Abstract
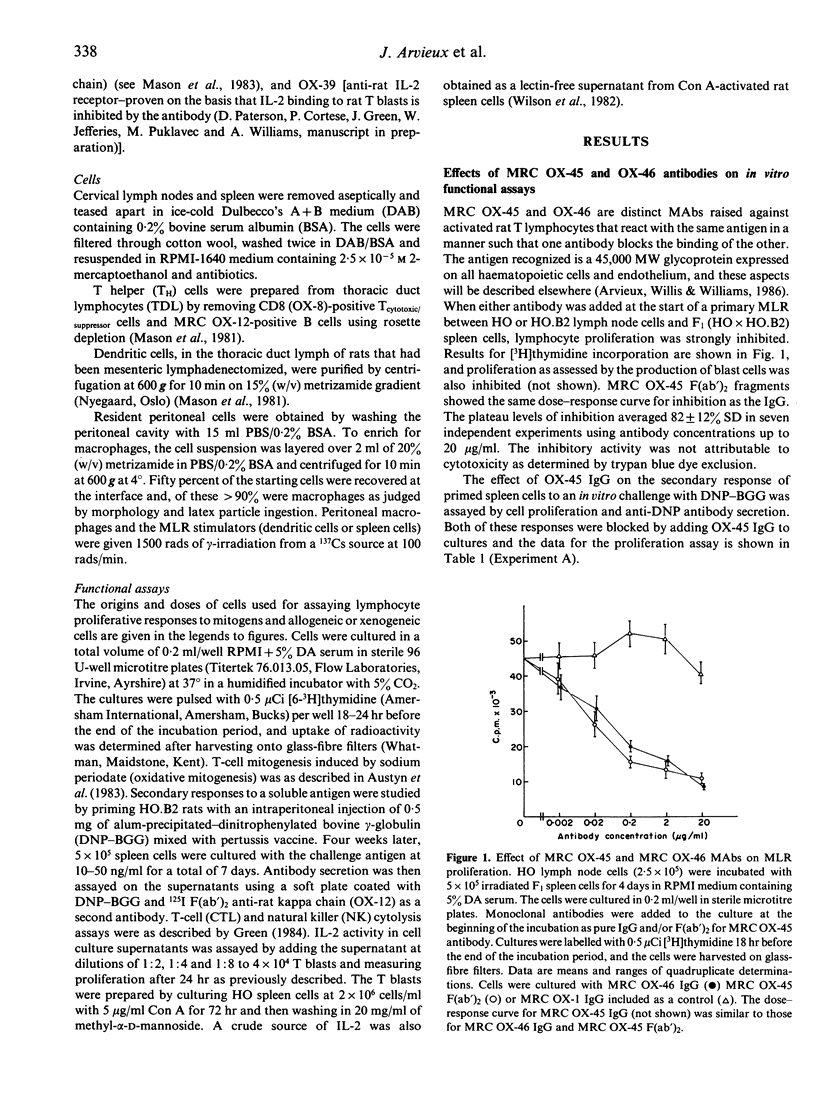
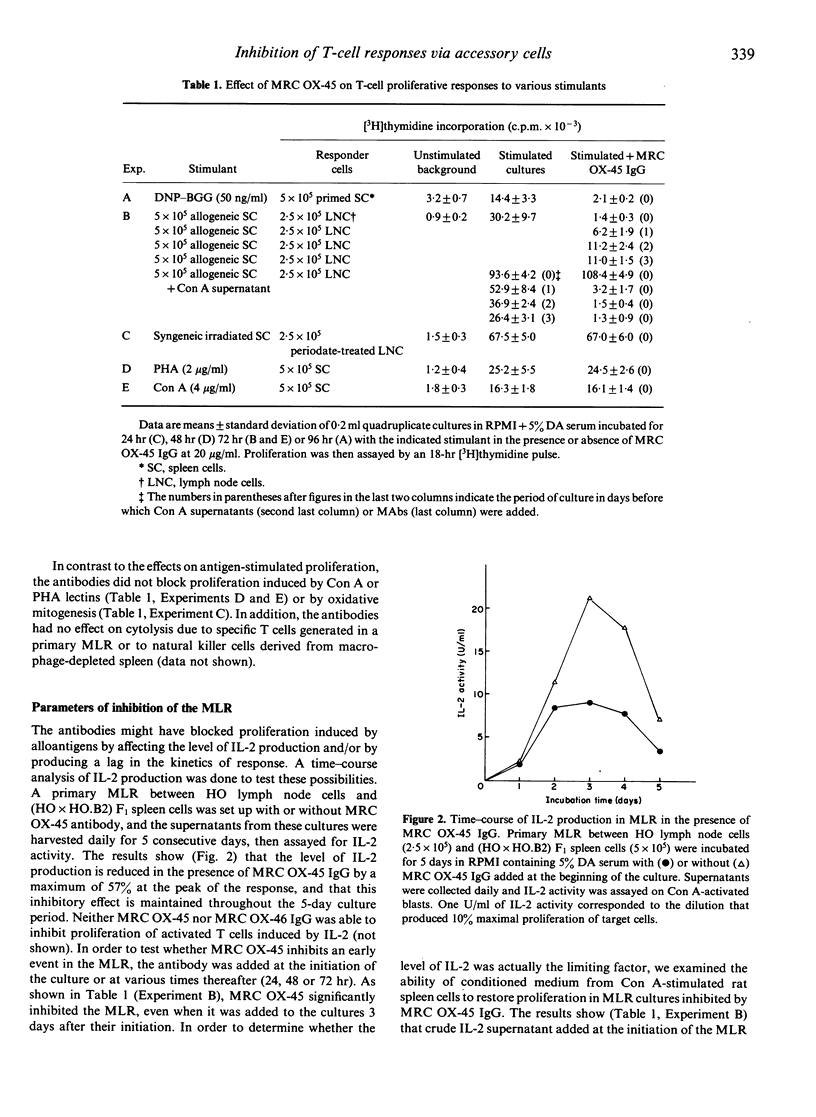
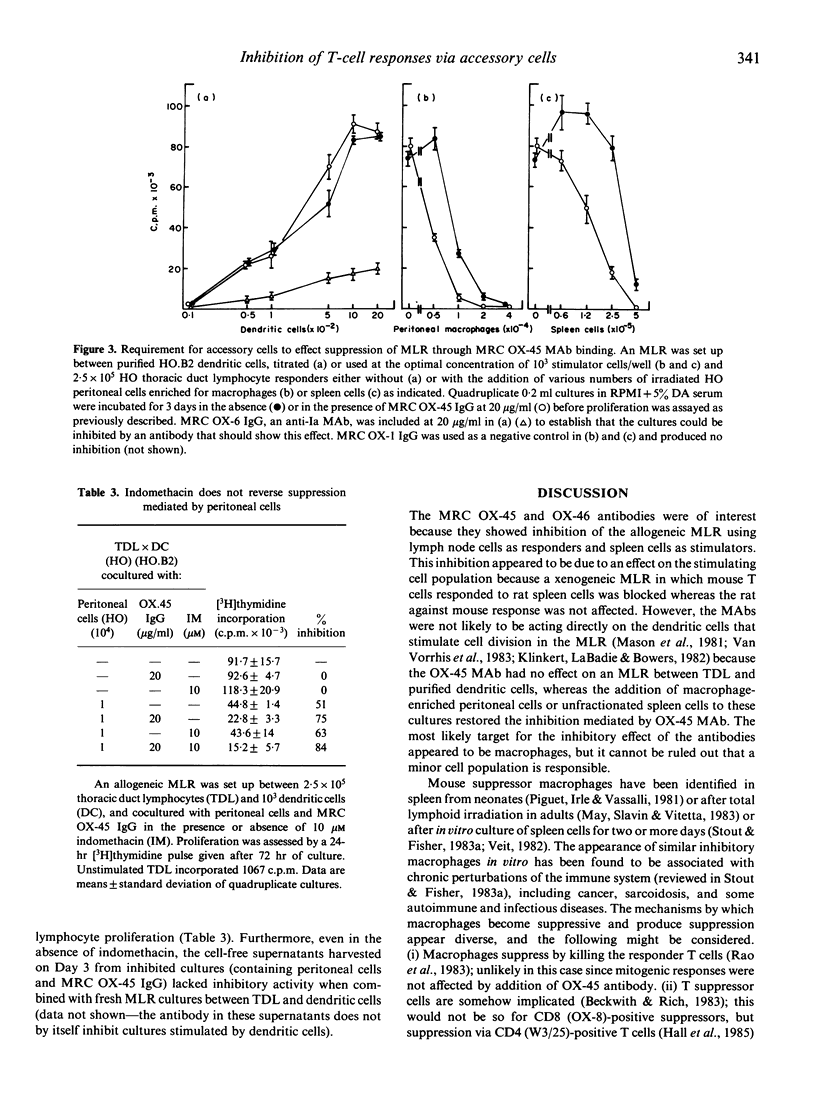
The MRC OX-45 and OX-46 mouse monoclonal antibodies recognize a rat cell surface glycoprotein of 45,000 MW that is present on a wide variety of haematopoietic cells and on endothelial cells. MRC OX-45 IgG or F(ab')2 blocked the primary mixed lymphocyte response (MLR) and the secondary response of T lymphocytes to the soluble antigen DNP-BGG. In contrast, the antibodies had no effect on the cytotoxic activity of specific (CTL) or non-specific (NK) killer cells or on proliferative responses stimulated by lectins or oxidative mitogenesis. The inhibitory effect was at the level of stimulator cells rather than responders since mouse anti-rat xenogeneic MLRs were inhibited but rat anti-mouse responses were unaffected. However, the effect was not a direct one because inhibition was seen when irradiated spleen cells were used as stimulators but not when cell populations highly enriched for dendritic cells were used. In the latter case, inhibition potentiated by antibody could be restored if a peritoneal cell population enriched for macrophages was added back to the cultures. The inhibitory effects of these monoclonal antibodies seem most likely to be due to potentiation of nonspecific suppression by macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith M., Rich S. Suppressive mechanisms in alloantigen-induced T cell responses. J Exp Med. 1983 Dec 1;158(6):1853–1867. doi: 10.1084/jem.158.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Green J. R. Generation of cytotoxic T cells in the rat mixed lymphocyte reaction is blocked by monoclonal antibody MRC OX-8. Immunology. 1984 Jun;52(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Hall B. M., Jelbart M. E., Gurley K. E., Dorsch S. E. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Mediation of specific suppression by T helper/inducer cells. J Exp Med. 1985 Nov 1;162(5):1683–1694. doi: 10.1084/jem.162.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., Dorf M. E. Functional analysis of cloned macrophage hybridomas. IV. Induction and inhibition of mixed lymphocyte responses. J Immunol. 1985 Jun;134(6):3722–3730. [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. D., Slavin S., Vitetta E. S. A partial characterization of suppressor cells in the spleens of mice conditioned with fractionated total lymphoid irradiation (TLI). J Immunol. 1983 Sep;131(3):1108–1114. [PubMed] [Google Scholar]
- Piguet P. F., Irle C., Vassalli P. Immunosuppressor cells from newborn mouse spleen are macrophages differentiating in vitro from monoblastic precursors. Eur J Immunol. 1981 Jan;11(1):56–61. doi: 10.1002/eji.1830110112. [DOI] [PubMed] [Google Scholar]
- Rao A., Faas S. J., Miller L. J., Riback P. S., Cantor H. Lysis of inducer T cell clones by activated macrophages and macrophage-like cell lines. J Exp Med. 1983 Oct 1;158(4):1243–1258. doi: 10.1084/jem.158.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadecker M. J., Calderon J., Karnovsky M. L., Unanue E. R. Synthesis and release of thymidine by macrophages. J Immunol. 1977 Nov;119(5):1738–1743. [PubMed] [Google Scholar]
- Stout R. D., Fisher M. Suppression of lymphocyte proliferative responses: characterization of the suppressor and kinetics of suppression. J Immunol. 1983 Apr;130(4):1573–1579. [PubMed] [Google Scholar]
- Stout R. D., Fisher M. Suppression of lymphocyte proliferative responses: demonstration of two stages occurring in the in vitro generation of suppressor macrophages. J Immunol. 1983 Apr;130(4):1580–1585. [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B. C. Immunoregulatory activity of culture-induced suppressor macrophages. Cell Immunol. 1982 Sep 1;72(1):14–27. doi: 10.1016/0008-8749(82)90279-9. [DOI] [PubMed] [Google Scholar]
- Webb M., Mason D. W., Williams A. F. Inhibition of mixed lymphocyte response by monoclonal antibody specific for a rat T lymphocyte subset. Nature. 1979 Dec 20;282(5741):841–843. doi: 10.1038/282841a0. [DOI] [PubMed] [Google Scholar]
- Wilson A., Chen W. F., Scollay R., Shortman K. Semi-automated limit-dilution assay and clonal expansion of all T-cell precursors of cytotoxic lymphocytes. J Immunol Methods. 1982 Aug 13;52(3):283–306. doi: 10.1016/0022-1759(82)90002-3. [DOI] [PubMed] [Google Scholar]



