Abstract
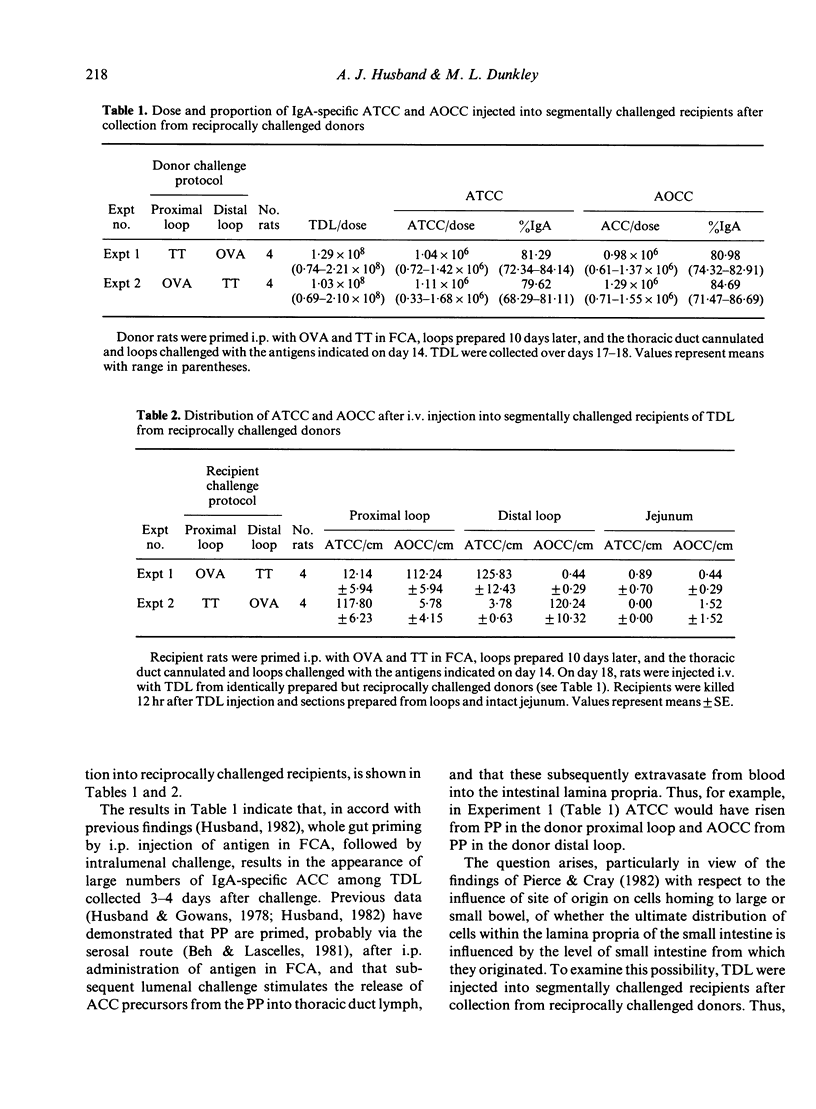
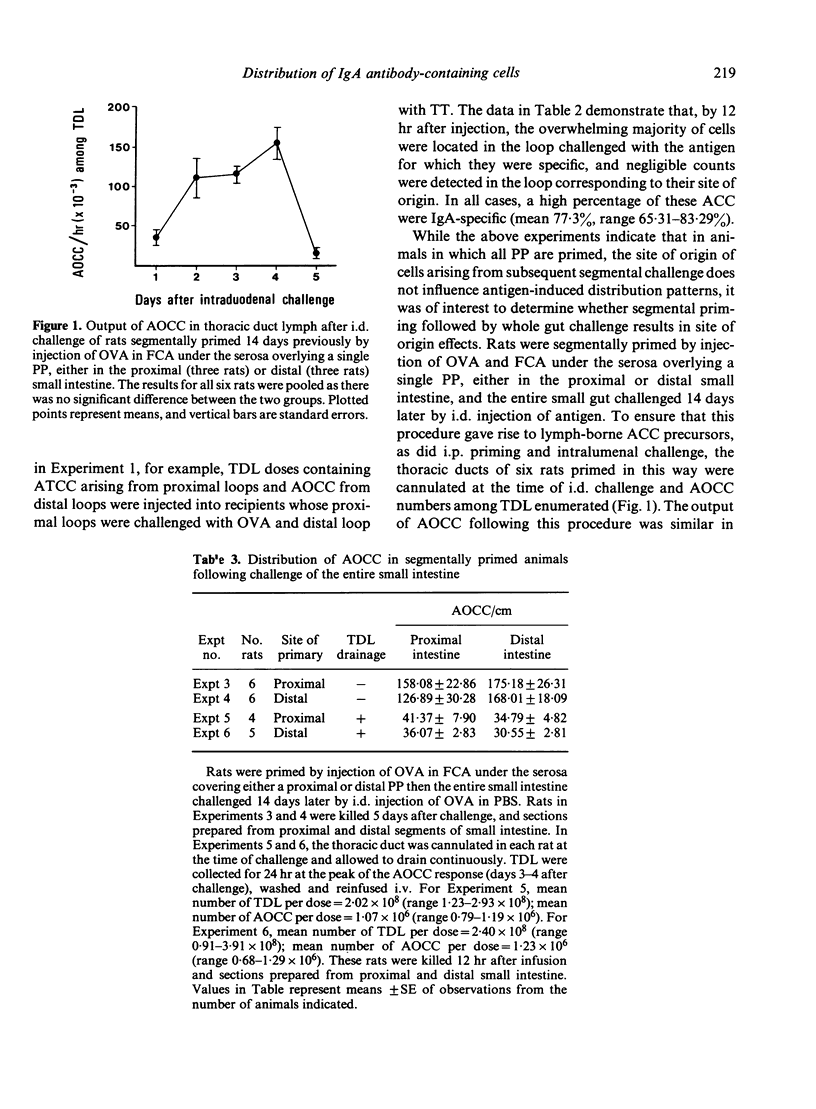
Previous experiments (Husband, 1982) indicated that IgA-specific antibody-containing cells (ACC), appearing among thoracic duct lymphocytes (TDL) following challenge of intestinal segments of i.p. primed rats, display antigen-dependent distribution in the small intestine by 12 hr after their i.v. injection into autologous recipients. Data are presented here which demonstrate that ACC among TDL collected from rats bearing double Thiry-Vella loops challenged with two different antigens still appear almost exclusively in loops immunized with the antigen corresponding to their antibody specificity, even when injected into recipients in which immunized loops were prepared from different levels of the small intestine to that of the donors. These experiments indicate that, in animals given whole gut priming (by i.p. immunization) and segmental challenge (by lumenal challenge of isolated loops), the site of origin of IgA-ACC precursors does not influence antigen-induced distribution patterns. To determine whether segmental priming followed by whole gut challenge results in site of origin effects, rats were primed by subserosal immunization of a single Peyer's patch, either in the proximal or distal intestine, and then challenged intraduodenally. The ultimate IgA-ACC distribution was similar, regardless of the site of priming. These results indicate that, in a model employing priming with antigen in Freund's complete adjuvant, there is no site of origin effect on ultimate IgA plasma cell location within the small intestine, whether whole gut priming and segmental challenge or segmental priming and whole gut challenge, are used.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J., Lascelles A. K. The effect of route of administration of antigen on the antibody-containing cell response in lymph of sheep. Immunology. 1981 Apr;42(4):577–582. [PMC free article] [PubMed] [Google Scholar]
- Bennell M. A., Husband A. J. Study of immunisation regimes for the stimulation of local immunity in the pig intestine. Res Vet Sci. 1981 May;30(3):353–356. [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Husband A. J. An immunisation model for the control of infectious enteritis. Res Vet Sci. 1978 Sep;25(2):173–177. [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J. Intestinal immunity following a single intraperitoneal immunisation in lambs. Vet Immunol Immunopathol. 1980 Aug;1(3):277–286. doi: 10.1016/0165-2427(80)90028-8. [DOI] [PubMed] [Google Scholar]
- Husband A. J. Kinetics of extravasation and redistribution of IgA-specific antibody-containing cells in the intestine. J Immunol. 1982 Mar;128(3):1355–1359. [PubMed] [Google Scholar]
- Husband A. J., Monié H. J., Gowans J. L. The natural history of the cells producing IgA in the gut. Ciba Found Symp. 1977 Apr 26;(46):29–54. doi: 10.1002/9780470720288.ch3. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Seaman J. T. Vaccination of piglets against Escherichia coli enteritis. Aust Vet J. 1979 Sep;55(9):435–436. doi: 10.1111/j.1751-0813.1979.tb05601.x. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Koster F. T. Priming and suppression of the intestinal immune response to cholera toxoid/toxin by parenteral toxoid in rats. J Immunol. 1980 Jan;124(1):307–311. [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]


