Abstract
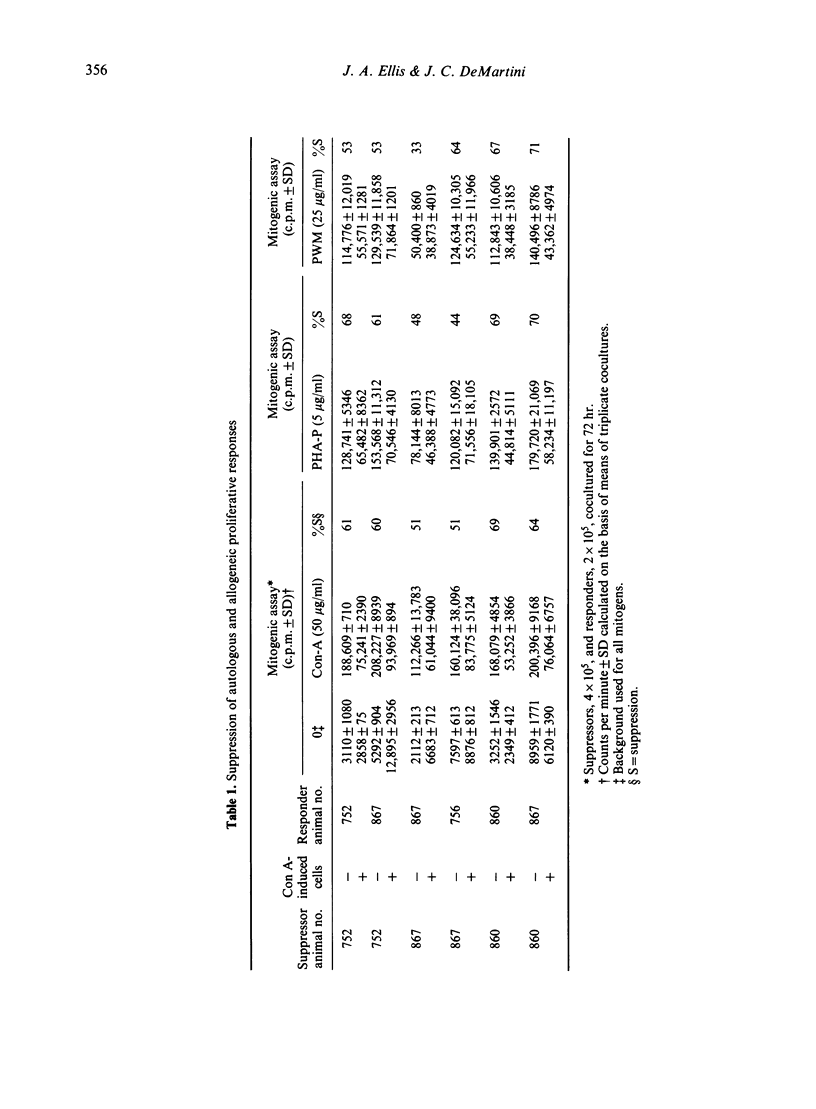
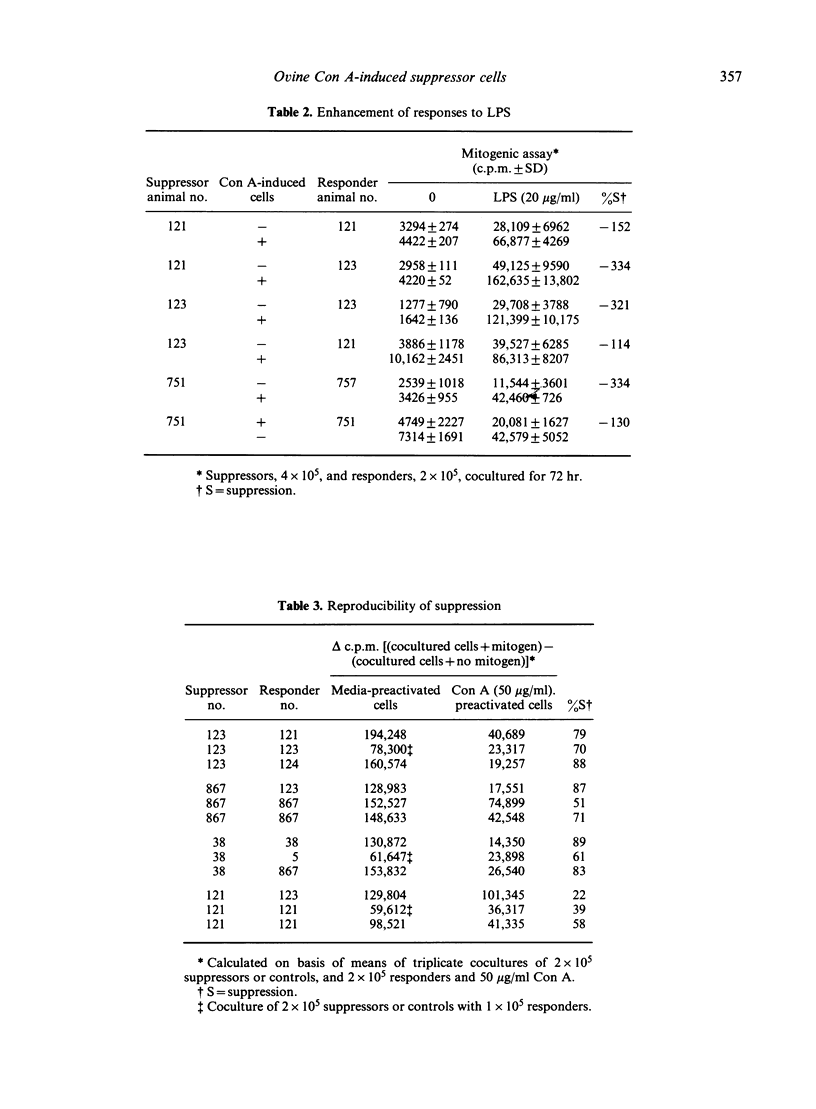
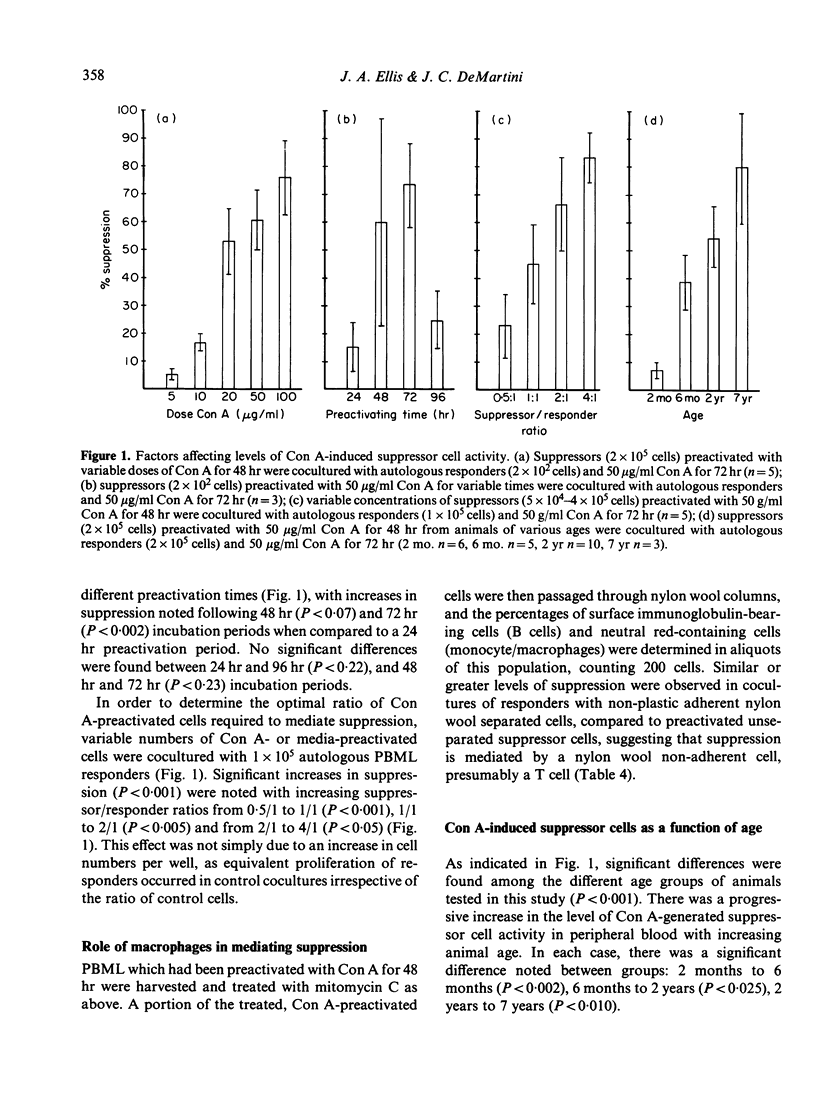
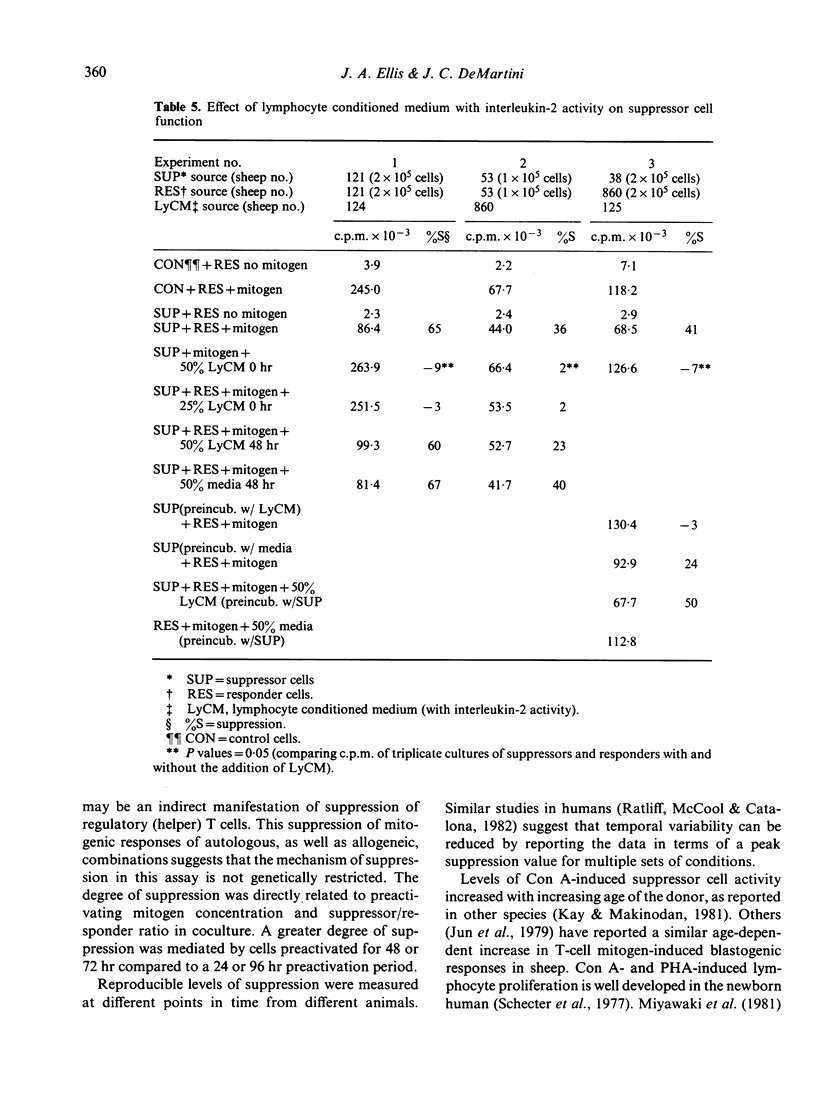
Preactivation of ovine peripheral blood and lymph node mononuclear cells with mitogenic doses of concanavalin A (Con A) induced cells which suppressed mitogen-stimulated proliferative responses of untreated autologous and allogeneic responder cells. The degree of suppression varied with preactivating doses of Con A, length of preactivation time, and ratio of preactivated to responder cells. The role of macrophages in generation of suppressor cells was not evaluated. However, macrophages were not required to mediate suppression in cocultures, as lymphoblasts depleted of macrophage by plastic adherence and nylon wool columns mediated equal, and often greater, suppression than unseparated, preactivated cells. Suppressor cell activity in peripheral blood increased from the neonatal period to adulthood. Supernatants from Con A preactivated cell cultures with detectable interleukin-2 activity abrogated suppression when added at 0 and 24 hr of the 72 hr coculture period, suggesting that Con A-induced suppressor cells exert their function by decreasing available levels of IL-2 in the cocultures.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells C., Wells P. W. In vitro stimulation of ovine lymphocytes by various mitogens. Res Vet Sci. 1977 Jul;23(1):84–86. [PubMed] [Google Scholar]
- Cahill R. N., Poskitt D. C., Frost H., Julius M. H., Trnka Z. Behaviour of sheep-immunoglobulin-bearing and non-immunoglobulin-bearing lymphocytes isolated by nylon wool columns. Int Arch Allergy Appl Immunol. 1978;57(1):90–96. doi: 10.1159/000232088. [DOI] [PubMed] [Google Scholar]
- De Martini J. C., Fiscus S. A., Pearson L. D. Macrophages in efferent lymph of sheep and their role in lectin-induced lymphocyte blastogenesis. Int Arch Allergy Appl Immunol. 1983;72(2):110–115. doi: 10.1159/000234851. [DOI] [PubMed] [Google Scholar]
- Eife R. F., Eife G., August C. S., Kuhre W. L., Staehr-Johansen K. Lymphotoxin production and blast cell transformation by cord blood lymphocytes: dissociated lymphocyte function in newborn infants. Cell Immunol. 1974 Dec;14(3):435–442. doi: 10.1016/0008-8749(74)90194-4. [DOI] [PubMed] [Google Scholar]
- Farrant J., Newton C. Relative ability to provide help: an explanation for Con A-induced suppression. Clin Exp Immunol. 1981 Sep;45(3):504–513. [PMC free article] [PubMed] [Google Scholar]
- Fernandez L. A., Macsween J. M. Generation of suppressor cells by concanavalin A: a new perspective. J Immunol. 1980 Jul;125(1):267–269. [PubMed] [Google Scholar]
- Fineman S. M., Mudawwar F. B., Geha R. S. Characteristics and mechanisms of action of the concanavalin A-activated suppressor cell in man. Cell Immunol. 1979 Jun;45(1):120–132. doi: 10.1016/0008-8749(79)90367-8. [DOI] [PubMed] [Google Scholar]
- Fiscus S. A., DeMartini J. C., Pearson L. D. Mitogen-induced blastogenesis of peripheral blood and efferent lymph lymphocytes from sheep. Am J Vet Res. 1982 Apr;43(4):629–632. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Fleisher T. A., Waldmann T. A. Soluble suppressor supernatants elaborated by concanavalin A-activated human mononuclear cells. I. Characterization of a soluble suppressor T cell proliferation. J Immunol. 1981 Mar;126(3):1185–1191. [PubMed] [Google Scholar]
- Herscowitz H. B., Sakane T., Steinberg A. D., Green I. Heterogeneity of human suppressor cells induced by concanavalin A as determined in simultaneous assays of immune function. J Immunol. 1980 Mar;124(3):1403–1410. [PubMed] [Google Scholar]
- Jun M. H., Johnson R. H., Mills J. M. In vitro response of lymphocytes of normal and ovine squamous cell carcinoma-bearing sheep to phytomitogens and tumour extracts. Res Vet Sci. 1979 Sep;27(2):144–148. [PubMed] [Google Scholar]
- Kay M. M., Makinodan T. Relationship between aging and immune system. Prog Allergy. 1981;29:134–181. [PubMed] [Google Scholar]
- Langweiler M., Cockerell G. L. Generation of concanavalin A-induced suppressor cells in the cat. Int Arch Allergy Appl Immunol. 1982;69(2):148–155. doi: 10.1159/000233163. [DOI] [PubMed] [Google Scholar]
- Lederman M. M., Ellner J. J., Rodman H. M. Generation of a soluble immune response suppressor factor (IRSF) by unstimulated leukocytes of healthy subjects. Clin Exp Immunol. 1981 Jul;45(1):191–200. [PMC free article] [PubMed] [Google Scholar]
- Maca R. D., Bonnard G. D., Herberman R. B. The suppression of mitogen- and alloantigen-stimulated peripheral blood lymphocytes by cultured human T lymphocytes. J Immunol. 1979 Jul;123(1):246–251. [PubMed] [Google Scholar]
- Mawas C., Charmot D., Comoy A., Sasportes M. Suppression of generation of human cytotoxic effectors by lectins or lectin-activated peripheral blood lymphocytes. Eur J Immunol. 1977 Jan;7(1):11–15. doi: 10.1002/eji.1830070104. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Moriya N., Nagaoki T., Taniguchi N. Maturation of B-cell differentiation ability and T-cell regulatory function in infancy and childhood. Immunol Rev. 1981;57:61–87. doi: 10.1111/j.1600-065x.1981.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Möller G. T cell growth factor abrogates concanavalin A-induced suppressor cell function. J Exp Med. 1981 May 1;153(5):1360–1365. doi: 10.1084/jem.153.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff T. L., McCool R. E., Catalona W. J. Optimum conditions for the reproducible measurement of concanavalin A-activated suppressor cell activity. Transplantation. 1982 May;33(5):505–509. doi: 10.1097/00007890-198205000-00008. [DOI] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. 3. Suppression of plaque-forming cell responses in vitro by supernatant fluids from concanavalin A-activated spleen cell cultures. J Immunol. 1974 Apr;112(4):1360–1368. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollwagen F. M., Stutman O. Culture-generated suppressor cells: evidence for an adherent cell component. Cell Immunol. 1981 Nov 1;64(2):371–380. doi: 10.1016/0008-8749(81)90488-3. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Schechter B., Handzel Z. T., Altman Y., Nir E., Levin S. Cellular immunity in newborn infants and children: stimulation of lymphocyte protein synthesis as a measure of immune competence. Clin Exp Immunol. 1977 Mar;27(3):478–484. [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]


