a low malaria transmission rate, or with sera from adults residing in San Dulakudar (DK110, DK111, DK116, DK165, and DK705), a village where P. falciparum malaria was hyperendemic. Mixed-color agglutinates were defined as agglutinates with at least three infected erythrocytes, with at least one erythrocyte of each color. Blank boxes indicate cases where no mixed-color agglutinates were found. Blank boxes marked with an asterisk indicate cases where no mixed-color agglutinates were found, even though both isolates are individually recognized. Mixed-color agglutinates containing up to 20 P. falciparum-infected erythrocytes with at least two infected erythrocytes of each color labeled were scored in 25 fields at ×40 magnification. Hatched boxes indicate that on average one to five such mixed-color agglutinates are observed per field, and black boxes indicate that on average more than five such mixed-color agglutinates are observed per field. Mixed-color agglutinates constitute 20 to 30% of all agglutinates in cases where mixed-color agglutinates are observed.
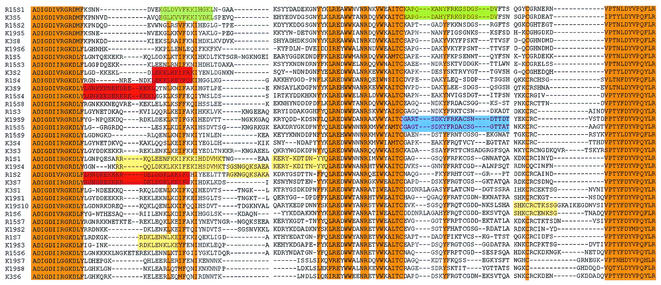
FIG. 4.
Multiple alignment of DBLα sequences from Indian and Kenyan P. falciparum isolates. A multiple-sequence alignment of DBLα sequences derived from var genes from two Indian P. falciparum isolates from Rourkela, R1 (R1S1 to R1S7) and R15 (R15S1 to R15S9), and two Kenyan P. falciparum isolates, K3 (K3S1 to K3S9) and K19 (K19S1 to K19S10, is shown. Orange boxes indicate conserved stretches of either identical or highly conserved residues shared by all var genes. Colored boxes indicate short segments of at least 10-amino-acid residues with greater than 70% identity within hypervariable regions that are common to some var genes from heterologous Indian and Kenyan isolates. Blue boxes indicate sequences common to R15 and K19 and red boxes indicate sequences common to R1 and K3, yellow boxes indicate sequences common to R1 and K19, and green boxes indicate sequences common to R1 and K3, yellow boxes indicate sequences common to R15 and K19, and green boxes indicate sequences common to R15 and K3..
PCR typing of P. falciparum patient isolates.
P. falciparum isolates used for the agglutination assays were typed by PCR using two polymorphic blood stage antigens, merozoite surface protein-1 (MSP-1) and merozoite surface protein-2 (MSP-2), to determine if they were genotypically distinct and contain single or multiple genotypes as described earlier (16, 27). PCR products encoding MSP-1 block 2 were subtyped by nested PCR using oligonucleotide primers specific for the K1, MAD20, and R033 subtypes of MSP-1 (21). PCR products encoding blocks 2 and 3 of MSP-2 were subtyped using oligonucleotide primers specific for FC27 and 3D7 subtypes as previously described (21).
Analysis of diversity in DBLα domain sequences of PfEMP-1 among P. falciparum isolates.
The extracellular region of PfEMP-1 contains multiple conserved, cysteine-rich domains that are referred to as Duffy binding-like (DBL) domains because they share homology with the binding domain of P. vivax Duffy binding protein (2, 5, 6, 18-20, 22). DBL domains are classified into five types, α, β, γ, δ, and ɛ, based on the presence of type-specific conserved sequences (19, 20). Genomic DNA isolated from P. falciparum isolates R1, R15 and R35 was used as template in PCRs with degenerate primers based on conserved sequences in DBLα domains of var genes as previously described (23). PCR products encoding the central region of DBLα domains were cloned in TA cloning vector (Invitrogen) and sequenced using an Automated ABI 310 DNA Sequencer (Perkin Elmer).
The var sequences identified from P. falciparum isolates R1, R15, and R35 and 19 var sequences reported previously from two Kenyan P. falciparum isolates, 3 and 19, referred to here as K3 and K19 (23; GenBank accession numbers from AF221811 to AF221819 and from AF221777 to AF221786), were used for analysis. Pairwise sequence comparison was performed using the SIM program with default values available on the server http://www.expasy.ch, which determines the percent identity between two amino acid sequences. A matrix-based analysis was performed using SIM in which each sequence from a parasite isolate was compared to all other sequences from the same isolate or from a different isolate. This resulted in a matrix containing the percent sequence identity of each sequence relative to every other sequence. Mean, range, and standard deviation values for the sequence comparison data were calculated and are shown in Table 2. A multiple-sequence alignment was carried out with var sequences from R1, R15, K3, and K19 (total number of sequences = 35) using the program CLUSATLW.
TABLE 2.
Pairwise comparison of DBLα sequences from var genes from P. falciparum isolates (R1, R15, and R35) from Rourkela and P. falciparum isolates (K3 and K19) from Kenyaa
| P. falciparum isolates | Range (%) | Avg identity (%) | SD |
|---|---|---|---|
| R1/R1 | 41.9-56.6 | 49.7 | 4.1 |
| R15/R15 | 36.7-67.5 | 48.5 | 6.2 |
| R35/R35 | 35.0-69.1 | 49.3 | 7.3 |
| R1/R35 | 35.2-64.6 | 50.0 | 6.4 |
| R1/R15 | 41.6-58.5 | 48.5 | 4.9 |
| K3/K3 | 37.0-58.5 | 47.7 | 5.5 |
| K3/K19 | 35.1-65.0 | 47.5 | 6.7 |
| K3/R35 | 34.1-62.5 | 48.2 | 6.2 |
Average, standard deviation, and range for percent sequence identity for pairwise comparison of DBLα sequences from P. falciparum isolates (R1, R15, and R35) from Rourkela and P. falciparum isolates (K3 and K19) from Kenya (23) are given.
Nucleotide sequence accession number.
Seven unique var gene sequences were identified from R1, and nine unique var gene sequences were identified from R15 and R35. These nucleotide sequences are available in the GenBank database and have accession numbers from AF404238 to AF404262.
RESULTS
Agglutination of homologous and heterologous P. falciparum isolates by acute- and convalescent-phase sera collected from adult malaria patients residing in Rourkela, an area with low malaria transmission rates.
The incidence of malaria in Rourkela is very low (see Materials and Methods). Acute- and convalescent-phase sera collected from P. falciparum malaria patients residing in Rourkela were tested for recognition of homologous and heterologous P. falciparum isolates in agglutination assays. Most acute-phase sera (12 out of 14 acute-phase sera tested) do not agglutinate homologous P. falciparum isolates (Fig. 1). All convalescent-phase sera agglutinate homologous P. falciparum isolates, indicating that individuals seroconvert following infection and develop antibodies that recognize surface antigens expressed by the infecting P. falciparum isolate (Fig. 1). Interestingly, in addition to agglutinating homologous parasite isolates, a significant number of convalescent-phase sera also agglutinate heterologous isolates (Fig. 1). In cases where both acute- and convalescent-phase sera agglutinate heterologous isolates, it is not possible to attribute agglutination by convalescent-phase sera to antibodies that develop in response to the recent infection. Such cases are therefore disregarded in the following analysis. The majority of convalescent-phase sera (11 out of 14 sera tested) agglutinate at least one heterologous isolate in addition to the homologous isolate. Convalescent-phase serum from patient 1 agglutinates five out of seven heterologous isolates tested, convalescent-phase serum from patient 16 agglutinates four heterologous isolates, and convalescent-phase serum from patient 11 agglutinates 3 heterologous isolates. The observation that convalescent-phase sera frequently agglutinate heterologous isolates suggests that these individuals develop cross-reactive antibodies that recognize common epitopes shared by surface antigens expressed by diverse P. falciparum isolates.
Agglutination of P. falciparum field isolates with sera from children and adults residing in San Dulakudar, a village where P. falciparum malaria is hyperendemic.
P. falciparum malaria is hyperendemic in the village of San Dulakudar (see Materials and Methods). Cryopreserved P. falciparum isolates collected from malaria patients admitted to IGH, Rourkela, were cultured for one cycle from ring to trophozoite or schizont stages and used for agglutination assays with sera collected from children and adults residing in San Dulakudar. Sera collected from nine children between 1 and 3 years of age do not agglutinate any of the eight P. falciparum isolates tested (Fig. 2). In contrast, six out of seven adult sera tested agglutinate at least four out of eight P. falciparum isolates (Fig. 2). These data indicate that, whereas sera from children in San Dulakudar lack antibodies directed against diverse PfEMP-1, sera from adults residing in the same village recognize PfEMP-1 variants expressed by a wide range of P. falciparum isolates. Pooled sera collected from individuals residing in areas of India where malaria is not endemic do not agglutinate any of the P. falciparum isolates tested (Fig. 2). Pooled immune sera collected from adults residing in a different area of P. falciparum endemicity of Orissa (10) agglutinate all eight P. falciparum isolates tested (Fig. 2).
Mixed-agglutination assays to detect presence of cross-reactive antibodies directed against PfEMP-1 variants expressed by diverse P. falciparum isolates.
Mixed-agglutination assays were used to examine presence of cross-reactive antibodies to PfEMP-1. Since P. falciparum isolates R1 and R35 were most commonly recognized by heterologous convalescent-phase sera, they were used in combination with other parasite isolates in mixed-agglutination assays. Convalescent-phase sera from all malaria patients whose parasites were used for agglutination with heterologous sera were used for mixed-agglutination assays. Adult sera from San Dulakudar that agglutinate at least four out of eight isolates tested were also tested in mixed-agglutination assays.
Both convalescent-phase sera from Rourkela and adult immune sera from San Dulakudar form mixed-color agglutinates with some of the P. falciparum isolate pairs tested (Fig. 3). For example, convalescent-phase serum 1c forms mixed-color agglutinates with each of the P. falciparum isolate pairs R1-R13, R1-R28, R1-R30, R1-R35, and R1-R40, suggesting that it contains cross-reactive antibodies to common epitopes shared by PfEMP-1 variants expressed by P. falciparum isolate R1 and five other P. falciparum isolates. In all cases where one of the isolates in the pair is not individually recognized, mixed-color agglutinates are not found. Such cases serve as negative controls. There are a number of cases where two P. falciparum isolates are individually recognized, but mixed-color agglutinates are not observed (marked with asterisk in Fig. 3). In such cases agglutination is attributed to the presence of variant-specific antibodies that react with variant epitopes that are not shared by PfEMP-1 expressed by these isolates.
PCR typing and var gene diversity in P. falciparum isolates used for agglutination assays.
PCR typing based on the polymorphic markers MSP-1 and MSP-2 indicates that each P. falciparum isolate used in the agglutination assays is unique and contains a single P. falciparum genotype (Table 1).
TABLE 1.
PCR typing of P. falciparum patient isolates based on polymorphic markers MSP-1 and MSP-2
| P. falciparum isolate | Length (bp) of:
|
||||
|---|---|---|---|---|---|
| MSP-1
|
MSP-2
|
||||
| K1 | MAD20 | R033 | FC27 | 3D7 | |
| R1 | 564 | 708 | |||
| R13 | 471 | 823 | |||
| R15 | 525 | 788 | |||
| R28 | 537 | 878 | |||
| R30 | 456 | 850 | |||
| R35 | 653 | 878 | |||
| R39 | 402 | 739 | |||
| R40 | 492 | 806 | |||
Sequence diversity in var genes from P. falciparum isolates used in the study was examined and compared to the diversity found in var genes from African isolates. Degenerate oligonucleotide primers based on conserved DBLα sequences were used to amplify var gene fragments encoding the central semiconserved segment of DBLα domains of PfEMP-1 by PCR from three P. falciparum isolates, i.e., R1, R15, and R35 (23). R1 and R35 were selected because they were most commonly recognized, and R15 was selected because it was least commonly recognized among isolates used in the agglutination assays. Amino acid sequences encoded by each of the cloned PCR-amplified var gene fragments from R1, R15, and R35 were compared to each other, and percent sequence identity was determined for each pair (Table 2). The average “percent sequence identity values” derived from pairwise comparison of var sequences from the same isolate are similar to those derived from pairwise comparison of var sequences from heterologous isolates (Table 2). Similar percent sequence identity values obtained upon comparison of homologous and heterologous var genes suggest that var sequences from a single isolate are as similar and/or dissimilar to each other as var sequences from heterologous isolates in Rourkela. Another sequence comparison analysis was performed on var sequences reported from P. falciparum isolates K3 and K19 from eastern Kenya (23). The average percent sequence identity values determined for pairwise comparison of var sequences from Kenyan isolates as well as those determined from comparison of var sequences from Indian isolates with Kenyan isolates are similar to average percent sequence identity values found for pairwise comparison of heterologous Indian isolates (Table 2). The level of sequence diversity in var genes from Indian and Kenyan isolates is thus comparable.
A multiple-sequence alignment of DBLα sequences derived from two Indian isolates from Rourkela and two Kenyan isolates is shown in Fig. 4. Orange boxes indicate conserved segments of either identical or highly conserved residues that are shared by all var genes. Such linear segments are found at both the amino and carboxyl termini of this region. A number of hydrophobic residues and at least two cysteines are conserved in this region. It is noteworthy that a number of hydrophilic residues are also found in these segments. The conserved segments shared by all var sequences are interspersed with “hypervariable segments” that vary significantly in both sequence and length. Within these hypervariable regions, a number of amino acid segments of limited conservation common to var sequences from heterologous isolates (colored boxes in Fig. 4) exist. The presence of such common amino acid segments in PfEMP-1 variants from diverse P. falciparum isolates may be responsible for the observed cross-reactivity of agglutinating antibodies.
DISCUSSION
Individuals residing in areas of endemicity acquire natural immunity following repeated infection with P. falciparum (14). Antibodies directed against PfEMP-1 are thought to a play a role in naturally acquired immunity to P. falciparum malaria (3, 13). Here we have studied the development of antibodies to PfEMP-1 following P. falciparum infection in adult residents of Rourkela, a town with a low malaria transmission rate, and the prevalence of antibodies to PfEMP-1 in residents of San Dulakudar, a village where P. falciparum malaria is hyperendemic.
Our data show that acute-phase sera from adult malaria patients from Rourkela have limited recognition of P. falciparum isolates. Residents of Rourkela are not exposed to a wide range of P. falciparum isolates and thus lack antibodies against diverse PfEMP-1. All malaria patients from Rourkela seroconvert following P. falciparum infection to agglutinate the homologous P. falciparum isolate. Interestingly, convalescent-phase sera from adult malaria patients in Rourkela also frequently agglutinate a limited number of heterologous P. falciparum isolates. These observations suggest that antibodies to PfEMP-1 that develop following infection recognize common epitopes shared by at least some PfEMP-1 variants. In a few cases convalescent-phase sera fail to agglutinate heterologous isolates, even though the corresponding acute-phase sera agglutinate that isolate. Such examples have been observed before and suggest that antibodies to the heterologous isolate in acute-phase serum may not be present in the corresponding convalescent-phase serum in these cases. In the case of the high-transmission study, children from San Dulakudar fail to agglutinate any of the P. falciparum isolates tested, whereas adult sera agglutinate diverse P. falciparum isolates. This is in line with previous observations that children in areas of endemicity have very limited recognition, whereas adults have wide recognition of diverse PfEMP-1 (4, 7-9, 12, 15). It is possible that children make more variant-specific antibodies than naïve adults upon infection with P. falciparum and therefore fail to agglutinate any heterologous parasites. This possibility could not be addressed in the present study since all acute- and convalescent-phase sera were collected from naïve adults.
Mixed-agglutination assays using P. falciparum isolate pairs stained with distinguishable DNA intercalating dyes were used to examine presence of cross-reactive antibodies to PfEMP-1. Mixed-color agglutinates were frequently found with different P. falciparum isolate pairs tested with convalescent-phase sera from Rourkela. The presence of mixed-color agglutinates indicates presence of cross-reactive antibodies that recognize common epitopes shared by PfEMP-1 expressed by both isolates. In some cases, mixed- color agglutinates were not observed even though both parasite isolates were individually recognized. In such cases, convalescent-phase sera are likely to contain variant-specific antibodies that recognize variant epitopes that are not shared by PfEMP-1 expressed by the two isolates tested. Previous studies using acute- and convalescent-phase sera have also reported that convalescent-phase sera agglutinate some heterologous isolates (4, 9). However, mixed-agglutination assays were not performed and the presence of cross-reactive antibodies was not explicitly demonstrated in these studies (4, 9). A study using adult sera from an area where P. falciparum malaria is mesoendemic also suggested that a single P. falciparum infection might elicit cross-reactive antibodies against diverse PfEMP-1 (8). However, these investigators failed to detect cross-reactive antibodies in mixed-agglutination assays probably because they used a limited number of P. falciparum isolate pairs (8). Our study is the first to explicitly demonstrate that both variant-specific antibodies as well as antibodies with limited cross-reactivity for diverse PfEMP-1 develop following a single infection with P. falciparum.
Initial studies with sera from Gambian adults reported the presence of cross-reactive antibodies to PfEMP-1 in immune sera (12). In contrast, a later study reported that mixed-color agglutinates were rarely observed with pooled immune sera from Gambian adults (15). The presence of some high-titer variant-specific antibodies in pooled immune sera may have led to the predominant formation of single-color agglutinates in this study. Individual Gambian adult sera were also used for mixed-agglutination assays in this study (15). However, only three pairs of P. falciparum laboratory isolates were tested and mixed-color agglutinates were observed only in a limited number of cases, leading these investigators to conclude that antibodies to PfEMP-1 are predominantly variant specific (15). In the present study, we have tested individual immune sera from adult residents of an area of hyperendemicity with 13 different pairs of P. falciparum field isolates in mixed-agglutination assays. Mixed-color agglutinates are observed frequently with adult immune sera from San Dulakudar. In some cases mixed-color agglutinates are not observed even though both parasite isolates are individually recognized. We therefore conclude that adult immune sera contain both variant-specific and partially cross-reactive antibodies to PfEMP-1.
Are cross-reactive antibodies observed in our studies because the P. falciparum isolates used have limited diversity? P. falciparum isolates used in this study were collected from malaria patients in Rourkela, an area with a low malaria transmission rate. It is possible that only a small number of P. falciparum isolates circulate and are repeatedly sampled in an area with a low entomological inoculation rate. However, PCR typing based on two polymorphic markers, MSP-1 and MSP-2, demonstrates that each P. falciparum isolate used in our study is genotypically unique, ruling out this possibility.
Do P. falciparum isolates used in our study have limited sequence diversity in var genes compared to isolates from areas of endemicity in Africa with high entomological inoculation rates? In order to address this question, we have determined the extent of sequence diversity in the central, semiconserved region of DBLα domains of PfEMP-1 from three P. falciparum isolates used in our study. DBLα sequences from the Indian isolates were compared with each other and with DBLα sequences reported from Kenyan isolates (23). The average sequence identity derived from pairwise comparison of DBLα sequences from Indian and Kenyan isolates is comparable (Table 2). This analysis indicates that the level of conservation and/or diversity in var sequences from Indian and Kenyan P. falciparum isolates is comparable. Moreover, var sequences from parasite isolates from the same geographical region are as similar and/or dissimilar to each other as they are to var sequences from parasite isolates from distant geographical regions.
We further performed comparative sequence analyses of DBLα sequences from two Indian isolates and two Kenyan isolates to explore the molecular basis of cross-reactivity observed in our field studies. A multiple-sequence alignment reveals the presence of highly conserved segments (grey boxes, Fig. 4). These segments contain conserved cysteines and hydrophobic amino acid residues, suggesting that they may be structurally important. A number of charged, potentially exposed amino acid residues are also present in these segments. Antibodies directed against such highly conserved segments would be widely cross-reactive. However, since widely cross-reactive antibodies are not observed in the field, it is likely that these highly conserved segments of DBLα are not immunogenic during natural infection. The conserved segments are interspersed with hypervariable segments that vary in both sequence and length. These divergent segments contain multiple hydrophilic residues, suggesting that they are likely to be exposed and may therefore serve as epitopes for agglutinating antibodies. Sets of common sequences shared by diverse PfEMP-1 from heterologous isolates can be found in these divergent segments of DBLα domains. Colored boxes illustrate such common sequences shared by var genes from heterologous Indian and Kenyan isolates (Fig. 4). Antibodies directed against such shared amino acid sequences may be responsible for the limited cross-reactivity observed with naturally acquired antibodies. Similar common stretches of amino acid sequences are also found between var genes from heterologous Indian isolates (data not shown). The presence of common sequences in var genes from geographically distant P. falciparum isolates has been previously noted (26) and explains the ability of adult immune sera to agglutinate parasite isolates from distant locations (1).
Following infection with P. falciparum, the infected individual is exposed to a series of PfEMP-1 variants as the blood-stage parasite multiplies and undergoes antigenic variation in vivo. As a result, one can expect that a set of agglutinating antibodies directed against multiple PfEMP-1 will develop. These antibodies are likely to be directed against divergent segments of PfEMP-1 as discussed earlier. However, as our sequence analysis suggests, divergent segments contain amino acid sequences that are shared by subsets of PfEMP-1 variants. We may therefore expect that the set of naturally acquired antibodies elicited by a single infection should agglutinate at least some heterologous isolates that express PfEMP-1 molecules, which share epitopes with the homologous isolate (colored boxes, Fig. 4). Indeed, agglutination assays using acute- and convalescent-phase sera from Rourkela demonstrate the development of antibodies with limited cross-reactivity.
As is well known, adults in areas of endemicity are typically exposed to a wide range of P. falciparum isolates and thereby develop antibodies that together recognize a majority of PfEMP-1 variants. We suggest that the basis of such wide recognition may not entirely be due to the acquisition of a repertoire of variant-specific antibodies. Our analysis of PfEMP-1 sequences reveals the presence of common segments even in divergent regions of DBLα domains, and given the hydrophilic character of such segments, it is likely that they are both exposed and form epitopes for antibody recognition. Antibodies to such epitopes on PfEMP-1 are likely to be partially cross-reactive. Sets of such naturally acquired partially cross-reactive antibodies in immune sera will have an additive effect enabling recognition of a wide range of PfEMP-1 variants. Indeed, adult sera from San Dulakudar agglutinate diverse P. falciparum isolates, and mixed-agglutination assays confirm the presence of both variant-specific and partially cross-reactive antibodies. We propose that the molecular basis of wide recognition of PfEMP-1 variants by immune serum from areas of endemicity lies in the prevalence of multiple sets of partially cross-reactive antibodies that together may cover most of the “var epitope space.”
Acknowledgments
This work was supported by Wellcome Trust International Senior Research Fellowships to C.E.C. and A.S.
We thank Shobhona Sharma for providing pooled immune sera, Virander S. Chauhan for comments on the manuscript, V. P. Sharma for encouraging us to initiate these studies, and Sarala Subbarao for continued support. We also thank the staff of Malaria Research Centre Field Station, Rourkela, and clinicians at the Ispat General Hospital, Rourkela, for their cooperation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aguiar, J. C., G. R. Albrecht, P. Cegielski, B. M. Greenwood, J. B. Jensen, G. Lallinger, A. Martinez, I. A. McGregor, J. N. Minjas, J. Neequaye, M. E. Patarroyo, J. A. Sherwood, and R. J. Howard. 1992. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 47:621-632. [DOI] [PubMed] [Google Scholar]
- 2.Baruch, D., B. Pasloske, H. Singh, B. Xiahui, X. Ma, M. Feldman, T. Taraschi, and R. J. Howard. 1995. Cloning of the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and cytoadherence receptor on the surface of parasitised human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 3.Bull, P., B. Lowe, M. Kortok, C. Molyneux, C. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P., B. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitnis, C. E., and L. H. Miller. 1994. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 180:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitnis, C. E., P. Sinnis, and L. H. Miller. 1999. The sporozoite, the merozoite and the infected red cell: parasite ligands and host receptors, p. 249-285. In M. Wahlgren and P. Perlmann (ed.), Malaria: molecular and clinical aspects. Harwood Academic Publishers, Newark, N.J.
- 7.Forsyth, K. P., G. Phillip, T. Smith, E. Kum, B. Southwell, and G. V. Brown. 1989. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 83:464-469. [PubMed] [Google Scholar]
- 8.Giha, H. A., T. Staalsoe, D. Dodoo, I. M. Elhassan, C. Roper, G. M. H. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 1999. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology 119:7-17. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal, J., P. Perlmann, and K. Berzin. 1993. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 87:583-588. [DOI] [PubMed] [Google Scholar]
- 10.Lobo, C. A., S. K. Kar, B. Ravindran, L. Kabilan, and S. Sharma. 1994. Novel proteins of Plasmodium falciparum identified by differential immunoscreening using immune and patient sera. Infect. Immun. 62:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacPherson, G., M. Warrell, N. White, W. Looareesuwan, and D. Warrell. 1985. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 13.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood-stage antigens of Plasmodium falciparum in rural Gambia and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 14.Marsh, K. 1992. Malaria—a neglected disease? Parasitology 104:S53-S69. [DOI] [PubMed] [Google Scholar]
- 15.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 16.Okoyeh, J. N., C. R. Pillai, and C. E. Chitnis. 1999. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect. Immun. 67:5784-5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patnaik, J. K., B. S. Das, S. K. Mishra, S. Mohanty, S. K. Satpathy, and D. Mohanty. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 51:642-647. [PubMed] [Google Scholar]
- 18.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudson-Taylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, J. D., G. Subramanian, B. Gamain, D. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 20.Smith, J. D., B. Gamain, D. Baruch, and S. Kyes. 2001. Decoding the language of var gene and Plasmodium falciparum sequestration. Trends Parasitol. 17:538-545. [DOI] [PubMed] [Google Scholar]
- 21.Snounou, G., X. Zhu, N. Siripoon, W. Jarra, S. Thaithong, K. N. Brown, and S. Viriyakosoi. 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:369-374. [DOI] [PubMed] [Google Scholar]
- 22.Su, X., V. Heatwole, S. Wertheimer, F. Guinet, J. Herrfeldt, D. Peterson, J. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, H., S. Kyes, and C. I. Newbold. 2000. Var gene diversity in Plasmodium falciparum is generated by frequent recombination events. Mol. Biochem. Parasitol. 110:391-397. [DOI] [PubMed] [Google Scholar]
- 24.Turner, G., H. Morrison, M. Jones, T. Davis, S. Looareesuwan, I. Buley, K. Gatter, K. C. I. Newbold, S. Pukritayakamee, B. Nagachinta, N. J. White, and A. R. Berendt. 1994. An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role of intercellular adhesion molecule-1. Am. J. Pathol. 145:1057-1069. [PMC free article] [PubMed] [Google Scholar]
- 25.Walter, P., Y. Garin, and P. Blot. 1982. Placental pathological changes in malaria. Am. J. Pathol. 109:330-342. [PMC free article] [PubMed] [Google Scholar]
- 26.Ward, C. P., G. T. Clottey, M. Dorris, D. Ji, and D. E. Arnot. 1999. Analysis of Plasmodium falciparum PfEMP-1/var genes suggests that recombination rearranges constrained sequences. Mol. Biochem. Parasitol. 102:167-177. [DOI] [PubMed] [Google Scholar]
- 27.Wooden, J., S. Kyes, and C. Sibley. 1993. PCR and strain identification in Plasmodium falciparum. Parasitol. Today 9:303-305. [DOI] [PubMed] [Google Scholar]