Abstract
Guy's 13 is a mouse monoclonal antibody which recognizes streptococcal antigen I/II (SA I/II), a major cell surface glycoprotein of Streptococcus mutans. In a number of clinical trials, this antibody has been shown to prevent colonization in the human oral cavity. The aim of this study was to identify the SA I/II epitope recognized by Guy's 13. The data suggest that the epitope is conformational, delimited by two noncontiguous regions of the antigen: residues 45 to 457, within the N-terminal half of SA I/II, and residues 816 to 983, within the C-terminal half. In fluid-phase immunoassays a strict requirement for the simultaneous presence of both regions was demonstrated for antibody binding. Furthermore, these two regions of SA I/II were shown to have the ability to interact with each other in the absence of Guy's 13 antibody, suggesting that the normal conformation of SA I/II might be determined by the interaction of these two regions.
Initial adhesion to the tooth surface by Streptococcus mutans, the main etiological agent of dental caries (9), is mediated by a cell-surface protein variously termed streptococcal antigen I/II (SA I/II) (32), antigen B (34), P1 (6), or PAc (29). The spaP gene encoding SA I/II from S. mutans NG5 has been cloned (16) and sequenced (11, 12). In common with the other members of the antigen I/II family, SA I/II contains a region (residues 121 to 447) consisting of alanine-rich repeats (the A-region) and a proline-rich repeat region (residues 840 to 983) known as the P-region (10).
Antibodies directed against SA I/II inhibit adhesion in vitro (2) and prevent oral colonization in vivo (17, 22). The mouse monoclonal antibody (MAb) Guy's 13, which specifically recognizes SA I/II of S. mutans and Streptococcus sobrinus (38), has been used successfully to prevent S. mutans colonization and the development of dental caries in nonhuman primates (17) and prevents bacterial colonization in human clinical trials (20, 21).
Immunoblotting of SA I/II under denaturing conditions (25) suggested that the epitope for Guy's 13 might be nonconformational. However, when a gene fragment phage display library of the spaP gene was constructed and panned against Guy's 13 in an attempt to delineate the Guy's 13 epitope, none of the enriched clones showed specific binding to Guy's 13 (data not presented). Phage display of random hexamer peptides with the fUSE phage display system (37) was also employed in an effort to delineate the Guy's 13 epitope. However, following panning against Guy's 13 immunoglobulin G (IgG), none of the enriched phage contained sequences which had homology to SA I/II (C. G. Kelly, unpublished).
The expression of Guy's 13 antibody in transgenic plants (18, 23) has led to its potential application in passive immunotherapy for the prevention of dental caries (14, 19). The ability of this antibody to recognize SA I/II homologues from a number of streptococcal species (38) underlines the importance of understanding the mechanism of Guy's 13-mediated prevention of S. mutans colonization, particularly in relation to the molecular interaction between antibody and antigen (SA I/II). The aims of this work were to delineate the Guy's 13 epitope by cloning, expression, and immunoblotting of progressively smaller fragments of the spaP gene. The nature of the Guy's 13 epitope was also investigated using direct and inhibition-based enzyme-linked immunosorbent assays (ELISAs). This work established that the Guy's 13 epitope is conformational, being assembled from two noncontiguous regions of SA I/II, and that these two regions are able to interact with each other.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli HB2151 was from Pharmacia Biotech. E. coli BL21(DE3)pLysS was from Novagen. S. mutans Guy's c strain (serotype c) is a clinical isolate (38).
Antibodies.
Mouse MAb Guy's 13 (IgG1κ) was purified from ascites fluid by protein A affinity chromatography by Biogenesis, Poole, United Kingdom. The antibody was stored at −20°C in 0.05 M boric acid (pH 8.3 with NaOH)-50% (vol/vol) glycerol. Mouse anti-E tag antibody was from Amersham-Pharmacia. Isotype-matched (IgG1κ) control murine antibody (MOPC 31C) was purchased from Sigma. Detection of murine antibodies was achieved with a 1/1,000 dilution of an alkaline phosphatase (AP)-conjugated goat anti-mouse IgG antibody (Sigma) in immunoblotting experiments or with a 1/2,500 dilution of a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibody (The Binding Site) in ELISAs.
Preparation of SA I/II.
SA I/II was prepared from S. mutans Guy's strain (serotype c) as described previously (33).
Vectors.
Expression vector pScFvΔ is a derivative of the pCANTAB 5 E phagemid display vector (Amersham-Pharmacia). The cloning of a Guy's 13 single-chain Fv (scFv) gene into pCANTAB 5 E resulted in the introduction of a unique NcoI site just downstream of the vector-encoded SfiI site. This plasmid was further modified by deleting a PstI fragment of the scFv, resulting in the pScFvΔ plasmid. The vector, digested with NcoI and NotI, was used for cloning fragments of the spaP gene. Expression is controlled by the lac promoter.
Expression vector pEXssΔ3 is based on pET-32a(+) (Novagen). The vector was constructed by replacing pET-32a(+) sequences between the NcoI and HindIII sites with the open reading frame of pScFvΔ (i.e., signal sequence-scFvΔ-E tag). Thus, spaP gene fragments can also be cloned using the NcoI and NotI restriction sites. Expression from pEXssΔ3 is controlled by the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible T7lac promoter.
Both vectors encode the 13-amino-acid E tag peptide sequence (36) located C terminal to the cloned fragments. In the nonrecombinant vectors the E tag is out of frame with respect to the initiation codon. Cloning of spaP gene fragments restores the reading frame, and the recombinant polypeptide is produced as a fusion protein with a C-terminal E tag.
Cloning and expression of recombinant spaP gene fragments.
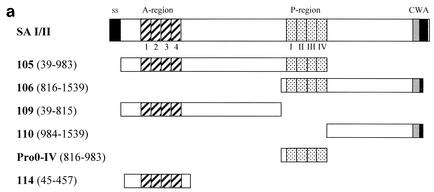
PCR was used to amplify specific regions of the spaP gene of S. mutans Guy's c strain (Table 1). Genomic DNA of S. mutans was isolated according to the method of Bollet et al. (1). Sequences of the oligonucleotide primers used are shown (Table 2) as well as the residues encoded by each derived clone. Figure 1a shows the residues encoded by each clone in diagrammatic form. The products of the PCRs were digested with NcoI-NotI and cloned into pScFvΔ. For some clones, expression from pScFvΔ resulted in poor levels of recombinant protein. In these situations, the amplicon was cloned into pEXssΔ3.
TABLE 1.
Nomenclature of cloned fragments of the spaP genea
| PCR clone | 5′ primer | 3′ primer | Duration of PCR condition
|
Amino acids of SA I/II expressed by cloneb | ||
|---|---|---|---|---|---|---|
| Denaturation (94°C) | Annealing (50°C) | Extension (72°C) | ||||
| 105 | SA-D39-5′ | SA-I983-3′ | 10 s | 10 s | 5 min | 39-983 |
| 106 | SA-G816-5′ | SA-M1539-3′ | 10 s | 10 s | 5 min | 816-1539 |
| 109 | SA-D39-5′ | SA-N815-3′ | 10 s | 10 s | 5 min | 39-815 |
| 110 | SA-P984-5′ | SA-M1539-3′ | 10 s | 10 s | 5 min | 984-1539 |
| Pro0-IV | SA-G816-5′ | SA-1983-3′ | 10 s | 10 s | 1 min | 816-983 |
| 114 | SA-S45-5′ | SA-A457-3′ | 10 s | 10 s | 1 min | 45-457 |
Cloning of the spaP gene was performed by PCR using the indicated primers. Amplifications were performed in a Techne Progene thermal cycler in the presence of 2.5 mM MgCl2 and deoxyribonucleoside triphosphates (0.25 mM). Taq polymerase (2.5 U per reaction mixture; Roche) was added at 94°C, and the reaction was cycled 12 times at 94°C for 10 s; 72°C (−2°C per cycle) for 10 s; and 72°C for 5 minutes, which was followed by 30 cycles under the conditions indicated in the table. The amplicons were cloned into pScFvΔ and/or pEXssΔ3 expression vectors.
Residue numbering from SA I/II of S. mutans NG5.
TABLE 2.
Cloning of spaP gene fragmentsa
| Primer | Sequenced | Nucleotide coordinates |
|---|---|---|
| 5′b | ||
| SA-D39-5′ | 5′-tgtagcGgCCcaGCCggCCAtGgccgatgaaacgaccactactagt-3′ | 189-234 |
| SA-S45-5′ | 5′-aaacgaccaTGGcCagtgatgtagatactaaagta-3′ | 218-252 |
| SA-G816-5′ | 5′-CTAtcCAtGGCCggtaaaatccgtgcggttaa-3′ | 2533-2564 |
| SA-P984-5′ | 5′-cacctccCATGGCCccaactgttcatttccattac-3′ | 3035-3069 |
| 3′c | ||
| SA-A457-3′ | 5′-tatttggcGGCCGctgcttgatacttagcaagttt-3′ | 1450-1484 |
| SA-N815-3′ | 5′-gcacggGCGGCCGcatttaatgaataccaaatatt-3′ | 2524-2558 |
| SA-I983-3′ | 5′-GCaCCTGcGgCCgCtatagatggaggtgttggaa-3′ | 3029-3062 |
| SA-M1539-3′ | 5′-ataccaGCGGCCgCcatataagcattgtttgttact-3′ | 4695-4730 |
Oligonucleotide primers used for the amplification of regions of the spaP gene are shown. Primer sequences were based on the nucleotide sequence of the spaP gene from S. mutans NG5 (12) (GenBank accession number X17390).
Primers which bind to the noncoding strand (5′ primers).
Primers which bind to the coding strand (3′ primers).
Primer nucleotides which differ from the spaP gene template are shown in uppercase letters. The NcoI and NotI restriction sites are shown in boldface type.
FIG. 1.
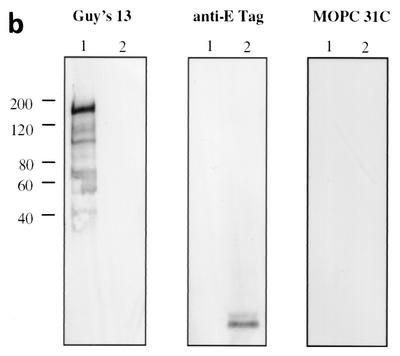
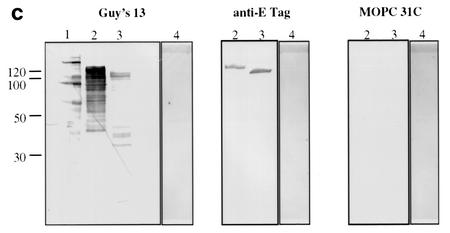
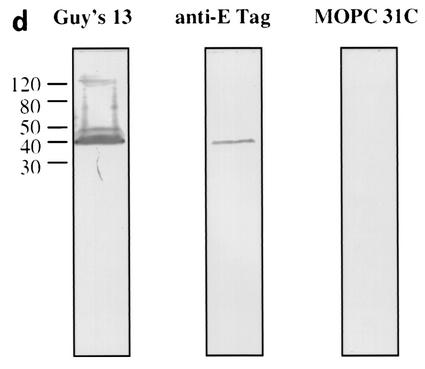
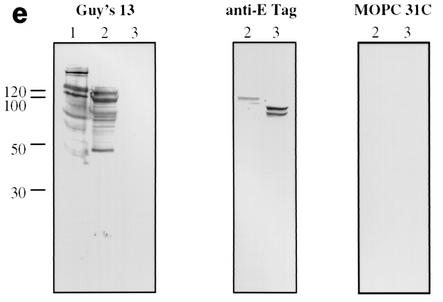
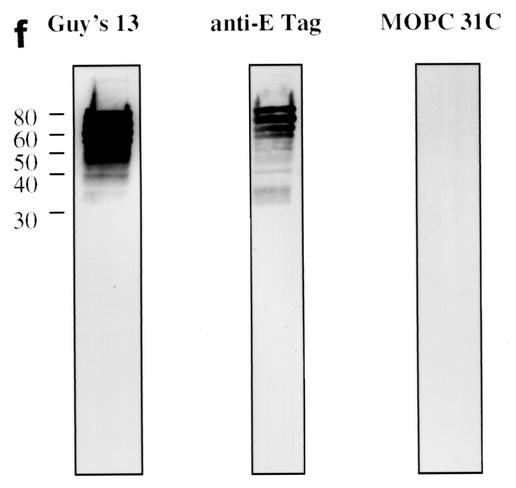
Immunoblotting of SA I/II and recombinant SA I/II fragments. (a) Diagrammatic representation of the amino acid residues of SA I/II encoded by the recombinant fragments of SA I/II. The open reading frames expressed by each clone are aligned below the SA I/II sequence. Amino acid residues of SA I/II encoded by each clone are shown in parentheses. The signal sequence (ss) (residues 1 to 38) and sequences associated with anchoring of the protein to the bacterial cell wall CWA (residues 1464 to 1561) as well as the alanine-rich and proline-rich repeat regions (A- and P-regions, respectively) are indicated. The four A-region repeats comprise residues 121 to 201, 202 to 283, 284 to 365, and 366 to 447 of SA I/II, while the four P-region repeats consist of residues 840 to 878, 879 to 917, 918 to 956, and 957 to 983. (b) Immunoblotting of SA I/II (20 ng) (lane 1) and E. coli lysate of Guy's 13-scFv (produced using the pScFvΔ expression system) (lane 2). (c) Immunoblotting of SA I/II (20 ng) (lane 1), E. coli lysates expressing recombinant fragments 105 (lane 2) and 106 (lane 3) cloned in pScFvΔ, or nonrecombinant pScFvΔ (lane 4). (d) Immunoblotting of Pro0-IV cloned in pScFvΔ. (e) Immunoblotting of SA I/II (20 ng) (lane 1) or E. coli lysates expressing fragments 109 (lane 2) and 110 (lane 3) cloned in pScFvΔ. (f) Immunoblotting of 114 cloned inpEXssΔ3. E. coli lysates were electrophoresed on SDS-acrylamide gels, blotted to nitrocellulose, and probed with a 0.5-μg/ml concentration of either Guy's 13 IgG, mouse anti-E tag serum, or MOPC 31C as indicated, followed by detection with AP-conjugated goat anti-mouse IgG. The theoretical Mrs of the following recombinant polypeptides are as indicated: 105 (106,600), 106 (83,900), 109 (88,300), 110 (65,500), Pro0-IV (22,600), and 114 (51,100). The positions of molecular mass standards (BRL 10-kDa ladder) are shown.
Recombinant clones in pScFvΔ were transformed into E. coli HB2151 for expression and preparation of cell lysates, while amplicons cloned into pEXssΔ3 were transformed into E. coli BL21(DE3)pLysS for expression and preparation of cell lysates.
Recombinant protein expression and cell lysis.
E. coli clones were grown and their proteins were expressed according to the Novagen pET system manual or the Pharmacia recombinant phage antibody system manual. Following induction using 1 mM IPTG the cultures were shaken at 30°C and 250 rpm for 3 to 4 h and clarified (3,500 × g for 30 min at 4°C), and the supernatant was discarded. The cell pellet from a 500-ml culture was resuspended in 12 ml of phosphate-buffered saline (PBS). Lysis of E. coli BL21(DE3)pLysS cells was achieved by freeze-thawing, while E. coli HB2151 cells were lysed using lysozyme and deoxycholic acid (35). The viscosity of the cell lysate was reduced by the addition of DNase I and incubation at room temperature. The cell lysate was centrifuged (20,000 × g for 30 min at 4°C), and the supernatant was stored at −20°C.
Immunoblotting.
Proteins in E. coli lysates (10 μl) were separated on sodium dodecyl sulfate (SDS)-acrylamide gels under reducing conditions and electroblotted (700 mA, 50 V, for 45 to 90 min) to nitrocellulose. Free protein binding sites on the nitrocellulose were blocked by gently agitating the membrane overnight at 4°C in 50 ml of PBS containing 10% nonfat milk powder. The blocking solution was removed, and the membrane was washed several times in H2O. Primary antibody (Guy's 13, MOPC 31C, or anti-E tag antiserum) was diluted in PBS containing 10% (wt/vol) nonfat milk powder and 0.1% (vol/vol) Tween 20 (PBSMT). For each membrane, 5 ml of diluted primary antibody was used, and the membrane was incubated for 90 min at room temperature with gentle agitation. The membrane was washed in H2O containing 0.1% (vol/vol) Tween 20 (H2OT). AP-conjugated secondary antibody was diluted in PBSMT, and 5 ml was incubated with the membrane for 1 h at room temperature. Following washing the blot was developed using 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Sigma).
Coating ELISA plates.
Maxisorp 96-well ELISA plates (Nunc, Roskilde, Denmark) were coated with a 50-μl/well concentration of either a 2-μg/ml SA I/II solution in PBS or a dilution series (made in PBS) of cell lysates from E. coli cultures expressing recombinant SA I/II fragments. The plates were incubated overnight at 4°C, and the wells were washed three times with H2O. Free protein binding sites were blocked with blocking buffer (2.5% [wt/vol] bovine serum albumin [fraction V; Sigma] in PBS) for 2 h at 37°C. The plates were washed three times with H2OT, air dried, and stored at −20°C until required.
Competition ELISA to measure the ability of lysates to inhibit the binding of Guy's 13 IgG to solid-phase SA I/II.
For competition ELISAs (4, 39), SA I/II-coated ELISA plates were incubated overnight at 4°C with a mixture of inhibitor(s) and Guy's 13 IgG (at a predetermined optimal dilution designed to give a final optical density at 450 nm [OD450] of 0.7 to 1.0). Unless otherwise stated, the inhibition ELISAs were performed according to the following protocol. Typically, 65 μl of Guy's 13 IgG {1 μg/ml in 5× dilution buffer (DB; 12.5% [wt/vol] bovine serum albumin [fraction V; Sigma], 0.5% [vol/vol] Tween 20 in PBS)} was added to 130 μl of inhibitor 1 (either lysate or PBS) and 130 μl of inhibitor 2 (lysate or PBS). The final concentrations of reagents were 0.2 μg/ml for Guy's 13 IgG and 0.4 times undiluted for lysate. Aliquots were added in triplicate to an SA I/II-coated ELISA plate. The plate was incubated overnight at 4°C and then washed six times with H2OT. Detection of bound Guy's 13 IgG was achieved with HRP-conjugated goat anti-mouse IgG secondary antibody (50 μl/well; The Binding Site) and incubation at 37°C for 2 h. Following six washes with H2OT, HRP activity was quantitated by the addition of 3,3′,5,5′-tetramethylbenzidine substrate (Sigma). Enzyme activity was stopped by the addition of 1 M H2SO4, and the OD450 was measured.
Direct ELISA to measure the ability of Guy's 13 to bind to individual recombinant SA I/II fragments immobilized on the solid phase.
Aliquots (100 μl) of Guy's 13 IgG dilutions (made in 1× DB) were added to ELISA plates coated in advance with lysates from E. coli expressing recombinant SA I/II fragments. The plates were incubated overnight at 4°C to allow interaction between the antibody and immobilized recombinant polypeptide to take place. The plates were then washed six times with H2OT, and bound antibody was detected as described above.
Direct ELISA to measure the ability of recombinant SA I/II fragments to promote the binding of Guy's 13 IgG to immobilized recombinant SA I/II fragments.
Lysates from E. coli HB2151 or BL21(DE3)pLysS cells expressing recombinant fragments of SA I/II were mixed with Guy's 13 IgG (5 μg/ml; dilution made in 1× DB). As a negative control, an isotype-matched mouse IgG1κ MAb (MOPC 31C) was used. The final concentrations of reagents were 0.2 μg/ml for the antibody and 0.96 times the initial dilution for lysate. Aliquots were added in duplicate to the wells of ELISA plates coated in advance with E. coli lysates containing recombinant fragments of SA I/II. The plates were incubated overnight at 4°C to allow interaction between the three components (antibody, immobilized fragment, and fluid phase fragment) to take place. The wells were washed, and bound antibody was detected as described above.
Direct ELISA to examine the interaction between recombinant subclones and SA I/II.
Lysates from E. coli HB2151 or E. coli BL21(DE3)pLysS cells expressing recombinant fragments of SA I/II were added to the wells of an SA I/II-coated ELISA plate. The plates were incubated overnight at 4°C to allow any interaction between the fluid-phase recombinant SA I/II fragment and immobilized SA I/II to take place. Unbound fragments were removed by washing six times with H2OT, and any fragment which remained bound was detected by the addition of either mouse anti-E tag antibody (0.2 μg/ml; Pharmacia) or, as a negative control, an isotype-matched mouse IgG1κ antibody (MOPC 31C). Following overnight incubation at 4°C, the plates were washed and bound antibody was detected as described above.
Direct ELISA to examine the interaction between recombinant SA I/II fragments.
Lysates from HB2151 or BL21(DE3)pLysS cells, expressing recombinant fragments of SA I/II, were added to the wells of ELISA plates previously coated with lysates containing expressed recombinant SA I/II fragments. The plates were incubated overnight at 4°C. The following day unbound recombinant polypeptides were removed by washing six times with H2OT, and the formation or exposure of a Guy's 13 epitope was detected by the addition of either Guy's 13 IgG (0.2 μg/ml) or, as a negative control, an isotype-matched mouse IgG1κ antibody (MOPC 31). Following overnight incubation at 4°C, the plates were washed, and bound murine antibody was detected as described above.
Statistical methods.
All experimental results were reproduced in at least two independent experiments. ELISA data are presented as either the means of results for duplicate wells (where the duplicates did not differ from the mean by more than 4%) or as the mean ± 1 standard deviation for triplicate wells.
RESULTS
Guy's 13 recognizes SA I/II under denaturing conditions.
Immunoblotting with Guy's 13 IgG of SA I/II purified from S. mutans Guy's strain (serotype c) (Fig. 1b) revealed a range of immunoreactive bands. The highest-molecular-mass species had a mobility of approximately 180 kDa, which corresponds to the previously determined mobility of SA I/II from S. mutans serotypes c (16, 29) and f (27) and confirms earlier work (25) which demonstrated the ability of Guy's 13 to recognize SA I/II from S. mutans Guy's strain under denaturing conditions. Blotting with either mouse anti-E tag antiserum or with mouse MAb MOPC 31C, as a specificity control, produced no immunoreactive bands. The observation that Guy's 13 IgG is able to recognize SA I/II under denaturing conditions suggested that the epitope might be nonconformational. This conclusion was further supported by the presence of many other bands of lower molecular mass, down to approximately 40 kDa, that were recognized.
Immunoblotting of E. coli lysates containing recombinant SA I/II fragments.
Figure 1c demonstrates the recognition of two recombinant SA I/II fragments by Guy's 13 IgG. Fragment 105 is an N-terminal fragment of SA I/II (amino acid residues 39 to 983), whereas fragment 106 is a C-terminal fragment (residues 816 to 1539).
Expression of the recombinant SA I/II fragments was confirmed by detection with the anti-E tag antiserum. Guy's 13 recognized the full-length fragments as well as a number of lower-molecular-mass fragments which were not recognized by the anti-E tag antiserum. These are hypothesized to be degradation products. No nonspecific binding was detected using the control MAb. Lysate from cells harboring the nonrecombinant pScFvΔ vector showed no reactivity with any of the antisera, as expected (lane 4). The results suggested that the P-region sequences (residues 816 to 983) present in both fragments 105 and 106 contain the epitope for Guy's 13. The observation that the lysates of both fragments 105 and 106 contain a range of bands which show immunoreactivity with Guy's 13 suggested that the Guy's 13 binding site could be localized to a smaller fragment.
Immunoblotting of fragments Pro0-IV, 109, and 110 are shown in Fig. 1d to f. As before, the expression of the cloned fragment was confirmed using the anti-E tag antiserum, but with these smaller fragments, even though the gene sequences had been confirmed, a discrepancy between the expected and observed molecular sizes was consistently found. Discrepancies between the deduced polypeptide Mr and that determined by SDS-polyacrylamide gel electrophoresis (PAGE) have been reported for native antigen I/II from S. mutans strains NG5 (12), OMZ 175 (28), and MT8148 (30) and from S. sobrinus 6715 (15). Goldschmidt et al. (8) also observed anomalous migration of recombinant fragments of the SpaA protein of S. sobrinus. They suggested that the accumulation, within the recombinant polypeptides, of strings of either adjacent charged residues or multiple β-turns might significantly alter the polypeptide conformation, resulting in slower migration on SDS-acrylamide gels. The anomalous migration of a recombinant polypeptide containing the P-region of P1 has also been described (3). Following electrophoresis on SDS-acrylamide gels, the recombinant polypeptide (with a theoretical Mr of 22,000) migrated with an apparent Mr of 36,000. The abundance of alanine and proline, both residues which bind poorly to SDS (24), may confer a lower negative charge density on polypeptides in which they occur and thereby account for the lower electrophoretic mobility. The presence of proline has also been reported to affect the conformation of the SDS-protein complex (5).
As predicted, the lysate from Pro0-IV (residues 816 to 983), which represents the overlapping region between fragments 105 and 106, contains a Guy's 13-immunoreactive band (Fig. 1d). The C-terminal fragment, fragment 110, which does not contain the P-region, was not recognized by Guy's 13 (Fig. 1e, lane 3) although its expression (as two major bands) was confirmed using the anti-E tag serum. However, the N-terminal fragment 109, which, like fragment 110, lacks the P-region, unexpectedly showed binding to Guy's 13 (Fig. 1e, lane 2).
The Guy's 13 binding site within fragment 109 was further delimited and was found to be present on a recombinant polypeptide spanning amino acid residues 45 to 457 of SA I/II—fragment 114 (Fig. 1f). This fragment, which spans the A-region of SA I/II, was produced using the pEXssΔ3 expression system. As expected, lysate from cells harboring nonrecombinant pEXssΔ3 showed no reactivity with any of the antisera (data not shown).
In summary, the Guy's 13 binding site was mapped to two regions of SA I/II (amino acids 45 to 457 and amino acids 816 to 983) by immunoblotting. These share no sequence similarity, and the minimal sequences representing these determinants were defined by recombinant fragments 114 and Pro0-IV, respectively. Henceforth in this work, the binding site within amino acid residues 45 to 457 of SA I/II will be referred to as the A-region binding site and the epitope within amino acid residues 816 to 983 will be referred to as the P-region binding site.
Inhibition of Guy's 13 binding to solid-phase SA I/II using recombinant fragments of SA I/II.
In an attempt to confirm the Western blotting results, a different experimental system was employed, which was not reliant upon the denaturing conditions utilized in the Western blotting experiments. The 105, 106, 109, and 110 recombinant fragments were tested for their ability to inhibit the binding of Guy's 13 to solid-phase SA I/II. Fluid-phase SA I/II was used as a control.
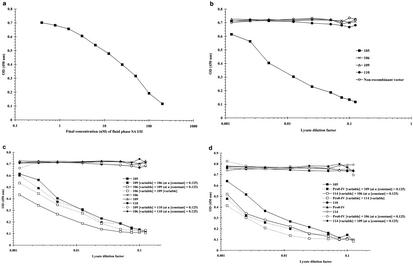
The results show that fluid-phase SA I/II inhibits Guy's 13 binding to immobilized SA I/II (Fig. 2a), with nearly complete inhibition achieved with 200 nM SA I/II in the fluid phase. The concentration of free SA I/II which resulted in 50% inhibition of Guy's 13 IgG binding was determined to be ∼38 nM, which is consistent with the previously determined value of 43 nM (19).
FIG. 2.
Inhibition of Guy's 13 binding to solid-phase SA I/II using fluid-phase SA I/II or recombinant fragments of SA I/II. To the wells of an SA I/II-coated ELISA plate were added a range of SA I/II dilutions (a), a range of lysate dilutions (b), or a mixture of two lysates (c and d). In each experiment, a control, containing only PBS (and no inhibitor), was also included and gave an OD450 of 0.72 (not shown). The nonrecombinant vector samples represent lysate from IPTG-induced cells harboring nonrecombinant pScFvΔ. Similar results were obtained with lysate from cells harboring the nonrecombinant pEXssΔ3 vector (not shown). Guy's 13 IgG bound to the solid phase was detected by the addition of an HRP-conjugated anti-mouse IgG secondary antibody. Results are presented as the mean OD450s for duplicate wells. Lysates of fragments 105, 106, 109, 110, and Pro0-IV were produced using the pScFvΔ expression system, while that of fragment 114 was produced using the pEXssΔ3 vector.
Figure 2b shows that, of the recombinant fragments, only fragment 105 was able to effectively inhibit Guy's 13 binding. The inhibitory effect of the 105 lysate titrated out at a >100-fold dilution. Control lysates from 110 and nonrecombinant vectors did not cause inhibition. The lack of inhibition shown by fragments 106 and 109 is in apparent conflict with the immunoblotting data, which suggested that both these fragments should be able to inhibit binding of Guy's 13 to SA I/II, since both were able to bind to Guy's 13 in the immunoblotting experiments.
As fragment 105 contains both of the Guy's 13 binding sites identified from the immunoblotting, it was reasoned that it might be possible to achieve inhibition by mixing together 106 lysate (containing the P-region binding site) and 109 lysate (containing the A-region binding site). The results of this competition experiment (Fig. 2c) showed that this was indeed the case, with a level of inhibition comparable to that achieved using the 105 lysate alone. The inhibition was dose dependent for both 106 and 109 lysates. Once again, lysates 106, 109, and 110 on their own were ineffective as inhibitors. Mixing either 106 or 109 lysate with control 110 lysate did not result in inhibition of binding of Guy's 13 to immobilized SA I/II.
The lysates from E. coli expressing recombinant fragments Pro0-IV and fragment 114 (the smallest immunoreactive fragments defined by immunoblotting) were mixed with lysates 106 and 109 and used in the inhibition ELISAs. This experiment (Fig. 2d) shows that mixing of recombinant fragments containing heterologous binding sites (such as Pro0-IV with either fragment 114 or 109, or mixing fragment 114, which spans the A-region, with fragment 106, which spans the P-region) always resulted in effective concentration-dependent inhibition of Guy's 13 binding to immobilized SA I/II, comparable to that achieved using fragment 105. However, mixing recombinant fragments containing homologous binding sites (e.g., Pro0-IV with fragment 106 or fragment 114 with fragment 109) had no inhibitory effect on Guy's 13 binding. Furthermore, these fragments on their own were unable to inhibit Guy's 13 IgG binding to immobilized SA I/II.
In an additional control experiment, a phage-displayed scFv fragment of Guy's 13 was employed in the inhibition ELISAs. The inhibition of binding of the phage-displayed Guy's 13 scFv to solid-phase SA I/II by using recombinant SA I/II fragments mirrored the pattern of inhibition shown by Guy's 13 IgG; i.e., there was also a codependency for both A- and P-region binding sites (data not shown). This result rules out the possibility that the inhibition of binding is mediated by the Fc region of Guy's 13.
Guy's 13 binds to a solid-phase-associated binding site in the presence of the cognate, liquid-phase binding site.
In these assays, the solid phase of an ELISA plate was coated with a lysate containing one of the binding sites and this was followed by incubation with a mixture of Guy's 13 IgG (or negative control antibody) and lysate from E. coli expressing another recombinant SA I/II fragment. Bound Guy's 13 IgG was detected using an affinity-purified, HRP-conjugated anti-mouse IgG antibody.
When lysate from E. coli expressing fragment 105 (which encodes both binding sites) was used to coat the solid phase, Guy's 13 IgG was able to bind directly, without the need for additional liquid-phase components (Fig. 3a). This result mimics the binding of Guy's 13 to SA I/II-coated ELISA plates. ELISA plate wells coated with either 110 lysate or control lysates from IPTG-induced cells harboring the nonrecombinant pScFvΔ or pEXssΔ3 vector were not recognized by Guy's 13. The MOPC 31C specificity control antibody did not recognize immobilized 105 lysate (data not shown). An optimal Guy's 13 concentration of 0.2 μg/ml was used in subsequent assays.
FIG. 3.
Guy's 13 binds to a solid-phase-associated binding site in the presence of the cognate fluid-phase binding site. (a) Dilutions (made in PBS) of lysates from E. coli expressing fragment 105 or 110 or from IPTG-induced cells harboring the nonrecombinant pScFvΔ or pEXssΔ3 vector were used to coat ELISA plate wells. Following blocking and washing, the wells were incubated with Guy's 13 (0.2 μg/ml). Detection of bound Guy's 13 was achieved with an HRP-conjugated anti-mouse IgG secondary antibody. ELISA plates were also coated with dilutions of lysate from E. coli expressing recombinant fragment 106 (b), 109 (c), or 110 (d). Following blocking and washing, these plates were incubated with a mixture of Guy's 13 and the indicated fluid-phase lysate. Detection of bound Guy's 13 was with an HRP-conjugated anti-mouse IgG secondary antibody. Lysates 105, 106, 109, and 110 were produced using the pScFvΔ expression system.
When the solid phase of an ELISA plate was coated with lysate from clone 106 (encoding the P-region binding site) (Fig. 3b), Guy's 13 IgG was only able to bind when lysate from a clone expressing the cognate A-region binding site (109) was included in the liquid phase together with Guy's 13. Addition to the fluid phase of either 106 or 110 lysate was unable to promote binding of Guy's 13 IgG to the immobilized 106 fragment. Guy's 13 binding exhibited dose dependency for both the solid-phase 106 lysate and the 109 lysate included in the liquid phase.
Conversely, when the solid phase was coated with 109 lysate (Fig. 3c), Guy's 13 IgG was only able to bind when lysate containing the cognate P-region binding site (fragment 106) was included in the liquid phase together with Guy's 13. Once again, addition to the fluid phase of the homologous binding site (fragment 109) or the negative control 110 fragment was unable to promote binding of Guy's 13 IgG to the immobilized 109 fragment. Guy's 13 binding exhibited dose dependency for both the solid-phase and liquid-phase lysates.
Coating the solid phase of the negative control with 110 lysate (Fig. 3d) gave no appreciable binding of Guy's 13 IgG, irrespective of the recombinant fragment included in the fluid phase. The MAb control demonstrated no binding (not shown).
The results of these direct binding experiments support the earlier inhibition ELISA results and confirm that there is a strict requirement for the presence of both binding sites in order for binding by Guy's 13 to take place. Furthermore, the binding of Guy's 13 takes place irrespective of which binding site is immobilized on the solid phase.
The recombinant fragments bind to solid-phase SA I/II in the absence of Guy's 13.
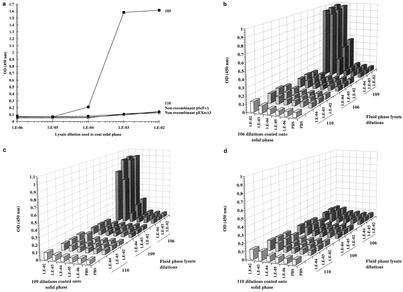
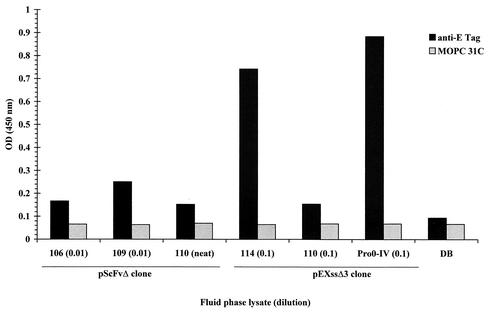
In order to determine whether recombinant SA I/II fragments are themselves able to bind to native SA I/II, lysates containing the recombinant SA I/II fragments were added to the wells of an SA I/II-coated ELISA plate. Detection of any recombinant fragment which had bound to SA I/II was by the addition of the mouse anti-E tag antibody followed by an affinity-purified, HRP-conjugated anti-mouse IgG secondary antibody.
The results for several recombinant fragments are shown in Fig. 4. The isotype-matched mouse IgG1κ negative control antibody (MOPC 31C) exhibited very low levels of binding (OD450 < 0.1). Addition of fragment 110, expressed from either the pScFvΔ or pEXssΔ3 plasmid, also resulted in low levels of binding of Guy's 13 IgG. Binding was, however, achieved by the addition of fragment 114 (which contains the A-region binding site) and by the addition of Pro0-IV lysate (which contains the P-region binding site).
FIG. 4.
Binding of recombinant SA I/II fragments to solid-phase SA I/II. Recombinant lysates (at the indicated dilution) were added to the wells of an SA I/II-coated ELISA plate. Following washing, bound recombinant fragments were detected by the addition of either the anti-E tag serum or MOPC 31C as a specificity control. Detection of bound antibody was achieved with an HRP-conjugated secondary antibody. Results are presented as the mean OD450s of duplicate wells. Dilution buffer, DB (see Materials and Methods for composition), was used for dilution of cell lysates and antibodies.
This result demonstrates, firstly, that recombinant SA I/II fragments containing either the A- or P-region binding sites are able to interact directly with SA I/II and, secondly, that Guy's 13 is not required for this interaction to take place.
Curiously, the 106 and 109 lysates (which contain the P- and A-region binding sites, respectively) showed little evidence of binding to SA I/II. A possible explanation for this absence of binding may lie in a comparison of the observed breakdown products when these lysates are immunoblotted with either Guy's 13 IgG or anti-E tag antibodies (Fig. 1e). The immunoblotting shows that many of the smaller fragments present in the Guy's 13 immunoblot have no counterpart in the E tag immunoblot. Therefore, in the proposed model for the interaction of the A- and P-regions, many of the non-E-tagged Guy's 13-immunoreactive fragments could compete with the E-tagged Guy's 13-immunoreactive fragments for binding to SA I/II, thereby leading to a reduction in the ability to detect any interaction using the anti-E tag reagent.
The cognate binding sites bind to each other in the absence of Guy's 13.
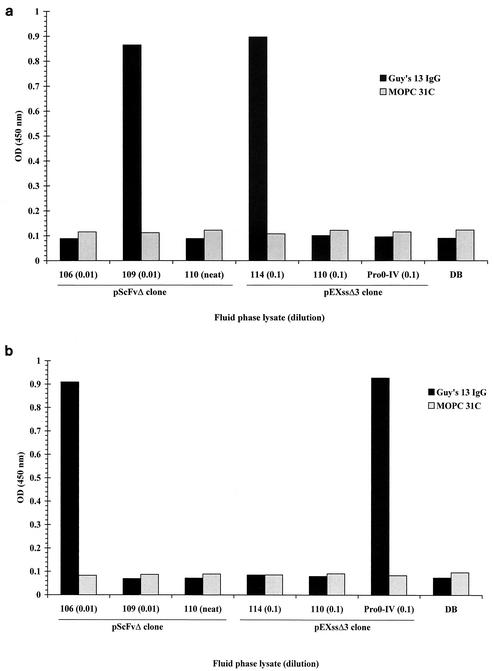
The solid phase of an ELISA plate was coated with lysates containing recombinant SA I/II fragments, followed by the addition of the second cognate binding site in the fluid phase. After incubation, unbound fluid-phase fragment was removed by extensive washing, and any stable interaction between the two binding sites was detected by the addition of Guy's 13 IgG (0.2 μg/ml), followed by an affinity-purified, HRP-conjugated anti-mouse IgG secondary antibody.
When the solid phase was coated with the P-region binding site (recombinant fragment 106) (Fig. 5a), only the addition of lysates containing the cognate A-region binding site (from fragment 109 or 114) led to an interaction that was recognized by Guy's 13. Conversely, when the solid phase (Fig. 5b) of the ELISA plate was coated with the A-region binding site (recombinant fragment 109), only the addition of lysates containing the cognate P-region binding site (fragment 106 or Pro0-IV) led to an interaction that was recognized by Guy's 13. In both experiments, homologous fluid-phase fragments were not able to promote the binding of Guy's 13 IgG to the immobilized fragment.
FIG. 5.
Direct ELISA demonstrating the specific interaction between A- and P-region binding sites. ELISA plates coated with recombinant lysate 106 (a) or 109 (b) were incubated with the indicated recombinant lysates (at the indicated dilution), followed by either Guy's 13 or MOPC 31C as a specificity control. Detection of bound antibody was achieved with an HRP-conjugated secondary antibody. Results are presented as the mean OD450s of duplicate wells. Dilution buffer, DB (see Materials and Methods for composition), was used for dilution of fluid-phase cell lysates and antibodies.
The results of these ELISAs show that the two binding sites are able to interact specifically with each other, in the absence of Guy's 13, in a manner which leads to the formation or exposure of a Guy's 13 conformational epitope.
No interaction between 106 or 109 fragments and immobilized SA I/II was discernible when probed with the anti-E tag antibody (Fig. 4). The lack of binding in this case was attributed to competition by non-E-tagged fragments. This explanation is supported by the present experiments (Fig. 5), which reveal significant levels of binding of the 106 and 109 fragments when the interaction is probed using Guy's 13 instead of the anti-E tag antibody.
DISCUSSION
In an attempt to delineate the Guy's 13 epitope, cloned fragments of the spaP gene were expressed in E. coli, and the cell lysates were immunoblotted with Guy's 13 IgG. In this way the epitope was shown to reside on two separate regions of SA I/II which showed no sequence homology. A partial epitope was shown to be present within residues 45 to 457 of SA I/II and was designated the A-region binding site. The other partial epitope was localized to a fragment spanning residues 816 to 983 and was designated the P-region binding site.
The results of the ELISA experiments suggested that the Guy's 13 epitope was discontinuous, being assembled from two noncontiguous stretches of SA I/II sequence. The inability to isolate a Guy's 13 epitope from phage display libraries (see the introduction) can be attributed to the conformational and bipartite nature of the epitope. Furthermore, the direct ELISAs suggest that the interaction of Guy's 13 with SA I/II proceeds via a two-stage mechanism, with the two partial epitopes interacting with each other to form or expose the conformational epitope which is subsequently bound by Guy's 13. The interaction of the A-region binding site with the P-region binding site may, or may not, involve a concomitant conformational change in one or both binding partners which then forms, or exposes, the epitope and allows Guy's 13 to bind.
Antibodies recognizing conformational epitopes of antigen I/II have been described before. Goldschmidt et al. (8) used rabbit antisera to characterize immunodominant determinants of the SpaA protein of S. sobrinus. By comparing the differential abilities of the antisera to bind to recombinant fragments of SpaA in either immunoblotting or immunoprecipitation experiments, they showed that a major immunodeterminant consisted of two distinct, noncontiguous fragments of SpaA and that the immunoreactivity of the major determinant resulted from the topographical assembly of distantly located amino acid residues. They observed that this discontinuous immunodeterminant retained the ability to be recognized by the antisera under the denaturing conditions employed in the immunoblotting. This mirrors the findings for Guy's 13 presented in this work. As an explanation, Goldschmidt et al. (8) suggested that the immunodominant determinant of SpaA was either stable under the denaturing conditions of the immunoblotting or renatured upon transfer of the proteins to nitrocellulose. Brady et al. (3) found that deletion of the P-region of P1 abolished the binding of several anti-P1 MAbs. Because these MAbs did not bind to the P-region itself, these results suggested that these MAbs recognized conformational epitopes which were dependent on the P-region sequences for the formation of the protein's native conformation.
The inhibition observed in the competition ELISAs was termed codependent, since there was a simultaneous requirement for the presence of both A- and P-region binding sites. The presence of one or the other of the binding sites was insufficient to cause inhibition.
While the ELISAs showed a strict requirement for the presence of both binding sites, this codependency of binding was in conflict with the results of the immunoblotting, which showed that Guy's 13 was able to bind independently to either binding site. The reason for this is unclear, although several ideas are proposed below. In any event, the recombinant fragments which were positive in the immunoblotting experiments also turned out to be required in the ELISAs. Similarly, the fragments which did not exhibit binding in the immunoblotting were also negative in the ELISAs. This consistency between the two experimental systems lends further support to the identification of the nature of the Guy's 13 epitope.
While the localized high concentration of a fragment on a nitrocellulose filter may provide an explanation for the binding of Guy's 13 to an individual fragment in Western blotting and the absence of binding in the ELISAs (where the fragment would be at a lower local concentration), it does not explain the ability of the antibody to bind to mixed fragments in the ELISAs, where the fragments would still be at a low concentration.
Two explanations for the disparity between the immunoblotting and ELISA results are proposed. Both ideas draw on the finding by Kneissel et al. (13) that antibodies recognizing the native conformation of the phage fd major coat protein (g8p), which is known to adopt an α-helix conformation (7, 31) and is particularly rich in alanine residues at the N terminus, were still able to bind to the protein under denaturing (SDS-PAGE) conditions. The work suggests that local α-helical epitopes can retain their structure under SDS-PAGE conditions. This may be due to the low SDS-binding capacity of alanine. The similarly low SDS-binding capability (and the concomitant slow migration on SDS-acrylamide gels) of polypeptides containing P-region sequences may also result in the retention of P-region secondary structure, while serving to unfold the remainder of the polypeptide under immunoblotting conditions.
In the first explanation for the disparity, it is envisaged that the P-region and A-region sequences retain their local conformations when immobilized on nitrocellulose under denaturing conditions (13). Furthermore, this immobilization reduces the conformational flexibility and stabilizes the conformation of these local epitopes. The net result is that the A- and P-region binding sites adopt fewer alternative (nonepitope) conformations, and therefore, the energy requirement for Guy's 13 to bind to each individual binding site is favorable. In contrast, in the ELISA system the immobilization event may be quite different, such that each individual binding site retains a high degree of conformational flexibility. In this case, a significant proportion of the structures might not be in the correct conformation for Guy's 13 recognition. The energy required for Guy's 13 to stabilize the correct epitope conformation might be prohibitive in this case. However, addition of the cognate binding site may help to stabilize the conformation, permitting Guy's 13 to bind.
In the second explanation, the disparity is proposed to be dependent upon the differences in conformation of the recombinant SA I/II polypeptides when expressed in E. coli and under the denaturing conditions used in the immunoblotting. When expressed in E. coli, the recombinant SA I/II polypeptides may adopt a conformation that buries the binding sites within the folded protein and prevents recognition by Guy's 13. Guy's 13 would only be able to recognize these buried binding sites if these misfolded polypeptides were unfolded. Unfolding can be achieved in two ways. In the immunoblotting experiments, the denaturing conditions employed serve to unfold the polypeptides without unfolding the A- and P-region binding sites (13) and Guy's 13 is able to bind. In the fluid-phase assays, interaction between sequences present in the A- and P-regions of the misfolded polypeptides (as demonstrated in this work) may drive the unfolding of the proteins (or the adoption of new conformations), which exposes the buried epitopes for binding to Guy's 13.
Further structural studies will be required to examine these hypotheses, but whatever the mechanism, it is clear that the Guy's 13 epitope requires the presence of both the A- and P-region binding sites.
Curiously, when Munro et al. (25) used Guy's 13 as a probe in Western blotting of recombinant fragments of SA I/II, Guy's 13 binding was only observed to a recombinant fragment spanning residues 816 to 1161 (containing the P-region) of SA I/II and not to a fragment spanning residues 39 to 481 (containing the A-region). The recombinant A-region-containing fragment produced by Munro et al. (25), fragment 1, was expressed with additional vector-encoded N-terminal residues, whereas the recombinant polypeptides produced in this work were expressed as fusions with a C-terminal E tag. It is possible that these differences could affect the conformation of the recombinant polypeptides, and they might therefore be responsible for the differences in immunoreactivity of Guy's 13 observed between this work and that of Munro et al. (25).
Nakai et al. (26) used a sandwich assay in an effort to map a saliva-binding region of antigen I/II. In their experiments, recombinant, truncated PAc fragments adsorbed to the solid phase of an ELISA plate were used to capture human salivary components, and this interaction was measured by the addition of biotinylated PAc as a probe. While the main aim of their study was to map a saliva-binding epitope, they showed that biotinylated PAc was also able to bind directly to some of the immobilized PAc fragments in the absence of any saliva, suggesting that the PAc molecule might be able to self-aggregate or form an intramolecular interaction. In particular they showed that biotinylated PAc bound directly to recombinant PAc fragments which contained P-region (but not A-region) sequences. Furthermore, they reported that native purified PAc formed aggregates in solution, with a molecular mass of >1,000 kDa, suggesting that the PAc protein may be a pentamer or hexamer. However, in contrast to our findings, biotinylated PAc did not bind to recombinant PAc fragments containing A-region (but not P-region) sequences. The reasons for this are unclear, although the PAc fragments were produced as C-terminal fusions with β-galactosidase, which may have had an influence on their results.
The implications of our present findings for the structure of SA I/II and its role as an adhesin as well as the mechanism of Guy's 13-mediated prevention of colonization still need to be elucidated. Although recombinant polypeptides containing the A- and P-regions are able to interact with each other, our findings do not indicate whether this interaction takes place in the native SA I/II conformation and what role, if any, this might have in the adhesin function of SA I/II. Furthermore, it is not known whether SA I/II undergoes any conformational changes upon binding to the host receptor(s).
If the A- and P-regions interact with each other in the native molecule, then the fact that Guy's 13 is able to bind to an epitope assembled from the A- and P-regions could provide an explanation for its protective action: Guy's 13 may hold SA I/II in a conformation which prevents the adhesin activity of SA I/II.
The work described here has established the conformational nature of the Guy's 13 epitope and has provided an experimental system for dissecting it into two separate fragments. SA I/II can be divided into two separate fragments which, only when added together, reconstitute the Guy's 13 epitope. To our knowledge this is the first characterization of an epitope of this kind, and it is unclear whether this might be a generalized feature of antibodies recognizing discontinuous epitopes.
The ELISA-based methods allow each binding site to be studied in turn and should enable each fragment to be more finely mapped by cloning and expression of successively smaller recombinant fragments and possibly through the use of synthetic peptides. This should allow the identification of critical residues involved in the interaction between the A- and P-region binding sites and the interaction of the assembled epitope with Guy's 13. In turn, this could help in understanding the ability of Guy's 13 to recognize SA I/II homologues from different streptococcal species and provide a molecular basis for understanding streptococcal adhesion and Guy's 13-mediated prevention of colonization.
Acknowledgments
We thank Guy's and St. Thomas's Charitable Foundation for partly funding this work.
We thank Charles G. Kelly for helpful discussions.
Editor: V. J. DiRita
REFERENCES
- 1.Bollet, C., M. J. Gevaudan, X. de Lamballerie, C. Zandotti, and P. de Micco. 1991. A simple method for the isolation of chromosomal DNA from Gram positive or acid-fast bacteria. Nucleic Acids Res. 19:1955.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady, L. J., D. A. Piacentini, P. J. Crowley, P. C. Oyston, and A. S. Bleiweis. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 60:1008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady, L. J., D. G. Cvitkovitch, C. M. Geric, M. N. Addison, J. C. Joyce, P. J. Crowley, and A. S. Bleiweis. 1998. Deletion of the central proline-rich repeat domain results in altered antigenicity and lack of surface expression of the Streptococcus mutans P1 adhesion molecule. Infect. Immun. 66:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chargelegue, D., O. E. Obeid, S. C. Hsu, M. D. Shaw, A. N. Denbury, G. Taylor, and M. W. Steward. 1998. A peptide mimic of a protective epitope of respiratory syncytial virus selected from a combinatorial library induces virus-neutralizing antibodies and reduces viral load in vivo. J. Virol. 72:2040-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong, W. W., A. Zweers, and L. H. Cohen. 1978. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem. Biophys. Res. Commun. 82:532-539. [DOI] [PubMed] [Google Scholar]
- 6.Forester, H., N. Hunter, and K. W. Knox. 1983. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J. Gen. Microbiol. 129:2779-2788. [DOI] [PubMed] [Google Scholar]
- 7.Glucksman, M. J., S. Bhattacharjee, and L. Makowski. 1992. Three-dimensional structure of a cloning vector. X-ray diffraction studies of filamentous bacteriophage M13 at 7 Å resolution. J. Mol. Biol. 226:455-470. [DOI] [PubMed] [Google Scholar]
- 8.Goldschmidt, R. M., M. Thoren-Gordon, and R. Curtiss III. 1990. Regions of the Streptococcus sobrinus spaA gene encoding major determinants of antigen I. J. Bacteriol. 172:3988-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkinson, H. F., and D. R. Demuth. 1997. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23:183-190. [DOI] [PubMed] [Google Scholar]
- 11.Kelly, C., P. Evans, J. K.-C. Ma, L. A. Bergmeier, W. Taylor, L. J. Brady, S. F. Lee, A. S. Bleiweis, and T. Lehner. 1990. Sequencing and characterization of the 185 kDa cell surface antigen of Streptococcus mutans. Arch. Oral Biol. 35:33S-38S. [DOI] [PubMed]
- 12.Kelly, C., P. Evans, L. Bergmeier, S. F. Lee, A. Progulske-Fox, A. C. Harris, A. Aitken, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 258:127-132. [DOI] [PubMed] [Google Scholar]
- 13.Kneissel, S., I. Queitsch, G. Petersen, O. Behrsing, B. Micheel, and S. Dübel. 1999. Epitope structures recognised by antibodies against the major coat protein (g8p) of filamentous bacteriophage fd (Inoviridae). J. Mol. Biol. 288:21-28. [DOI] [PubMed] [Google Scholar]
- 14.Kruger, C., Y. Hu, Q. Pan, H. Marcotte, A. Hultberg, D. Delwar, P. J. van Dalen, P. H. Pouwels, R. J. Leer, C. G. Kelly, C. van Dollenweerd, J. K. Ma, and L. Hammarstrom. 2002. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat. Biotechnol. 20:702-706. [DOI] [PubMed] [Google Scholar]
- 15.LaPolla, R. J., J. A. Haron, C. G. Kelly, W. R. Taylor, C. Bohart, M. Hendricks, J. Pyati, R. T. Graff, J. K.-C. Ma, and T. Lehner. 1991. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect. Immun. 59:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. F., A. Progulske-Fox, and A. S. Bleiweis. 1988. Molecular cloning and expression of a Streptococcus mutans major surface protein, P1 (I/II), in Escherichia coli. Infect. Immun. 56:2114-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehner, T., J. Caldwell, and R. Smith. 1985. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect. Immun. 50:796-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma, J. K.-C., A. Hiatt, M. Hein, N. D. Vine, F. Wang, P. Stabila, C. van Dolleweerd, K. Mostov, and T. Lehner. 1995. Generation and assembly of secretory antibodies in plants. Science 268:716-719. [DOI] [PubMed] [Google Scholar]
- 19.Ma, J. K.-C., B. Y. Hikmat, K. Wycoff, N. D. Vine, D. Chargelegue, L. Yu, M. B. Hein, and T. Lehner. 1998. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4:601-606. [DOI] [PubMed] [Google Scholar]
- 20.Ma, J. K.-C., M. Hunjan, R. Smith, and T. Lehner. 1989. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin. Exp. Immunol. 77:331-337. [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, J. K.-C., M. Hunjan, R. Smith, C. Kelly, and T. Lehner. 1990. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect. Immun. 58:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, J. K.-C., R. Smith, and T. Lehner. 1987. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect. Immun. 55:1274-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, J. K.-C., T. Lehner, P. Stabila, C. I. Fux, and A. Hiatt. 1994. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur. J. Immunol. 24:131-138. [DOI] [PubMed] [Google Scholar]
- 24.Maley, F., and D. U. Guarino. 1977. Differential binding of sodium dodecyl sulfate to amino acids as evidenced by elution from Sephadex. Biochem. Biophys. Res. Commun. 77:1425-1430. [DOI] [PubMed] [Google Scholar]
- 25.Munro, G. H., P. Evans, S. Todryk, P. Buckett, C. G. Kelly, and T. Lehner. 1993. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect. Immun. 61:4590-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai, M., N. Okahashi, H. Ohta, and T. Koga. 1993. Saliva-binding region of Streptococcus mutans surface protein antigen. Infect. Immun. 61:4344-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogier, J. A., D. Wachsmann, M. Schöller, Y. Lepoivre, and J. P. Klein. 1990. Molecular characterization of the gene sr of the saliva interacting protein from Streptococcus mutans OMZ175. Arch. Oral Biol. 35:25S-31S. [DOI] [PubMed]
- 28.Ogier, J. A., M. Schöller, Y. Lepoivre, A. Pini, P. Sommer, and J. P. Klein. 1990. Complete nucleotide sequence of the sr gene from Streptococcus mutans OMZ 175. FEMS Microbiol. Lett. 56:223-228. [DOI] [PubMed] [Google Scholar]
- 29.Okahashi, N., C. Sasakawa, M. Yoshikawa, S. Hamada, and T. Koga. 1989. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol. Microbiol. 3:221-228. [DOI] [PubMed] [Google Scholar]
- 30.Okahashi, N., C. Sasakawa, M. Yoshikawa, S. Hamada, and T. Koga. 1989. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol. Microbiol. 3:673-678. [DOI] [PubMed] [Google Scholar]
- 31.Opella, S. J., P. L. Stewart, and K. G. Valentine. 1987. Protein structure by solid-state NMR spectroscopy. Q. Rev. Biophys. 19:5440-5451. [DOI] [PubMed] [Google Scholar]
- 32.Russell, M. W., and T. Lehner. 1978. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch. Oral Biol. 23:7-15. [DOI] [PubMed] [Google Scholar]
- 33.Russell, M. W., L. A. Bergmeier, E. D. Zanders, and T. Lehner. 1980. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell, R. R. B. 1979. Wall-associated protein antigens of Streptococcus mutans. J. Gen. Microbiol. 114:109-115. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Schier, R., R. F. Balint, A. McCall, G. Apell, J. W. Larrick, and J. D. Marks. 1996. Identification of functional and structural amino-acid residues by parsimonious mutagenesis. Gene 169:147-155. [DOI] [PubMed] [Google Scholar]
- 37.Smith, G. P. 1992. Cloning in fUSE vectors, ed. of February 10, 1992. Division of Biological Sciences, University of Missouri, Columbia.
- 38.Smith, R., and T. Lehner. 1989. Characterization of monoclonal antibodies to common protein epitopes on the cell surface of Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol. Immunol. 4:153-158. [DOI] [PubMed] [Google Scholar]
- 39.Steward, M. W., and D. Chargelegue. 1997. Antibody affinity: measurement and biological significance, p. 38.1-38.36. In L. A. Herzenberg, D. M. Weir, L. A. Herzenberg, and C. Blackwell (ed.), Weir's handbook of experimental immunology, 5th ed. Blackwell Scientific, Oxford, United Kingdom.