Abstract
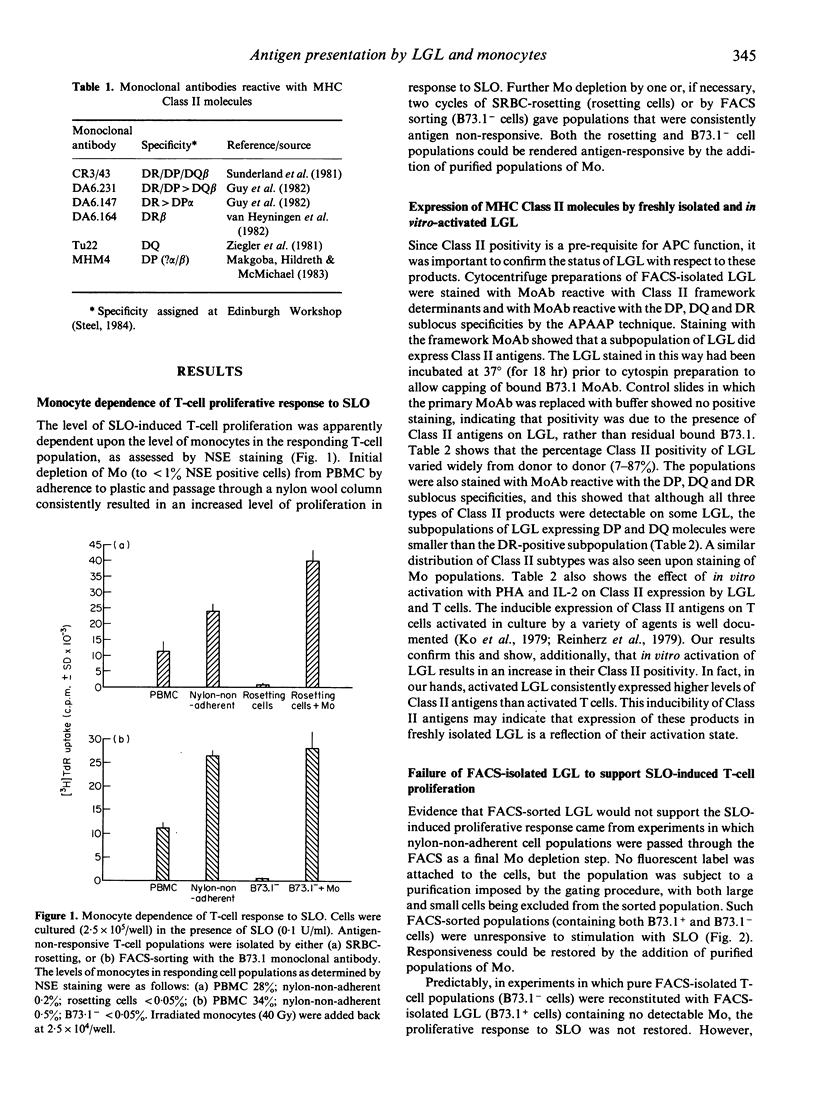
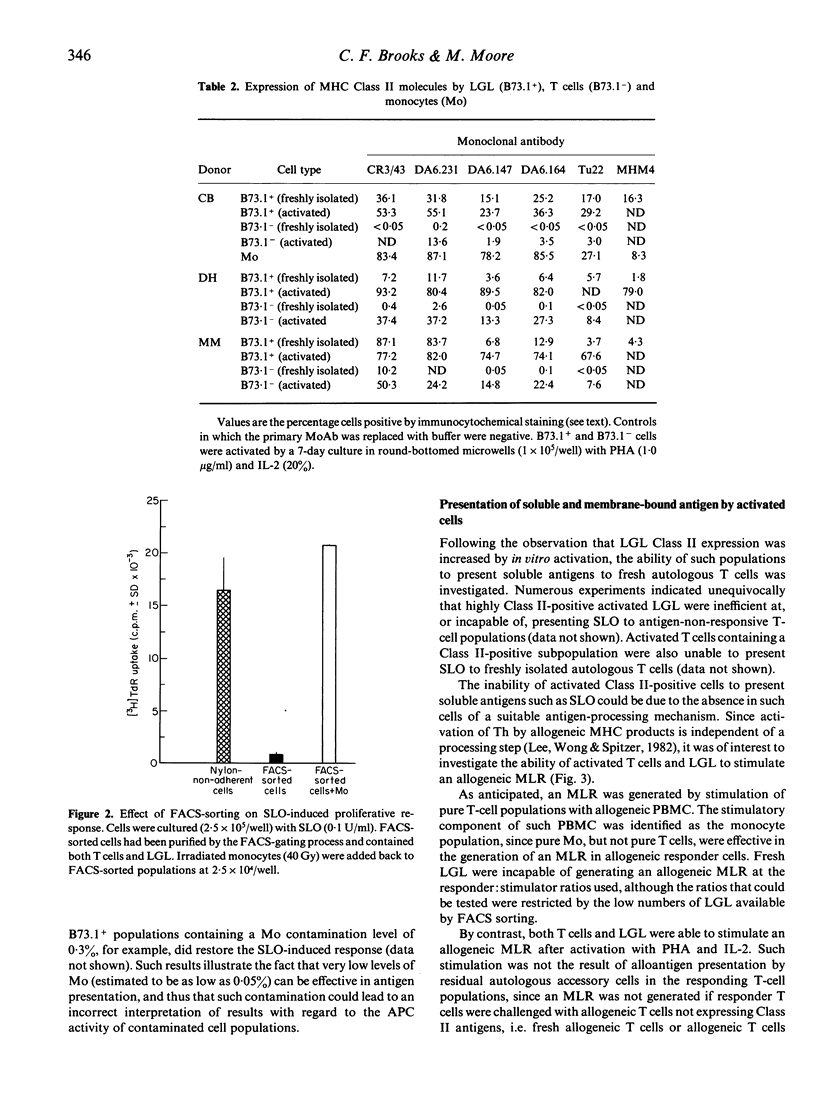
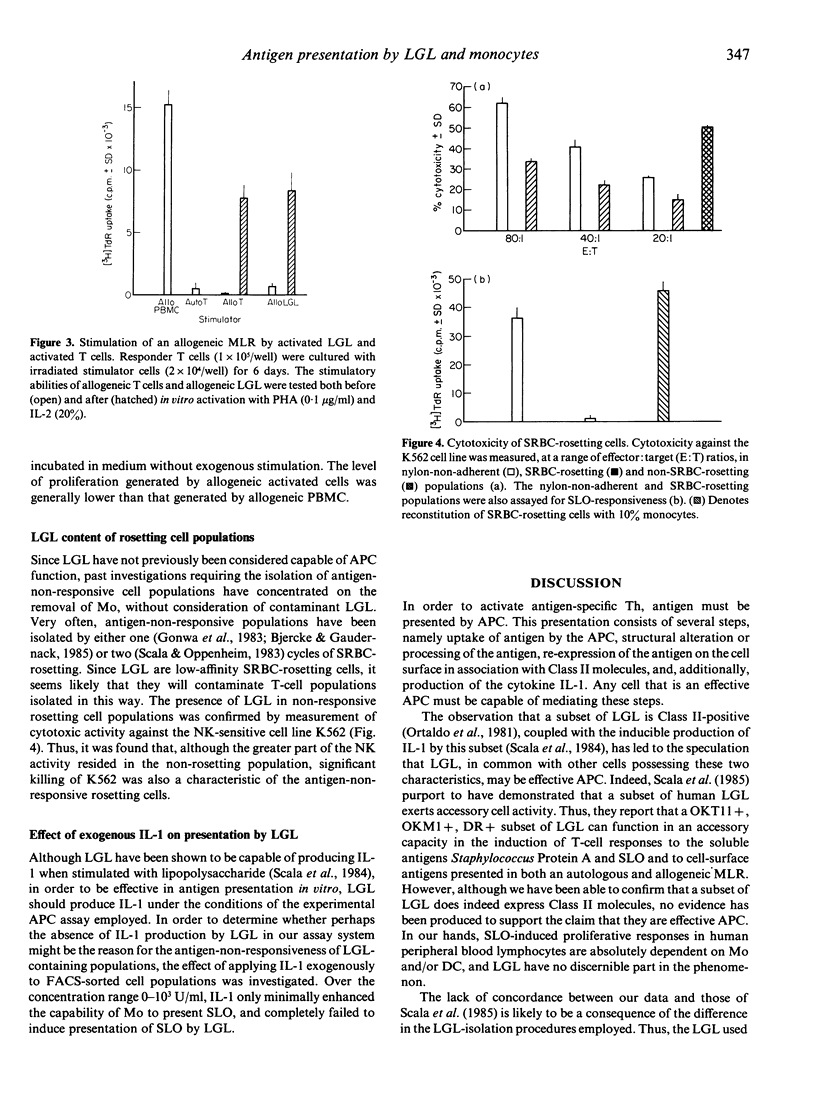
The ability of large granular lymphocytes (LGL) to function as antigen-presenting cells (APC) in the proliferative response to the soluble bacterial antigen streptolysin O (SLO) was investigated. Despite the fact that a subset of LGL isolated by sorting peripheral blood lymphocytes with the B73.1 monoclonal antibody on a fluorescence-activated cell sorter (FACS-IV) expressed MHC Class II molecules of the DP, DQ and DR subregion loci, presentation of SLO by LGL was not demonstrated. Thus, T-cell populations containing LGL but carefully depleted of monocytes, isolated either by sorting using the FACS-IV or by SRBC-rosetting, were unresponsive to antigenic stimulation with SLO. Application of exogenous interleukin-1 to FACS-IV-isolated LGL-containing T-cell populations did not elicit presentation of SLO by the LGL. In vitro activation with phytohaemagglutinin and interleukin-2, which induced Class II expression in T-cell populations, resulted in an increased expression of Class II molecules of the DP, DQ and DR specificities on LGL. Although such activated T-cell and LGL populations were incapable of presenting SLO to freshly isolated antigen-non-responsive T cells, both activated populations were able to act as stimulators in an allogeneic mixed lymphocyte reaction. The ability of highly Class II-positive activated LGL to present membrane-bound antigens suggests that their inability to present a soluble antigen may be related to the absence of effective antigen sequestration and/or processing mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Arai S., Yamamoto H., Itoh K., Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983 Aug;131(2):651–657. [PubMed] [Google Scholar]
- Bjercke S., Gaudernack G. Dendritic cells and monocytes as accessory cells in T-cell responses in man. II. Function as antigen-presenting cells. Scand J Immunol. 1985 May;21(5):501–508. doi: 10.1111/j.1365-3083.1985.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Brown M. F., Van M., Abramson S. L., Fox E. J., Rich R. R. Cellular requirements for induction of human primary proliferative responses to trinitrophenyl-modified cells. J Immunol. 1984 Jan;132(1):19–24. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Frohman M., Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol. 1985 Apr;134(4):2269–2275. [PubMed] [Google Scholar]
- Gerrard T. L., Volkman D. J., Jurgensen C. H., Fauci A. S. Activated human T cells can present alloantigens but cannot present soluble antigens. Cell Immunol. 1985 Oct 1;95(1):65–74. doi: 10.1016/0008-8749(85)90295-3. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B. Human fetal thymus and bone marrow contain target cells for natural killer cells. Eur J Immunol. 1981 Jan;11(1):8–12. doi: 10.1002/eji.1830110103. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Wong M., Spitzer D. Chloroquine as a probe for antigen processing by accessory cells. Transplantation. 1982 Sep;34(3):150–153. doi: 10.1097/00007890-198209000-00008. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Hildreth J. E., McMichael A. J. Identification of a human Ia antigen that is different from HLA-DR and DC antigens. Immunogenetics. 1983;17(6):623–635. doi: 10.1007/BF00366130. [DOI] [PubMed] [Google Scholar]
- Malissen B., Price M. P., Goverman J. M., McMillan M., White J., Kappler J., Marrack P., Pierres A., Pierres M., Hood L. Gene transfer of H-2 class II genes: antigen presentation by mouse fibroblast and hamster B-cell lines. Cell. 1984 Feb;36(2):319–327. doi: 10.1016/0092-8674(84)90225-3. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Ottosen P. D., Courtoy P. J., Farquhar M. G. Pathways followed by membrane recovered from the surface of plasma cells and myeloma cells. J Exp Med. 1980 Jul 1;152(1):1–19. doi: 10.1084/jem.152.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Pistoia V., Cozzolino F., Torcia M., Castigli E., Ferrarini M. Production of B cell growth factor by a Leu-7+, OKM1+ non-T cell with the features of large granular lymphocytes (LGL). J Immunol. 1985 May;134(5):3179–3184. [PubMed] [Google Scholar]
- Raff H. V., Picker L. J., Stobo J. D. Macrophage heterogeneity in man. A subpopulation of HLA-DR-bearing macrophages required for antigen-induced T cell activation also contains stimulators for autologous-reactive T cells. J Exp Med. 1980 Sep 1;152(3):581–593. doi: 10.1084/jem.152.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K., Moore M. A clonal analysis of human peripheral blood lymphocytes displaying natural killer-like activity. Eur J Immunol. 1985 May;15(5):448–456. doi: 10.1002/eji.1830150507. [DOI] [PubMed] [Google Scholar]
- Scala G., Allavena P., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Subsets of human large granular lymphocytes (LGL) exhibit accessory cell functions. J Immunol. 1985 May;134(5):3049–3055. [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Sunderland C. A., Naiem M., Mason D. Y., Redman C. W., Stirrat G. M. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981 Dec;3(6):323–331. doi: 10.1016/0165-0378(81)90048-6. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Abo T., Balch C. M. Suppressor cell function of human granular lymphocytes identified by the HNK-1 (Leu 7) monoclonal antibody. J Immunol. 1983 Mar;130(3):1171–1175. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ranki A., Saksela E., Häyry P. Human natural cell-mediated cytotoxicity against fetal fibroblasts. III. Morphological and functional characterization of the effector cells. Cell Immunol. 1979 Nov;48(1):121–132. doi: 10.1016/0008-8749(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E., Warner N. L., Chesnut R., Kappler J., Marrack P. Antigen-specific. I region-restricted interactions in vitro between tumor cell lines and T cell hybridomas. J Immunol. 1982 May;128(5):2164–2169. [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zembala M., Uracz W., Ruggiero I., Mytar B., Pryjma J. Isolation and functional characteristics of FcR+ and FcR- human monocyte subsets. J Immunol. 1984 Sep;133(3):1293–1299. [PubMed] [Google Scholar]
- van Heyningen V., Guy K., Newman R., Steel C. M. Human MHC class II molecules as differentiation markers. Immunogenetics. 1982;16(5):459–469. doi: 10.1007/BF00372104. [DOI] [PubMed] [Google Scholar]



