Abstract
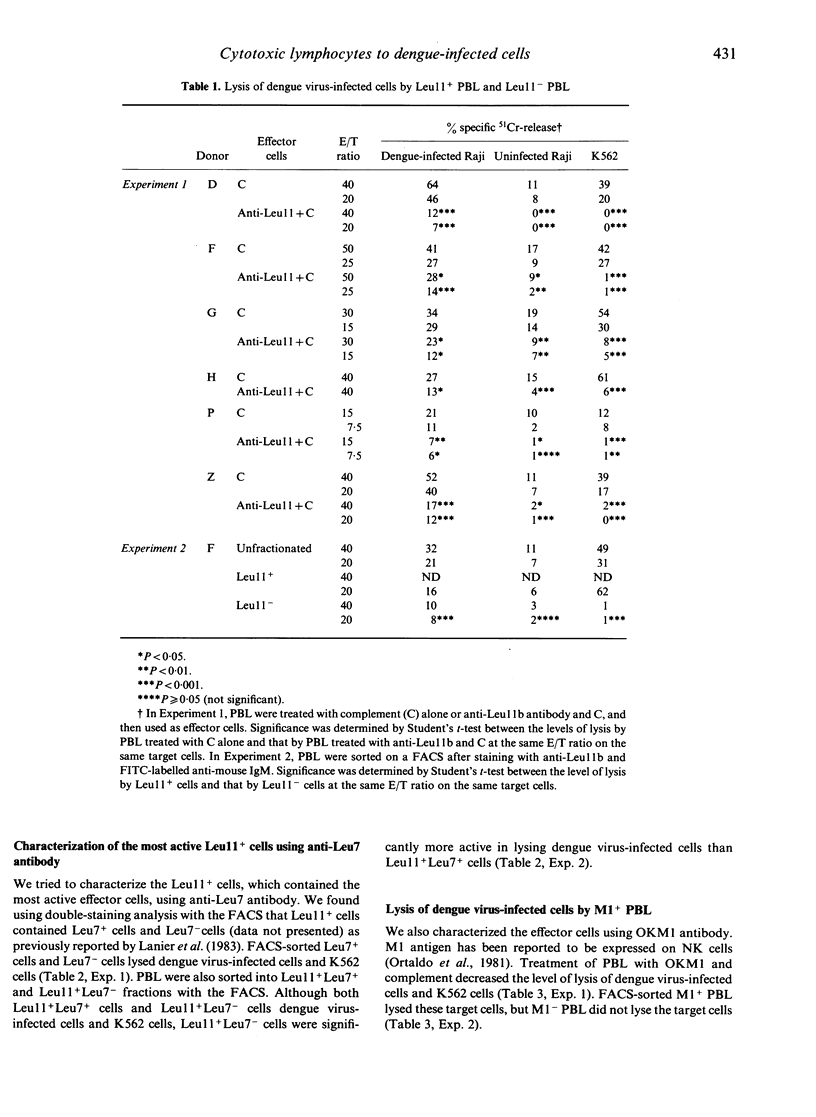
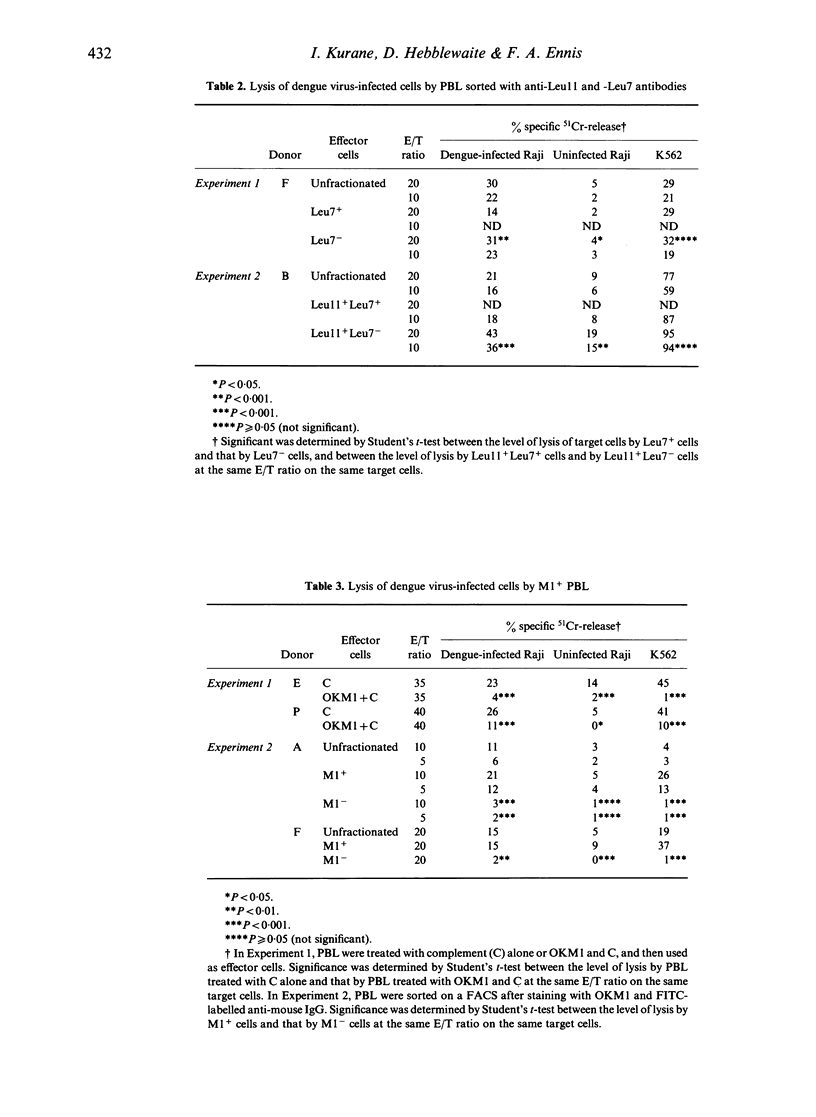
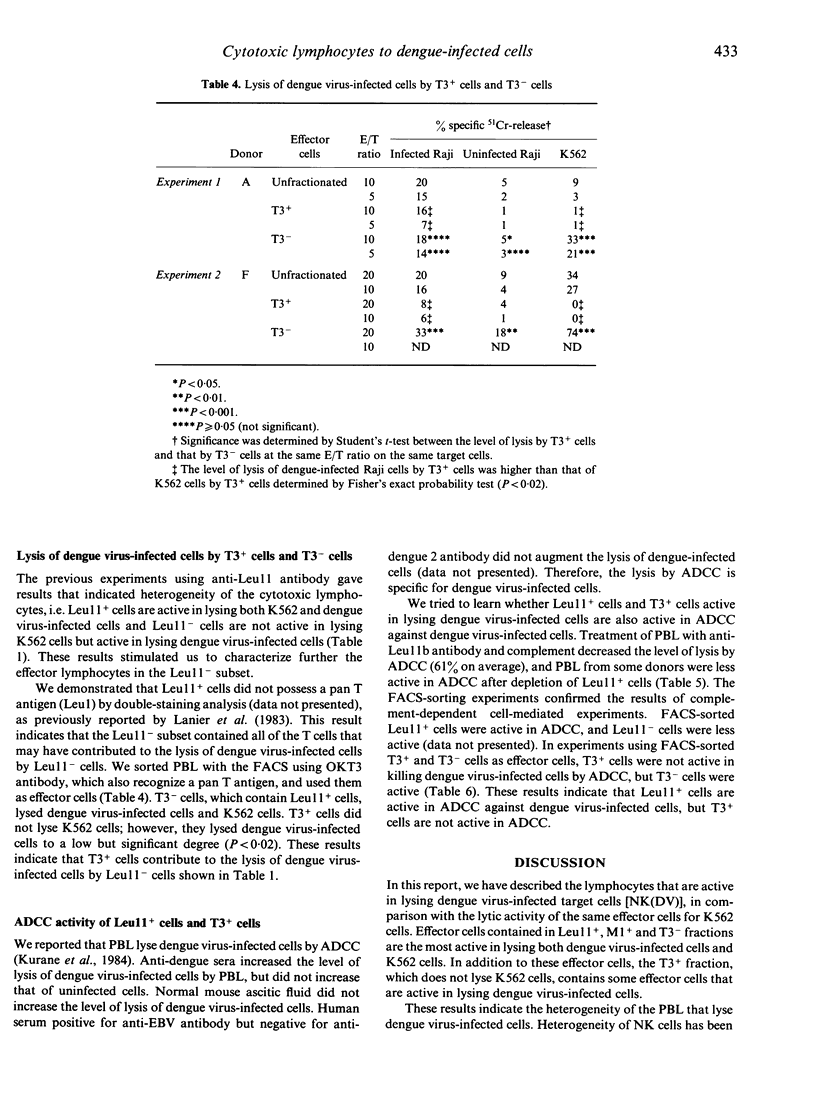
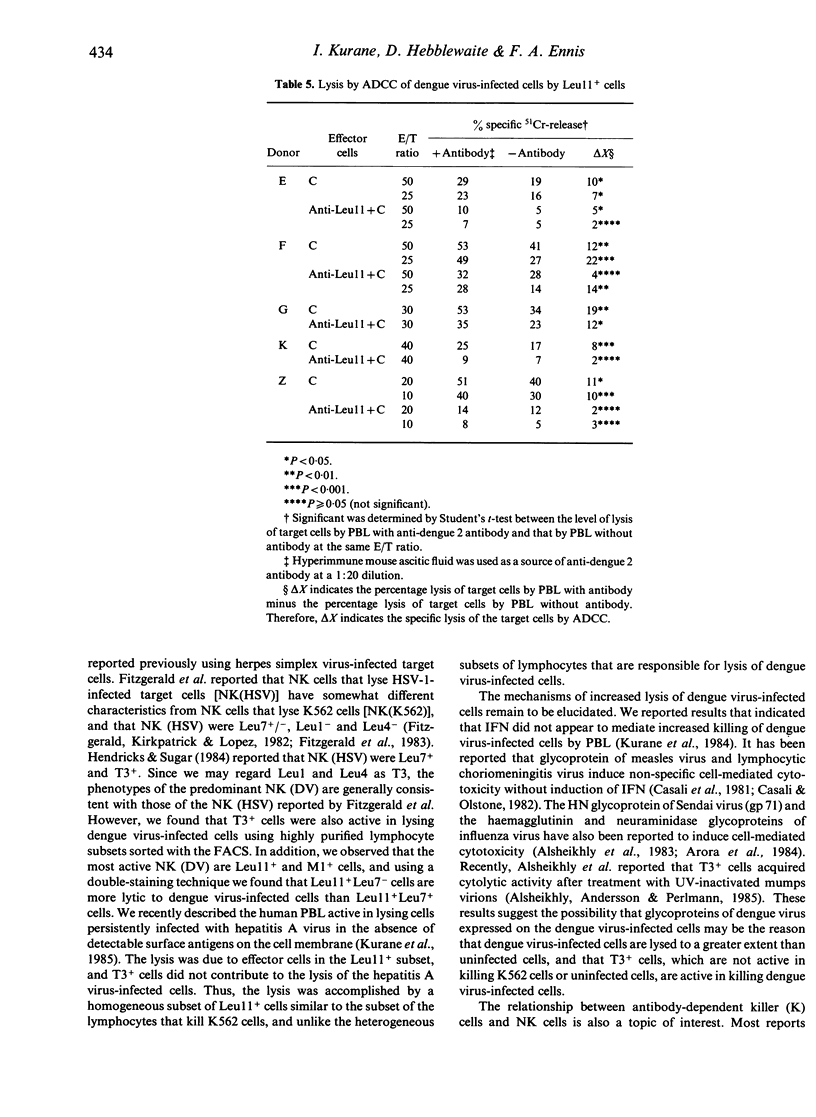
Non-immune human peripheral blood lymphocytes (PBL) lyse dengue virus-infected cells to a greater degree than uninfected cells. In the present study, the PBL active in lysing dengue virus-infected Raji cells are characterized using monoclonal antibodies and are compared to lymphocytes that lyse K562 cells. Leu11+ cells lyse dengue virus-infected cells and K562 cells. Leu11- cells lyse dengue virus-infected cells, but not K562 cells. In the Leu11+ fraction, Leu11+ Leu7- cells are more active than Leu11+ Leu7+ cells in lysing dengue virus-infected cells. T3+ cells also lyse dengue virus-infected cells, but they do not lyse K562 cells. T3- cells lyse both target cells. These results, along with the observation that Leu11+ cells and T3+ cells are different subsets of PBL, indicate that the PBL that are active in lysing dengue virus-infected cells are heterogeneous and are contained in Leu11+ and T3+ subsets. Leu11+ cells are more active than T3+ cells. Leu11+ cells are active in lysing dengue virus-infected cells by antibody-dependent cell-mediated cytotoxicity, whereas T3+ cells are not active.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Alsheikhly A. R., Andersson T., Perlmann P. Virus-dependent cellular cytotoxicity in vitro. Mechanisms of induction and effector cell characterization. Scand J Immunol. 1985 Apr;21(4):329–335. doi: 10.1111/j.1365-3083.1985.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Alsheikhly A., Orvell C., Härfast B., Andersson T., Perlmann P., Norrby E. Sendai-virus-induced cell-mediated cytotoxicity in vitro. The role of viral glycoproteins in cell-mediated cytotoxicity. Scand J Immunol. 1983 Feb;17(2):129–138. doi: 10.1111/j.1365-3083.1983.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Arora D. J., Houde M., Justewicz D. M., Mandeville R. In vitro enhancement of human natural cell-mediated cytotoxicity by purified influenza virus glycoproteins. J Virol. 1984 Dec;52(3):839–845. doi: 10.1128/jvi.52.3.839-845.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. P., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. IV. Natural killing and antibody-dependent cellular cytotoxicity can be mediated by the same human effector cell as determined by the two-target conjugate assay. J Immunol. 1982 Nov;129(5):2260–2265. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Casali P., Oldstone M. B. Mechanisms of killing of measles virus-infected cells by human lymphocytes: interferon associated and unassociated cell-mediated cytotoxicity. Cell Immunol. 1982 Jul 1;70(2):330–344. doi: 10.1016/0008-8749(82)90334-3. [DOI] [PubMed] [Google Scholar]
- Casali P., Sissons J. G., Buchmeier M. J., Oldstone M. B. In vitro generation of human cytotoxic lymphocytes by virus. Viral glycoproteins induce nonspecific cell-mediated cytotoxicity without release of interferon. J Exp Med. 1981 Sep 1;154(3):840–855. doi: 10.1084/jem.154.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday C. C., Brandt W. E., McCown J. M., Russell P. K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981 May;32(2):469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol. 1981 Nov;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Hendricks R. L., Sugar J. Lysis of herpes simplex virus-infected targets. II. Nature of the effector cells. Cell Immunol. 1984 Feb;83(2):262–270. doi: 10.1016/0008-8749(84)90305-8. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Kurane I., Binn L. N., Bancroft W. H., Ennis F. A. Human lymphocyte responses to hepatitis A virus-infected cells: interferon production and lysis of infected cells. J Immunol. 1985 Sep;135(3):2140–2144. [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Warner N. L., Babcock G. F. A human natural killer cell-associated antigen defined by monoclonal antibody anti-Leu (NKP-15): functional and two-color flow cytometry analysis. J Leukoc Biol. 1984 Jan;35(1):11–17. doi: 10.1002/jlb.35.1.11. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Neville M. E. Human killer cells and natural killer cells: distinct subpopulations of Fc receptor-bearing lymphocytes. J Immunol. 1980 Dec;125(6):2604–2609. [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]



