Abstract
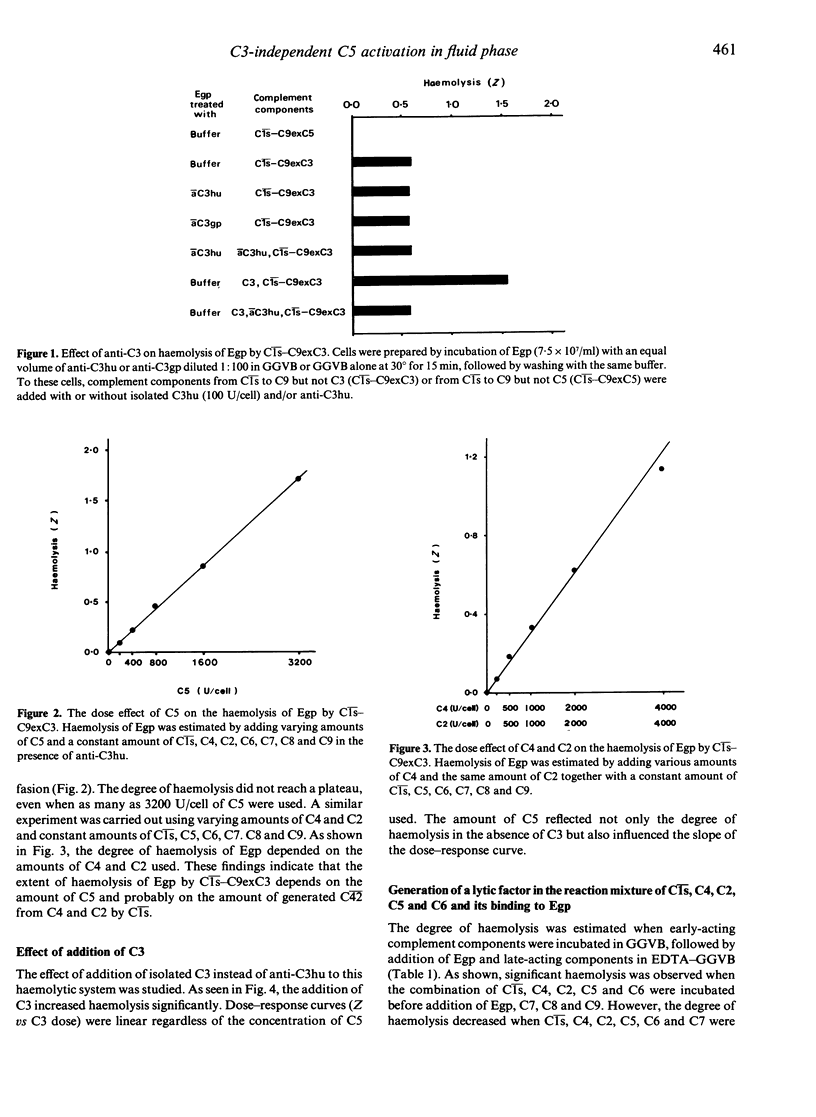
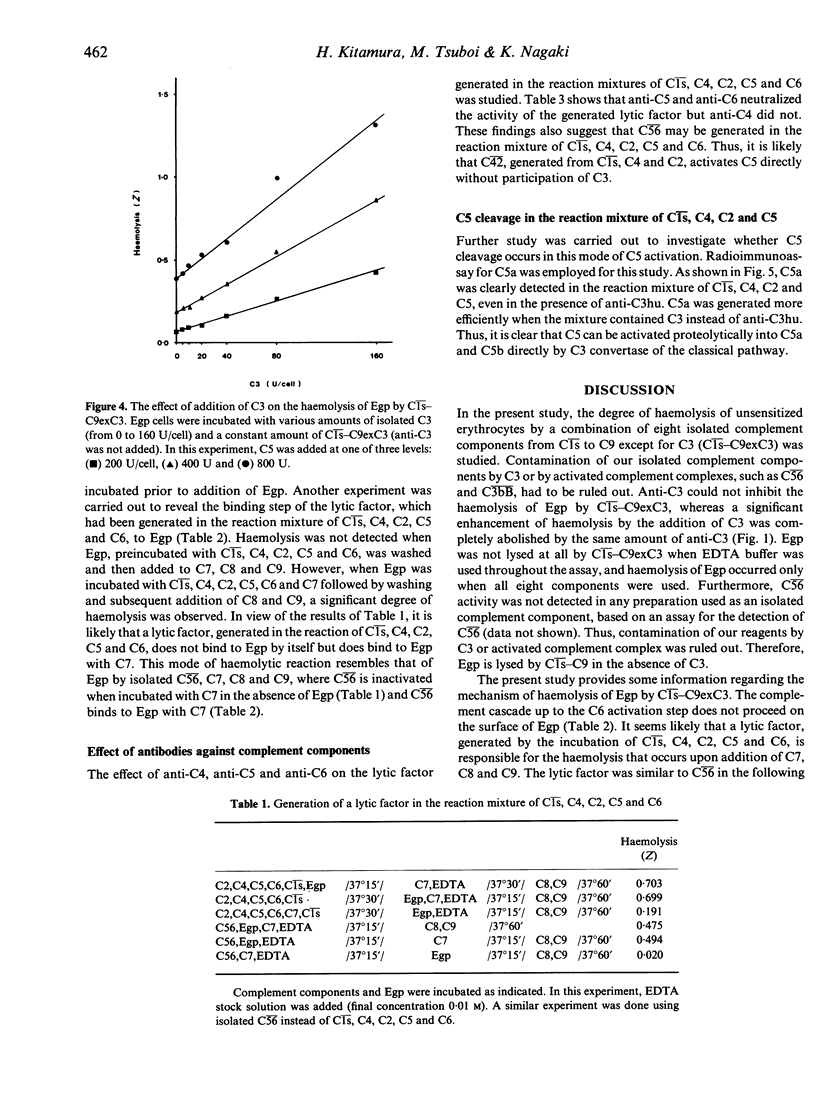
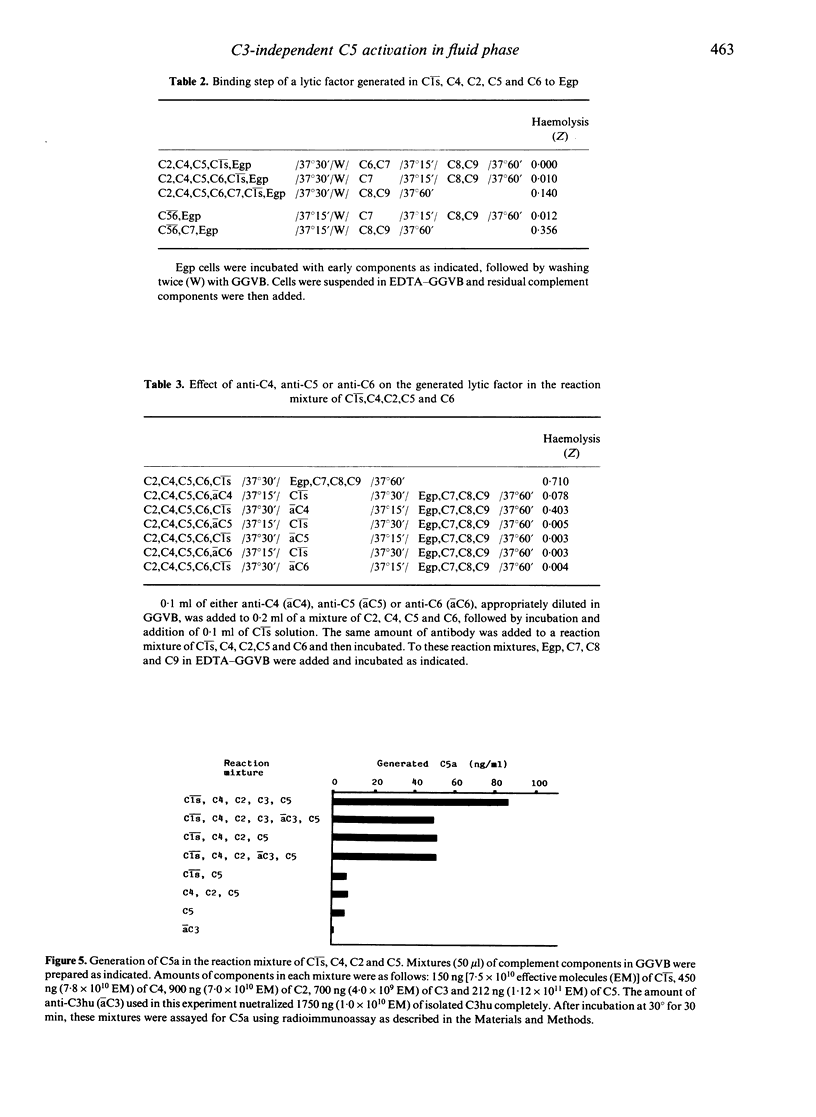
Unsensitized guinea-pig erythrocytes (Egp) were lysed by a combination of eight isolated, human-derived complement components, Cls, C4, C2, C5, C6, C7, C8 and C9 (Cls-C9exC3), even in the presence of anti-C3. It was determined that a factor was generated in the reaction mixture of Cls, C4, C2, C5 and C6, which had a lytic activity against Egp when C7, C8 and C9 were added. The lytic factor was similar to C56 in the following properties: the activity of the lytic factor decreased when incubated with C7 prior to its reaction with Egp, the lytic factor did not bind to Egp by itself but it did bind in the presence of C7, EDTA did not have any inhibitory effect on the lytic factor, and the activity of the lytic factor was lost by treatment with anti-C5 or anti-C6 but not by treatment with anti-C4. Furthermore, C5a, a cleavage product of C5, was clearly detected in the reaction mixture of Cls, C4, C2 and C5. These findings indicate that C5 can be activated proteolytically into C5a and C5b in the fluid phase solely by the classical pathway C3 convertase, C42, without any participation of C3.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 requirements for formation of alternative pathway C5 convertase. J Immunol. 1976 Aug;117(2):630–634. [PubMed] [Google Scholar]
- DiScipio R. G., Smith C. A., Muller-Eberhard H. J., Hugli T. E. The activation of human complement component C5 by a fluid phase C5 convertase. J Biol Chem. 1983 Sep 10;258(17):10629–10636. [PubMed] [Google Scholar]
- Goldlust M. B., Shin H. S., Hammer C. H., Mayer M. M. Studies of complement complex C5b,6 eluted from--EAC-6: reaction of C5b,6 with EAC4b,3b and evidence on the role of C2a and C3b in the activation of C5. J Immunol. 1974 Sep;113(3):998–1007. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadding U., Müller-Eberhard H. J. The ninth component of human complement: isolation, description and mode of action. Immunology. 1969 Jun;16(6):719–735. [PMC free article] [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Mayer M. M., Shin M. L. Activation of the fifth and sixth component of the complement system: similarities between C5b6 and C(56)a with respect to lytic enhancement by cell-bound C3b or A2C, and species preferences of target cell. J Immunol. 1981 Sep;127(3):999–1002. [PubMed] [Google Scholar]
- Inai S., Kitamura H., Hiramatsu S., Nagaki K. Deficiency of the ninth component of complement in man. J Clin Lab Immunol. 1979 Apr;2(1):85–87. [PubMed] [Google Scholar]
- Isenman D. E., Podack E. R., Cooper N. R. The interaction of C5 with C3b in free solution: a sufficient condition for cleavage by a fluid phase C3/C5 convertase. J Immunol. 1980 Jan;124(1):326–331. [PubMed] [Google Scholar]
- Kitamura H., Inai S. Molecular analysis of the reaction of C9 with EAC1-8: reaction of C9 with EAC1-8. J Immunol. 1974 Dec;113(6):1992–2003. [PubMed] [Google Scholar]
- Kitamura H., Matsumoto M., Nagaki K. C3-independent immune haemolysis: haemolysis of EAC14oxy2 cells by C5-C9 without participation of C3. Immunology. 1984 Nov;53(3):575–582. [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Nagaki K., Inai S. Further studies on C9 deficiency. J Clin Lab Immunol. 1981 Jul;6(1):7–11. [PubMed] [Google Scholar]
- Kitamura H., Nishimukai H., Sano Y., Nagaki K. Study on C3-like factor in the serum of a C3-deficient subject. Immunology. 1984 Feb;51(2):239–245. [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Tsuboi M., Nagaki K. C56 formation in the reaction mixture of isolated complement components through the classical complement pathway. Int Arch Allergy Appl Immunol. 1985;78(1):101–107. doi: 10.1159/000233870. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. The complement system. Sci Am. 1973 Nov;229(5):54–66. doi: 10.1038/scientificamerican1173-54. [DOI] [PubMed] [Google Scholar]
- Miyama A., Kato T., Horai S., Yokoo J., Kashiba S. Trypsin-activated complex of human factor B with cobra venom factor (CVF), cleaving C3 and C5 and generating a lytic factor for unsensitized guinea pig erythrocytes. I. Generation of the activated complex. Biken J. 1975 Dec;18(4):193–204. [PubMed] [Google Scholar]
- Nagaki K., Stroud R. M. The relationship of the hemolytic activity of active C'1s to its TAMe esterase action: a new method of purification and assay. J Immunol. 1969 Feb;102(2):421–430. [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. Purification of the sixth and seventh component of human complement without loss of hemolytic activity. J Immunol. 1976 Feb;116(2):263–269. [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Fifth component of human complement: purification from plasma and polypeptide chain structure. Biochemistry. 1979 Apr 17;18(8):1490–1497. doi: 10.1021/bi00575a016. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Takahashi S., Hirose S. Solubilization of antigen-antibody complexes: a new function of complement as a regulator of immune reactions. Prog Allergy. 1980;27:134–166. [PubMed] [Google Scholar]
- Vogel C. W., Smith C. A., Müller-Eberhard H. J. Cobra venom factor: structural homology with the third component of human complement. J Immunol. 1984 Dec;133(6):3235–3241. [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]



