Abstract
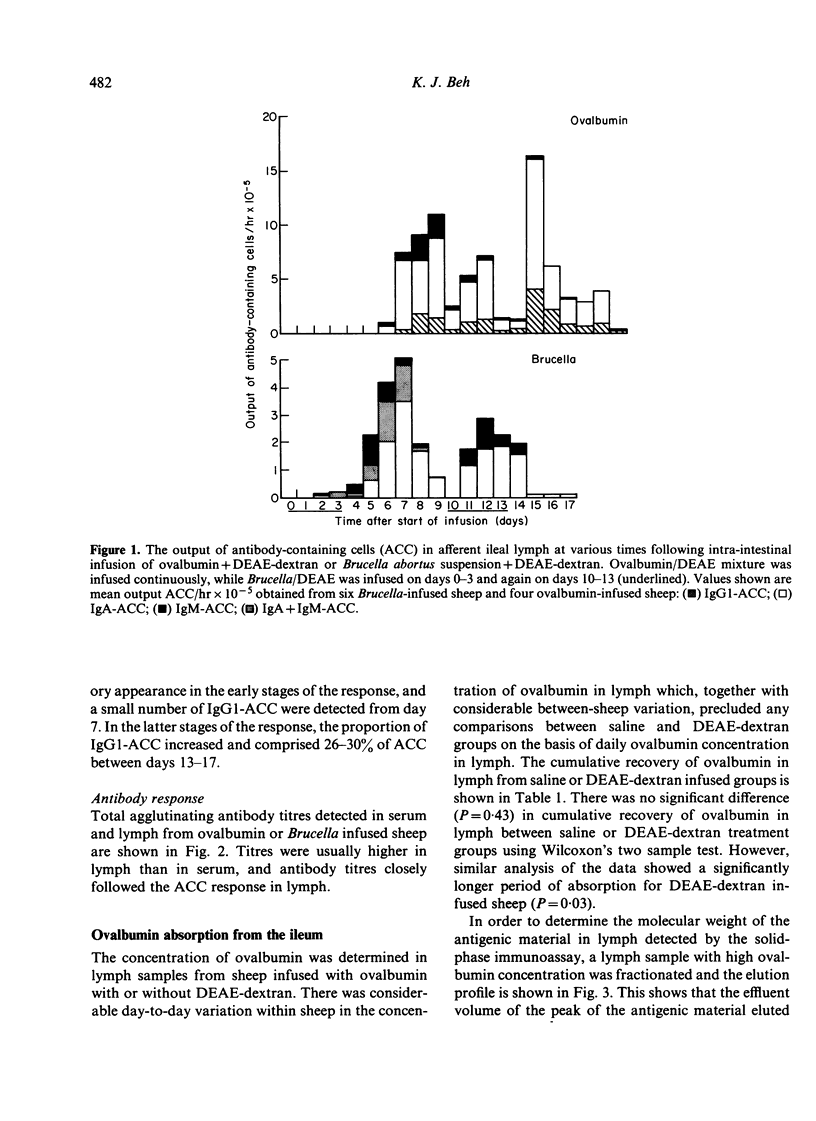
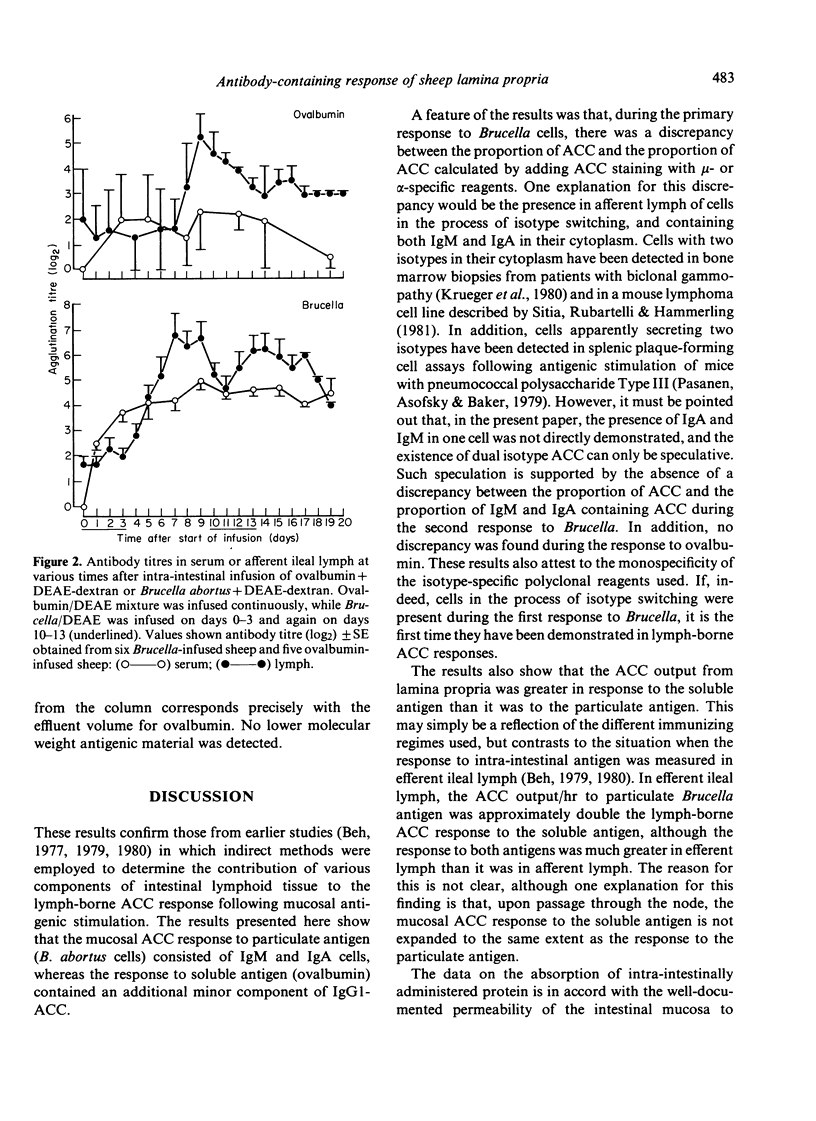
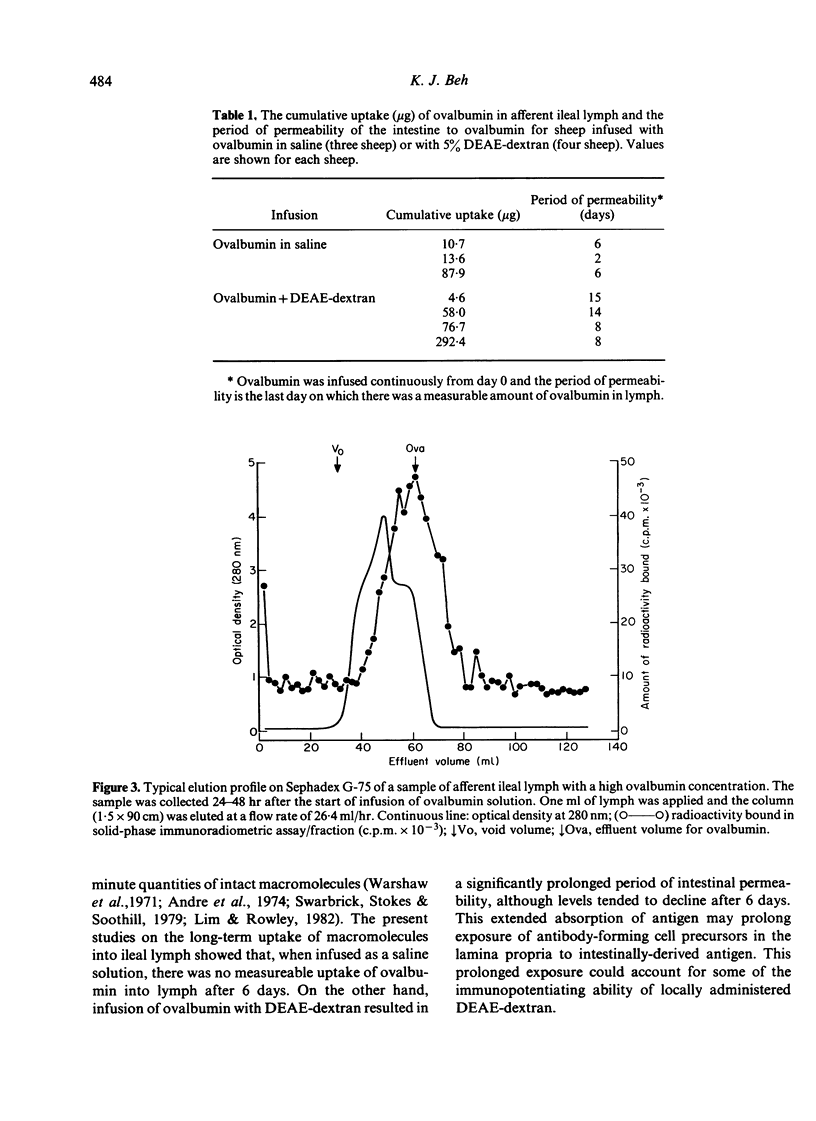
Experiments were carried out in sheep to determine the isotype distribution of antibody-containing cells (ACC) produced by the lamina propria of the intestine. Soluble or particulate antigen was infused intra-intestinally and the isotype specificity of the subsequent ACC response was monitored in afferent lymph collected from ileal lymphadenectomised sheep. Infusion with Brucella abortus cells+5% DEAE-dextran for 3 days elicited a peak lymph-borne ACC response of 5 X 10(5) ACC/hr on day 7, and a second 3-day infusion elicited a somewhat reduced response with a peak output of ACC of 2.9 X 10(5)/hr on day 12. Brucella-specific ACC were of either IgM or IgA isotype, and a percentage of cells apparently contained both isotypes during the primary response. Following continuous intra-intestinal infusion of ovalbumin with DEAE-dextran, peak levels of ACC in lymph occurred on day 9 when 10.7 X 10(5)/hr ACC were present in lymph, and on day 15 when 16.5 X 10(5) ACC/hr were discharged into lymph. IgA-ACC comprised 70-90% of ACC throughout the response, with 5-25% IgM-ACC produced in the early stages. IgG1-ACC assumed a greater proportion of the total ACC as the response progressed. A solid-phase radioimmunoassay was used to measure the long-term absorption of ovalbumin from the ileum. The cumulative uptake was similar, whether ovalbumin was infused with or without DEAE-dextran, although following infusion with DEAE-dextran, measurable amounts of ovalbumin were present in lymph for a longer period. These results confirm that the intestinal epithelium is permeable to minute amounts of macromolecules, and the prolonged permeability may contribute to the immunopotentiating effect of DEAE-dextran.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Lambert R., Bazin H., Heremans J. F. Interference of oral immunization with the intestinal absorption of heterologous albumin. Eur J Immunol. 1974 Oct;4(10):701–704. doi: 10.1002/eji.1830041013. [DOI] [PubMed] [Google Scholar]
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Beh K. J. Antibody-containing cell response in lymph of sheep after intra-intestinal infusion of ovalbumin with and without DEAE-dextran. Immunology. 1979 May;37(1):279–286. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J. Antibody-containing cell response of sheep to intestinal infusion of killed Brucella abortus cells. Cell Immunol. 1980 Aug 15;54(1):70–78. doi: 10.1016/0008-8749(80)90190-2. [DOI] [PubMed] [Google Scholar]
- Beh K. J. The origin of IgA-containing cells in intestinal lymph of sheep. Aust J Exp Biol Med Sci. 1977 Jun;55(3):263–274. doi: 10.1038/icb.1977.21. [DOI] [PubMed] [Google Scholar]
- Krueger R. G., Hilton P. M., Boehlecke J. M., Kyle R. A., Fair D. S. The cellular origin of multiple monoclonal immunoglobulins reflects the postulated pathways of isotype differentiation of antibody-forming cells. Cell Immunol. 1980 Sep 1;54(2):402–413. doi: 10.1016/0008-8749(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Lim P. L., Rowley D. The effect of antibody on the intestinal absorption of macromolecules and on intestinal permeability in adult mice. Int Arch Allergy Appl Immunol. 1982;68(1):41–46. doi: 10.1159/000233065. [DOI] [PubMed] [Google Scholar]
- Pasanen V. J., Asofsky R., Baker P. J. Synthesis of two classes of antibody, gammaM and gammaG or gammaM and gammaA, by identical cells. Amplification of the antibody response to pneumococcal polysaccharide type III. J Exp Med. 1979 May 1;149(5):1227–1237. doi: 10.1084/jem.149.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Hammerling U. Expression of 2 immunoglobulin isotypes, IgM and IgA, with identical idiotype in the B cell lymphoma I.29. J Immunol. 1981 Oct;127(4):1388–1394. [PubMed] [Google Scholar]
- Swarbrick E. T., Stokes C. R., Soothill J. F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut. 1979 Feb;20(2):121–125. doi: 10.1136/gut.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]



