Abstract
Synthetic oligodeoxynucleotides (ODN) containing CpG motifs (CpG-ODN) have been shown to be effective immunoprotective agents in murine models for a variety of viral, intracellular bacterial, and protozoan infections. Until now, the use of CpG-ODN to protect against extracellular bacterial infections has not been reported. The objective of this study was to investigate the effect of CpG-ODN against cellulitis and colibacillosis in broiler chickens, using a well-established model. At 22 days of age, birds received CpG-ODN by either the subcutaneous or intramuscular route. Three days later, a virulent isolate of Escherichia coli was applied to a scratch site on the caudal abdominal skin. Birds were examined for 10 days after the E. coli challenge, and pathological and bacteriological assessments were conducted on all birds. The control group of birds receiving no CpG-ODN(2007) had a survival rate of 15%. In contrast, groups that received CpG-ODN(2007), by either subcutaneous or intramuscular injection, had significantly higher survival rates (P < 0.0001). Furthermore, the size of the cellulitis lesion was significantly smaller in groups that received CpG-ODN(2007) by the subcutaneous route (P < 0.01). A dose of as little as 3.16 μg of CpG-ODN(2007), delivered 3 days prior to challenge by either the subcutaneous or intramuscular route, significantly protected birds against E. coli infection (P < 0.01). This study demonstrates that CpG-ODN(2007) has both local and systemic protective effects in broiler chickens. This is the first time that CpG-ODN(2007) has been demonstrated to have an immunoprotective effect against an extracellular bacterial infection in any food animal species.
The identification of events and signals that can boost immune responses to pathogens has been an active area of investigation for many decades. These studies have led to the discovery of various immunomodulators, as well as the cytokine pathways that allow cellular communication to direct the immune response. Initial studies demonstrated that specific DNA sequences from Mycobacterium bovis were effective in stimulating the production of cytokines and activating natural killer cells (28). These complementary studies clearly indicated that bacterial DNA had immunomodulatory effects. Furthermore, these immunostimulatory properties of bacterial DNA were shown to be due mainly to a specific sequence of cytosine-phosphodiester-guanine (CpG) motifs. They are present in the unmethylated state and occur at a higher frequency in bacterial DNA than in mammalian DNA (28). Other studies demonstrated that synthetic CpG-containing oligodeoxynucleotides (CpG-ODN) induce B-cell proliferation and immunoglobulin secretion, monocyte secretion of the Th1-like cytokines interleukin-12 and alpha, beta, and gamma interferons, and natural killer (NK) cell lytic activity (2, 4, 7, 12, 14, 19, 25, 27, 28, 30). Furthermore, a number of studies indicate that CpG-ODN can shift the immune response to a Th1-dominated cytokine pathway. This explains the predominance of Th1-like responses when CpG-ODN are used as an adjuvant with subunit or conventional vaccines (6, 15, 31).
It is now well established that two general immune mechanisms function against pathogens. Innate immunity (antigen-nonspecific) recognizes molecular patterns unique to and characteristic of infectious organisms. Adaptive or acquired (antigen-specific) immunity uses T and B lymphocytes that express distinct receptors to recognize pathogen-derived peptides or epitopes. Innate immunity has been thought to provide only a rapid, short-term, incomplete antimicrobial host defense until the slower, antigen-specific immune response develops. Recently, it has been suggested that the innate immune response may play a pivotal role in immune regulation and the development of host immunity by identifying which antigens require a specific response and determining the nature of that response (1, 9, 22). These studies have led to the evolution of the “danger” hypothesis, which proposes that cells of the immune system can respond rapidly to bacterial invaders, allowing the host to clear the infection even before specific immune responses are developed (6, 13, 16-18).
Escherichia coli causes a variety of disease syndromes in poultry, including yolk sac infection, omphalitis, respiratory tract infection, swollen head syndrome, septicemia, and cellulitis (21, 26). Cellulitis, first reported in 1984 by Randall et al. (26) in England, refers to inflammation of the subcutaneous tissue and is typically seen in the lower abdomens and thighs of broiler chickens. No clinical signs have been associated with cellulitis in living birds, but the presence of the lesion results in the condemnation of part of or the entire carcass at processing. Cellulitis has been reproduced in broilers by damaging the surface of the skin and applying virulent E. coli strains of serogroup O78 or O2 (10, 23, 24). A percentage of experimentally infected birds go on to develop colibacillosis, which is characterized in its acute form by septicemia resulting in death and in its subacute form by pericarditis, airsacculitis, and perihepatitis (5). The pathogenesis of colibacillosis is poorly understood, but the development of bacteremia appears to be essential (5, 20).
Although CpG-ODN have been used in laboratory animals and primates as inducers of innate immunity and as a vaccine adjuvant, their activity in chickens has not been reported to date. The objective of this study was to determine the effect of CpG-ODN as immunostimulants in broiler chickens by using an E. coli infection model.
MATERIALS AND METHODS
Bacteria.
A field isolate of E. coli serogroup O2 from a turkey with colisepticemia was used as the source of the challenge strain. This E. coli strain was nonhemolytic, was serum resistant, produced aerobactin, and had a K1 capsule and type I pili. Aliquots of this field isolate were stored at −70°C in brain heart infusion broth (BHI) (Difco, Detroit, Mich.) supplemented with 25% (wt/vol) glycerol (VWR Scientific Inc., Montreal, Quebec, Canada). Cells derived from a frozen culture were passaged once on sheep blood agar and then grown aerobically at 37°C in BHI for 12 to 14 h in 50-ml glass flasks with shaking at 200 rpm. A 1:10,000 dilution of the culture then was made in a 1-liter flask containing 100 ml of BHI. This was incubated for 8 to 9 h under the conditions described above until an absorbance of 0.8 at 600 nm was reached. This is known to produce a culture containing approximately 109 CFU per ml in the logarithmic phase of growth. The actual number of bacteria was determined by plating duplicate 10-fold serial dilutions of the culture on sheep blood agar and incubating at 37°C for 24 h. The challenge inoculum was washed once in normal saline and resuspended to an absorbance of 0.8 at 600 nm.
Synthetic CpG-ODN.
The sequence of CpG-ODN(2007) was TCGTCGTTGTCGTTTTGTCGTT, and the sequence of non-CpG-ODN(2041) was CTGGTCTTTCTGGTTTTTTTCTGG (underlining indicates CpG dinucleotides). Both ODN were produced with a phosphorothioate backbone. Synthetic CpG-ODN were diluted in sterile, pyrogen-free saline and administered in a 1-ml volume by either the subcutaneous or intramuscular route.
Animal model.
All procedures involving animals were done with the approval of the University of Saskatchewan Committee on Animal Care. Day-old broiler chicks were obtained from a local hatchery in Saskatchewan, Canada, and identified individually by wing bands. Groups of chicks were randomly allocated into individual animal isolation rooms at the Veterinary Infectious Disease Organization, University of Saskatchewan. Water and commercial broiler ration were provided ad libitum, and a photoperiod of 8 h per 24 h was established. Room temperature was maintained at 20 to 22°C. Each room was ventilated with filtered, nonrecirculated air at a rate of 10 to 12 changes/h. Air pressure differentials and strict sanitation were maintained in this isolation facility. The model used for the production of cellulitis and colibacillosis in broiler chickens was a modification of one previously described (11). At day 25 of age, two parallel 3-cm-long, full-thickness scratches were made through the skin on the left caudal abdominal region with a diabetic lancet (Lifescan Lancets; Lifescan Canada Ltd., Burnby, British Columbia, Canada). The challenge inoculum was applied with two cotton applicators (Hardwood Products Company LLC, Guilford, Maine) to the scratches. Application of E. coli was repeated 15 min later. In this model, cellulitis develops in 100% of birds, and pericarditis, airsacculitis, and perihepatitis develop in 50 to 90% of birds. Occasionally, a few birds also develop hepatitis. Mortality ranges between 50 and 90% of the birds.
Birds were observed twice daily for 9 days postchallenge for clinical signs, and each individual was assigned daily clinical scores as follows: 0, normal; 0.5, slightly abnormal appearance, slow to move; 1, depression, reluctant to move; 2, inability to stand or reach food or water; and 3, dead. All of the birds were scored daily for 9 days postchallenge. Birds that received a clinical score of 2 were euthanized by cervical dislocation.
Chickens that were found dead or were euthanized were necropsied immediately. Gross pathological examinations, including the measurement of the size of the cellulitis lesion, and bacteriological cultures were done. Bacterial swabs were taken from the cellulitis lesion, pericardium, and air sacs and cultured on MacConkey agar (Difco) and tryptic soy agar with sheep blood (PML Microbiologicals, Richmond, British Columbia, Canada). For isolation of E. coli, a semiquantitative estimate of E. coli was conducted on MacConkey agar (3). Any gross lesions were recorded. On day 10, the remaining birds were euthanized by cervical dislocation, and necropsy and bacteriological procedures were done as described above.
Experimental design. (i) Effect of CpG-ODN(2007) treatment on E. coli infection in chickens.
This large-scale experiment was designed after a preliminary experiment, using 80 birds, indicated a protective effect of CpG-ODN against cellulitis and colibacillosis. Two hundred birds were randomly allocated into five groups of 40 birds each, identified by wing band, injected with CpG-ODN(2007) on day 22, and randomly distributed to 10 isolation rooms. Two groups received either 10 or 50 μg of CpG-ODN(2007) in the subcutaneous tissues of the left caudal abdomen. Two other groups received either 10 or 50 μg of CpG-ODN(2007) intramuscularly in the left drumstick. Half of the control group received saline intramuscularly in the left drumstick, and the remaining birds in the control group received saline subcutaneously in the left caudal abdomen. All of the groups were challenged on day 25 with E. coli as described above. Clinical assessments, necropsy examinations, and bacteriology were done as previously described.
(ii) Dose titration of CpG-ODN(2007) by the subcutaneous route.
Birds were randomly allocated into seven groups of 20 birds each, identified by wing band, and injected with CpG-ODN(2007) on day 22. Four groups of birds received 100, 31.6, 10, or 3.16 μg of CpG-ODN(2007) subcutaneously in the left caudal abdomen. To determine if the effect of CpG-ODN treatment was localized, one group of birds received 31.6 μg of CpG-ODN(2007) in the subcutaneous tissues of the neck. The control group received saline in the subcutaneous tissue of the left caudal abdomen. All of the groups were challenged on day 25 with E. coli as described above. Clinical, bacteriological, and gross pathological evaluations were conducted as in the previous experiment.
(iii) Dose titration of CpG-ODN delivered by the intramuscular route.
Birds were randomly allocated into eight groups of 24 birds, wing banded, randomly distributed into isolation rooms, and injected intramuscularly with CpG-ODN(2007) on day 22. Four groups of birds received 100, 31.6, 10, or 3.16 μg of CpG-ODN(2007) in the left drumstick. Three negative control groups received normal saline or 10 or 3.16 μg of non-CpG-ODN(2041) in the left drumstick. All of the groups were challenged on day 25 with E. coli. Clinical, bacteriological, and gross pathological evaluations were conducted as described above.
(iv) Duration of protection against E. coli infection by administration of CpG-ODN(2007).
Birds were randomly allocated into five groups of 24 birds, wing banded, randomly distributed into isolation rooms, and injected with 10 μg of CpG-ODN(2007) subcutaneously in the left caudal abdomen on either day 10, 13, 16, 19, or 22 of age. All of the groups were challenged on day 25 with E. coli as described above. Clinical, bacteriological, and gross pathological evaluations were conducted as described above.
(v) Evaluation of tissue reactions at the site of CpG-ODN administration.
Fourteen-day-old birds were randomly allocated into seven groups of 15 birds each. Two groups of birds received CpG-ODN(2007) (100 or 3.16 μg) subcutaneously in the left caudal abdomen and saline in the right caudal abdomen. Two groups of birds received CpG-ODN(2007) (10 or 3.16 μg) in the left drumstick and saline in the right drumstick. Two groups of birds received 3.16 μg of non-CpG-ODN(2041) subcutaneously in the left caudal abdomen or intramuscularly in the left drumstick and received saline in either the right caudal abdomen or drumstick accordingly. A control group of birds were injected subcutaneously with saline in the right caudal abdomen and right drumstick. Three birds were euthanized on each of days 1, 3, 7, 10, and 14 postinoculation, and tissue sections were taken for histopathology. Tissue sections from the caudal abdominal subcutaneous tissue or drumstick were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5-μm thickness and stained with hematoxylin and eosin. A histological score from 0 to 4 (0, no visible lesions; 1, focal, mild mononuclear, and heterophil infiltrations or cellulitis or myositis; 2, locally extensive, moderate mononuclear and heterophil infiltrations or cellulitis or myositis; 3, locally extensive, moderate to severe mononuclear and heterophil infiltrations or cellulitis or myositis; 4, diffuse, severe mononuclear and heterophil infiltrations or cellulitis or myositis) was assigned based on the tissue reactions to the various doses of CpG-ODN.
(vi) E. coli septicemia in birds treated with CpG-ODN(2007).
Birds were randomly allocated into two groups of 20 birds, wing banded, randomly distributed into isolation rooms, and injected with 50 μg of CpG-ODN(2007) subcutaneously in the left caudal abdomen on day 22 of age. All of the birds were challenged on day 25 with E. coli as described above. One milliliter of blood was collected from all of the birds into 3-ml syringes containing heparin (Organon Teknika, Toronto, Ontario, Canada) on days 1, 2, 4, 7, and 8 postchallenge with E. coli. The actual number of bacteria in the blood sample was determined by plating 10-fold serial dilutions in duplicate on sheep blood agar and MacConkey agar (Difco) and incubating at 37°C for 24 h. Clinical evaluation and gross pathological evaluations were conducted as described above.
Statistical analysis.
Comparisons of survival patterns and median survivals were done by the log rank test. The significance of treatment effects on the size of cellulitis lesions was determined by one-way analysis of variance (nonparametric, Kruskal-Wallis) and the t test (Mann-Whitney). The significance of effect of treatment group on E. coli isolation score was determined by contingency table analysis and the chi-square test or Fisher's exact test as appropriate. All data were analyzed by using Prism 3.0 (GraphPad Software Inc., San Diego, Calif.), with a P of 0.05 indicating significance.
RESULTS
Effect of CpG-ODN(2007) treatment on E. coli infection in chickens.
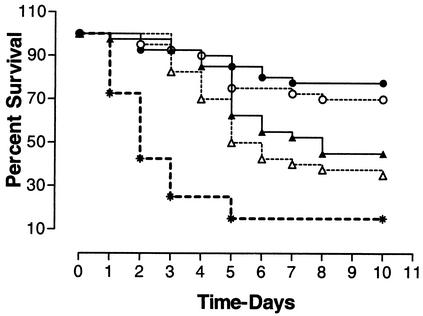
All of the birds in the experiment developed cellulitis following exposure to E. coli. Survival in all CpG-ODN(2007)-treated groups was significantly better than that in the control group (P < 0.0001) (Fig. 1). Treatment of birds with CpG-ODN(2007) by subcutaneous injection significantly increased survival compared to treatment with CpG-ODN(2007) by intramuscular injection (P < 0.01). The majority of the birds in the control group showed clinical signs of disease compared to groups treated with CpG-ODN(2007). Birds treated with CpG-ODN(2007) subcutaneously had the fewest clinical signs. Furthermore, the size of the cellulitis lesion was significantly smaller in birds treated subcutaneously (10 μg, 4.4 ± 3.0 cm2; 50 μg, 5.7 ± 3.7 cm2) than in the control group (8.5 ± 3.7 cm2) (P < 0.0001). In contrast, the size of the cellulitis lesion was not different between the control group and the group treated intramuscularly (10 μg, 8.6 ± 2.9 cm2; 50 μg, 8.9 ± 3.3 cm2) with CpG-ODN(2007) (P > 0.05). The majority of birds in the control group developed airsacculitis, pericarditis, and perihepatitis, either alone or in combination (polyserositis), in addition to cellulitis. Fewer birds developed similar lesions in the groups treated with CpG-ODN(2007) subcutaneously. Occasionally, some birds developed multifocal hepatitis. Birds which died peracutely within 24 h of challenge did not have gross lesions, but many bacteria were cultured from the air sacs, pericardium, and abdominal inoculation sites. In groups treated with CpG-ODN(2007) subcutaneously, bacteria were isolated less frequently from the cellulitis lesion, pericardium, and air sacs than in the control or intramuscularly CpG-ODN(2007)-treated groups (Table 1).
FIG. 1.
Survival of chickens following various treatments of CpG-ODN(2007). Groups of birds were treated subcutaneously (circles) or intramuscularly (triangles) with 50 μg (closed symbols) or 10 μg (open symbols) of CpG-ODN(2007), or with saline as a control (asterisks), 3 days prior to exposure to E. coli. Each group consisted of 40 birds. CpG-ODN(2007) treatment significantly protected against E. coli infection compared to the level of infection in the control group (P < 0.0001).
TABLE 1.
Bacterial isolation from different lesions
| Lesion sitea | E. coli growth scorec | No. of birds in treatment groupb:
|
||||
|---|---|---|---|---|---|---|
| Control | I/M, 10 μg | I/M, 50 μg | S/C, 10 μg | S/C, 50 μg | ||
| Cellulitis | 0 | 4 | 6 | 9 | 18 | 19 |
| 1+ | 4 | 14 | 9 | 11 | 13 | |
| 2+ | 7 | 8 | 11 | 4 | 1 | |
| 3+ | 15 | 11 | 6 | 6 | 6 | |
| 4+ | 10 | 1 | 5 | 1 | 1 | |
| Pericardium | 0 | 10 | 13 | 16 | 27 | 30 |
| 1+ | 4 | 4 | 5 | 3 | 1 | |
| 2+ | 6 | 7 | 4 | 5 | 2 | |
| 3+ | 13 | 18 | 11 | 4 | 3 | |
| 4+ | 7 | 3 | 4 | 1 | 4 | |
| Air sacs | ||||||
| 0 | 8 | 14 | 16 | 28 | 31 | |
| 1+ | 7 | 8 | 4 | 1 | 0 | |
| 2+ | 8 | 4 | 5 | 5 | 3 | |
| 3+ | 14 | 12 | 9 | 4 | 5 | |
| 4+ | 3 | 2 | 6 | 2 | 1 | |
Bacterial isolations from the cellulitis lesions, air sacs, and pericardiums, of birds in the large-scale experiment (see Materials and Methods).
Birds were treated with 10 or 50 μg of CpG-ODN(2007) by either the subcutaneous (S/C) or intramuscular (I/M) route.
The E. coli concentration in lesions was estimated on MacConkey agar by streaking sequentially in four distinct areas on agar plates. The enumeration was expressed on a scale of 0 to 4+ (0, no growth; 1+, growth of bacteria on area 1; 2+, growth of the bacteria on areas 1 and 2; 3+, growth of bacteria on areas 1, 2, and 3; 4+, growth of bacteria on areas 1, 2, 3, and 4). The effect of treatment on E. coli growth was very significant (P < 0.01). Heavy bacterial growth was observed more frequently in lesions from birds in the control group or groups treated with CpG-ODN(2007) by the intramuscular route than in the groups treated with CpG-ODN(2007) by the subcutaneous route.
Dose titration of CpG-ODN(2007) by the subcutaneous route.
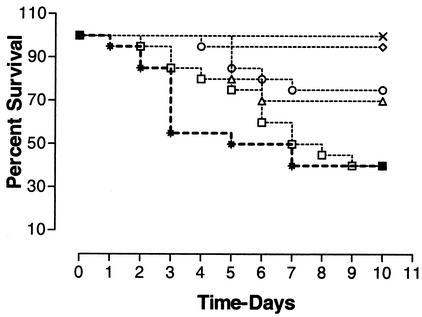
Since the subcutaneous administration of CpG-ODN was most effective, a second trial was conducted to evaluate the effect of dosage by this route. All of the groups given CpG-ODN(2007) subcutaneously in the left caudal abdomen had significantly better survival than the control group (P < 0.01) (Fig. 2). In contrast, birds that had received CpG-ODN(2007) in the neck did not have increased survival (P > 0.05). The pattern of clinical signs and bacterial culture results were similar to those observed in the previous experiment. The cellulitis lesions were significantly smaller in birds treated subcutaneously in the caudal abdomen with CpG-ODN(2007) (100, 31.6, and 3.16 μg) than in the control group (P < 0.05). The size of the cellulitis lesion in birds treated with CpG-ODN(2007) (31.6 μg) in the neck was not significantly different (P > 0.05) from that in the control group.
FIG. 2.
Survival of chickens following various doses of CpG-ODN(2007) administered by the subcutaneous route. Groups of birds (n = 20) were administered doses of CpG-ODN(2007) of 3.16 μg (◊), 10 μg (○), 31.6 μg (×), and 100 μg (▵) per bird in the caudal abdomen or of 31.6 μg per bird in the neck (□), by subcutaneous injection prior to infection with E. coli. All of the groups treated with CpG-ODN(2007) in the caudal abdomen were significantly protected against E. coli infection compared to the control group (*) (P < 0.01). The group of birds given CpG-ODN(2007) subcutaneously in the neck to study the local effect of CpG-ODN(2007) was not protected against E. coli infection (P > 0.05).
Dose titration of CpG-ODN delivered by the intramuscular route.
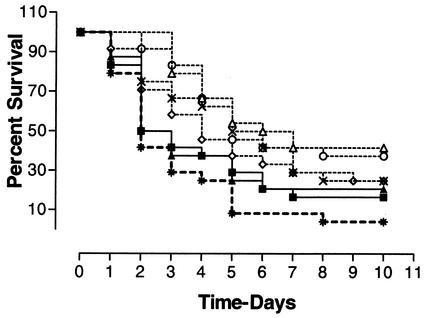
Since the intramuscular administration of CpG-ODN significantly protected birds against E. coli infections, a third trial was conducted to evaluate the effect of dosage by this route. Each group of birds that received CpG-ODN(2007), at doses ranging from 100 to 3.16 μg, had significantly increased survival compared to the control group (P < 0.01) (Fig. 3). The groups that received non-CpG-ODN(2041) did not show increased survival after E. coli infection (P > 0.05). The pattern of clinical signs and bacterial isolations were similar to those in the previous experiment. The size of the cellulitis lesion was not significantly different in birds treated with CpG-ODN(2007) by intramuscular injection from that in the control group (P > 0.05).
FIG. 3.
Survival of chickens given various doses of CpG-ODN(2007) by the intramuscular route. Groups of birds (n = 20) were administered doses of CpG-ODN(2007) of 3.16 μg (◊), 10 μg (○), 31.6 μg (×), and 100 μg (▵) per bird or non-CpG-ODN(2041) at 3.16 μg (▴) or 10 μg (▪) per bird by intramuscular injection 3 days prior to infection with E. coli. All groups treated with CpG-ODN(2007) were significantly protected against E. coli infection compared to the control group (*) (P < 0.01). Groups treated with non-CpG-ODN(2041) were not protected against E. coli infection (P > 0.05).
Duration of protection against E. coli infection by administration of CpG-ODN(2007).
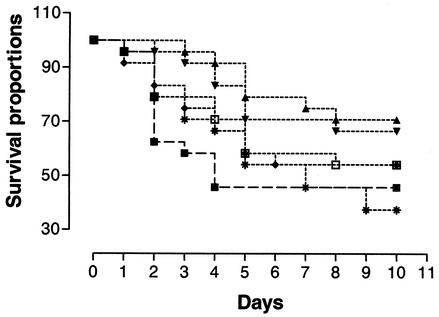
Birds were treated with CpG-ODN at different times prior to challenge to determine the duration of the protective effect. The group which received CpG-ODN(2007) 3 days prior to challenge had significantly better survival than the control group (P < 0.05) (Fig. 4). Although mortality was reduced, survival in the group that received CpG-ODN(2007) 6 days prior to challenge was not significantly improved (P = 0.06). The groups that received CpG-ODN(2007) from 9 to 15 days prior to challenge did not show increased survival after E. coli infection (P > 0.05). The pattern of clinical signs and bacterial isolations were similar to those in the previous experiment.
FIG. 4.
Duration of protection induced by CpG-ODN. Chickens were treated with 10 μg of CpG-ODN(2007) by the subcutaneous route on day 10 (□), 13 (*), 16 (⧫), 19 (▾), or 22 (▴) and challenged with E. coli on day 25 (n = 24). Birds were significantly protected (P < 0.01) by administration of CpG-ODN(2007) 3 days prior to challenge with E. coli, and the protection decreased with increasing interval between treatment and challenge. ▪, control.
Evaluation of tissue reactions at the site of CpG-ODN administration.
Since some of the effect of CpG-ODN administration appeared to be localized, a study was conducted to examine the tissue response at the site of injection (Fig. 5). In tissues that received CpG-ODN(2007) by subcutaneous injection, cellular infiltration was mild to severe for up to 10 days but had completely resolved by day 14 for the 3.16-μg treatment. In contrast, cellular infiltration was severe on day 1 and completely resolved by day 3 in birds that received 0.1 μg (Table 2).
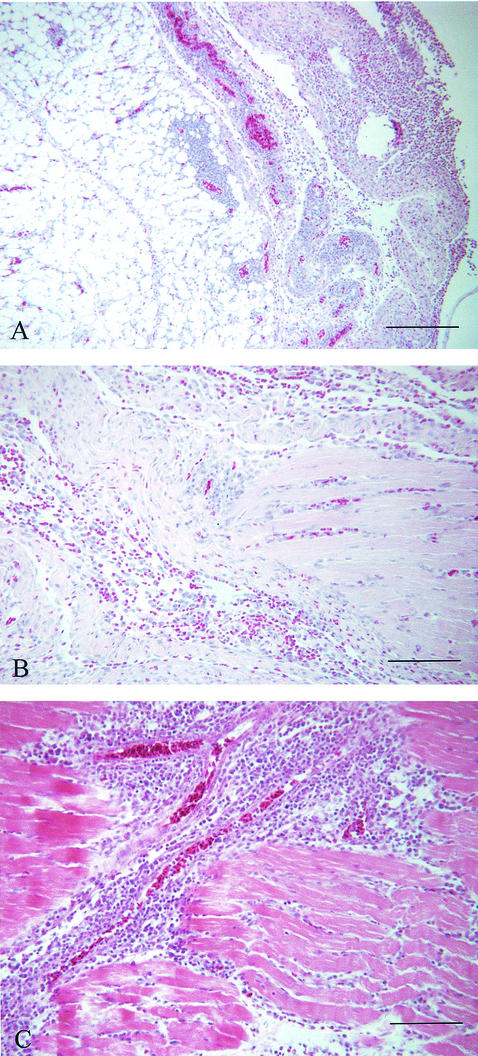
FIG. 5.
Histopathological evaluation of subcutaneous tissues of caudal abdomens of birds treated with CpG-ODN(2007). Tissues were taken 24 h after inoculation of CpG-ODN(2007), fixed in 10% neutral buffered formalin, sectioned at 5-μm thickness, and stained with hematoxylin and eosin. Both heterophils and mononuclear cells were infiltrated in the subcutaneous tissue and muscles without causing tissue necrosis. (A) Low magnification to demonstrate cellular infiltration in the subcutaneous tissue at 24 h after administration of CpG-ODN. Bar, 200 μm. (B) Higher magnification of panel A to demonstrate predominant heterophils (red). Bar, 100 μm. (C) Demonstration of predominant mononuclear cells (blue) in muscle tissue after 72 h of administration of CpG-ODN. Bar, 100 μm.
TABLE 2.
Histological changes in birds following administration of CpG-ODNa
| ODN | Dose (μg/bird) | Route(s) | Gross pathology scoreb on day:
|
||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 10 | 14 | |||
| CpG-ODN(2007) | 3.16 | S/C | 3 | 3 | 1 | 1 | 0 |
| 0.1 | S/C | 3 | 0 | 0 | 0 | 0 | |
| 10 | I/M | 3 | 3 | 3 | 3 | 1 | |
| 3.16 | I/M | 1 | 2 | 2 | 0 | 0 | |
| Non-CpG-ODN(2041) | 3.16 | S/C | 2 | 1 | 0 | 0 | 0 |
| 3.16 | I/M | 0 | 0 | 0 | 0 | 0 | |
| Control (saline) | I/M and S/C | 0 | 0 | 0 | 0 | 0 | |
Histopathological score (cellular infiltration) following administration of CpG-ODN by either subcutaneous (S/C) or intramuscular (I/M) injection. Cellular infiltration was most pronounced and persisted longest in birds treated with 10 μg of CpG-ODN(2007) by the intramuscular route.
0, no visible lesions; 1, focal, mild mononuclear and heterophil infiltrations; 2, locally extensive, moderate mononuclear and heterophil infiltrations; 3, locally extensive, moderate to severe mononuclear and heterophil infiltrations.
Cellular infiltration was detected up to day 14 after intramuscular injection of 10 μg of CpG-ODN(2007) but was mild to moderate until day 7 and had completely disappeared by day 10 for the 3.16-μg treatment.
To determine the contribution of the CpG motif to the inflammation, some birds received the non-CpG-ODN(2041). Treatment with this ODN by subcutaneous injection resulted in mild to moderate cellular infiltration at 3 days that had completely resolved by day 7 (Table 2). There was no cellular infiltration in response to treatment with 3.16 μg of non-CpG-ODN(2041) by intramuscular injection. No cellular infiltration was observed in any of the saline-injected sites.
E. coli septicemia in birds treated with CpG-ODN(2007).
To evaluate the systemic protective effect of CpG-ODN, the level of bacteremia was monitored following challenge. E. coli was isolated in the control group at a higher frequency than in the group that received CpG-ODN(2007) (P < 0.05) (Table 3), and the relative risk parameter indicated that 59% of birds that received this treatment became bacteremic, compared to controls. Also, the birds that received CpG-ODN(2007) had a lower number of E. coli than the control group (P < 0.01). Treatment also delayed the development of bacteremia. In the control group, of the 16 birds in which bacteremia was detected, 14 of those had E. coli isolated on day 1. In contrast, it was not until day 4 postchallenge that bacteremia was first detected in five of the nine birds which developed bacteremia in the CpG-ODN(2007)-treated group. The group that received CpG-ODN(2007) had significantly increased survival compared to the control group (P < 0.01), and the pattern of clinical signs and bacterial isolations from the internal lesions were similar to those in the previous experiments.
TABLE 3.
E. coli septicemia in birds challenged with E. coli following administration of 50 μg of CpG-ODN(2007) subcutaneouslya
| Group | Bird no. | CFU of E. coli/ml on day:
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 7 | 8 | ||
| Control | 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 × 105 | Dead | ||||
| 3 | 5 × 102 | 7 × 102 | Dead | |||
| 4 | 0 | 2 × 103 | 0 | 0 | 0 | |
| 5 | Dead | |||||
| 6 | 0 | 0 | 0 | 0 | 0 | |
| 7 | 1 × 105 | Dead | ||||
| 8 | 1 × 108 | Dead | ||||
| 9 | 1 × 104 | 1 × 104 | Dead | |||
| 10 | 1 × 105 | 1 × 103 | Dead | |||
| 11 | 1 × 103 | 3 × 102 | 1 × 102 | 0 | 0 | |
| 12 | 4 × 105 | Dead | ||||
| 13 | 1 × 104 | Dead | ||||
| 14 | 6 × 104 | Dead | ||||
| 15 | 1 × 105 | 1 × 105 | Dead | |||
| 16 | 1 × 104 | 2 × 103 | Dead | |||
| 17 | 7 × 102 | 2 × 102 | Dead | |||
| 18 | 0 | 1 × 105 | 0 | 0 | 0 | |
| 19 | 2 × 102 | Dead | ||||
| 20 | 0 | 0 | 0 | 0 | 0 | |
| Treatment | 21 | 0 | 0 | 2 × 104 | 0 | 0 |
| 22 | 0 | 0 | 0 | 0 | 0 | |
| 23 | 0 | 0 | 1 × 103 | Dead | ||
| 24 | 0 | 0 | 7 × 104 | 1 × 106 | Dead | |
| 25 | 2 × 102 | 6 × 102 | Dead | |||
| 26 | 0 | 0 | 0 | 0 | 0 | |
| 27 | 0 | 0 | 0 | 0 | 0 | |
| 28 | 0 | 4 × 102 | Dead | |||
| 29 | 0 | 0 | 0 | 0 | 0 | |
| 30 | 0 | 0 | 0 | 0 | 0 | |
| 31 | 0 | 6 × 103 | Dead | |||
| 32 | 0 | 0 | 1 × 102 | 0 | 0 | |
| 33 | 0 | 0 | 0 | 0 | 0 | |
| 34 | 0 | 0 | 0 | 0 | 0 | |
| 35 | 0 | 0 | 0 | 0 | 0 | |
| 36 | 0 | 0 | 0 | 0 | 0 | |
| 37 | 0 | 0 | 1 × 102 | 0 | 0 | |
| 38 | 0 | 0 | 0 | 0 | 0 | |
| 39 | 2 × 103 | 1 × 104 | Dead | |||
| 40 | 0 | 0 | 0 | 0 | 0 | |
CFU of E. coli in peripheral blood of birds treated with CpG-ODN(2007) and a control group on days 1, 2, 4, 7, and 8 following E. coli challenge (n = 20). E. coli was isolated on both sheep blood and MacConkey agar. The majority of birds in the control group had E. coli isolations on days 1 and 2 following E. coli challenge. CpG-ODN(2007) treatment significantly protected against E. coli infection compared to the level of infection in the control group (P < 0.01).
DISCUSSION
It is generally accepted that disease prevention by prophylactic measures or by immune stimulation is preferred to the use of therapeutic agents in the food animal industry. This has become especially important due to a number of food safety and human health issues. For example, the use of antibiotics by the animal industry has been linked to the emergence of resistant strains of bacteria. Also, the possibility of drug residues in meat products is an important issue, especially for international trade. For these reasons, the animal production industry is seeking to reduce the use of antibiotics. Novel immunostimulants may serve as alternative disease-preventive agents.
Synthetic CpG-ODN have been shown to be effective stimulators of the immune system and potent adjuvants in a number of species, including mice, primates, and humans (8, 29). However, this study demonstrates, for the first time, that CpG-ODN are efficacious as immunostimulants in poultry, a food animal species. Furthermore, while CpG-ODN have been effective in controlling intracellular protozoa, bacteria, and viruses, this is the first report showing CpG-ODN to be efficacious against a bacterial poultry disease, in which the bacteria do not have an intracellular phase.
The mortality rate in our animal model was generally above 50% and was associated with septicemia (11) and the development of systemic and local lesions. Our experiments demonstrated enhanced survival of birds that received CpG-ODN, by either the intramuscular or subcutaneous route, in a dose-dependent manner. In all instances, subcutaneous administration was more effective than intramuscular administration of CpG-ODN. Similarly, clinical disease scores, number of gross lesions, and amount of bacteria isolated from lesions were highest in the control group, intermediate in the group that received CpG-ODN intramuscularly, and lowest in the group that received CpG-ODN by subcutaneous injection. The mortality, clinical scores, and development of gross lesions are more severe in this model than in natural outbreaks of E. coli infections, where mortality is lower and subclinical infections cause major economic losses due to poor feed conversion and growth rate. Since CpG-ODN were able to significantly reduce the mortality in this severe model, we believe that CpG-ODN would be able to control naturally occurring, less severe E. coli infections.
Based on the histological data, doses of as low as 0.1 μg of CpG-ODN recruited both mononuclear and polymorphonuclear cells to the site of administration. Since we administered CpG-ODN and infected birds with E. coli at the same location in the subcutaneous groups, it is likely that inflammatory cells, recruited prior to challenge, reduced the number of bacteria that were available to enter systemic circulation. This local effect may explain why the administration of CpG-ODN by intramuscular injection resulted in a lower survival rate than the administration of CpG-ODN by the subcutaneous route. This explanation is supported by the observation that the administration of CpG-ODN by subcutaneous injection at a distant location (the neck) did not increase the survival rate (Fig. 2). The smallest cellulitis lesions were observed in birds that received CpG-ODN by the subcutaneous route, compared to either the control, intramuscular, or intravenous route. This observation also indicates that some bacteria are killed at the site of the CpG-ODN administration before they can establish the lesion.
Although there was some cellular infiltration to the site of non-CpG-ODN administration, cell recruitment was augmented by the presence of the CpG motif, demonstrating that similar motifs are recognized in chickens as in other species. Although this cellular infiltration was very high, occurred within 24 h, and lasted for up to 14 days, we did not see any indication of necrosis or cell death at the site of infiltration. This observation has also been reported for mice, and it was suggested that CpG-ODN failed to activate mast cell degranulation (30) and hence caused no tissue necrosis. Immunostimulation by CpG-ODN without causing tissue necrosis at the injection site would be a tremendous advantage to the poultry industry, since tissue damage due to injections leads to condemnation or trimming of parts of the carcass at slaughter and causes significant reduction of meat quality.
As well as having a local effect on the development of the cellulitis lesion, CpG-ODN treatment also had systemic effects as demonstrated by protection by intramuscular injection. These systemic effects were also demonstrated in that CpG-ODN treatment was able to minimize the incidence and the level of, and delay the development of, septicemia.
Although microbial contamination of poultry meat is controlled by modern sanitary slaughter procedures, bacteria commonly are found in retail poultry products. Reduction of the amount of bacteria present in the carcass is one way to reduce the risk that the meat products will become contaminated. We have shown that treatment with CpG-ODN reduced the amount of E. coli bacteria present in lesions of cellulitis, pericarditis, and airsacculitis. Reducing the amount of E. coli in the bird reduces the risk that poultry meat will become contaminated with potentially hazardous bacteria during processing and thus will improve food safety. This is especially important for those poultry processors that trim rather than condemn poultry with cellulitis.
CpG-ODN treatment was consistently effective when given 3 days prior to infection and appeared to have some effect for almost 6 days (Fig. 4). This duration of effect represents a significant proportion of the life span of a broiler chicken, which is from 35 to 42 days. Thus, work on the effect of repeated administration and development of feasible delivery methods such as aerosol or oral delivery are necessary to determine the utility of CpG-ODN use in the poultry industry. Moreover, E. coli infection-associated mortality in the first week of life is a significant problem. Currently, antibiotics are used as a preventative measure, but there is the potential to use CpG-ODN in ovo to reduce the incidence of E. coli infection in the first week of life.
In conclusion, CpG-ODN were effective immunoprotective agents in poultry, as in other species. Further research into the mechanism of action and testing in other poultry disease models is required to determine how the recognition of CpG motifs by the avian immune system compares to that by other species and how utility of these immunostimulatory agents in the poultry industry can be developed.
Acknowledgments
We are grateful to Barry Carroll, Sherry Tetland, Jan Erickson, and Tracy Bruneau for care and handling of the animals. The ODN were supplied by QIAGEN-GmbH.
Financial support was provided by grants from the Poultry Industry Council, Ontario; the Saskatchewan Agriculture Development Fund; the Natural Sciences and Engineering Research Council; and QIAGEN-GmbH, Hilden, Germany.
Editor: V. J. DiRita
Footnotes
Published with the permission of the Director of VIDO as journal series no. 317.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 3.Barry, A. L. 1972. Clinical specimens for microbiologic examination, p. 103-107. In P. D. Haeprich (ed.), Infectious disease: a guide to the understanding and management of infectious processes. Harper and Row, New York, N.Y.
- 4.Behboudi, S., D. Chao, P. Klenerman, and J. Austyn. 2000. The effects of DNA containing CpG motif on dendritic cells. Immunology 99:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheville, N. F., and L. H. Arp. 1978. Comparative pathologic findings of Escherichia coli infection in birds. J. Am. Vet. Med. Assoc. 173:584-587. [PubMed] [Google Scholar]
- 6.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowdery, J. S., J. H. Chace, A. K. Yi, and A. M. Krieg. 1996. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156:4570-4575. [PubMed] [Google Scholar]
- 8.Davis, H. L., I. I. Suparto, R. R. Weeratna, Jumintarto, D. D. Iskandriati, S. S. Chamzah, A. A. Ma'ruf, C. C. Nente, D. D. Pawitri, A. M. Krieg, Heriyanto, W. Smits, and D. D. Sajuthi. 2000. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine 18:1920-1924. [DOI] [PubMed] [Google Scholar]
- 9.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 10.Glunder, G. 1990. Dermatitis in broilers caused by Escherichia coli: isolation of Escherichia coli from field cases, reproduction of the disease with Escherichia coli O78:K80 and conclusions under consideration of predisposing factors. Zentbl. Vet. B 37:383-391. [DOI] [PubMed] [Google Scholar]
- 11.Gomis, S. M., T. Watts, C. Riddell, A. A. Potter, and B. J. Allan. 1997. Experimental reproduction of Escherichia coli cellulitis and septicemia in broiler chickens. Avian Dis. 41:234-240. [PubMed] [Google Scholar]
- 12.Hartmann, G., G. J. Weiner, and A. M. Krieg. 1999. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 96:9305-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heeg, K., and S. Zimmermann. 2000. CpG DNA as a Th1 trigger. Int. Arch. Allergy Immunol. 121:87-97. [DOI] [PubMed] [Google Scholar]
- 14.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 15.Klinman, D. M., A. K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg, A. M. 1999. Mechanisms and applications of immune stimulatory CpG oligodeoxynucleotides. Biochim. Biophys. Acta 1489:107-116. [DOI] [PubMed] [Google Scholar]
- 17.Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12:35-43. [DOI] [PubMed] [Google Scholar]
- 18.Krieg, A. M., G. Hartmann, and A. K. Yi. 2000. Mechanism of action of CpG DNA. Curr. Top. Microbiol. Immunol. 247:1-21. [DOI] [PubMed] [Google Scholar]
- 19.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 20.Leitner, G., and E. D. Heller. 1992. Colonization of Escherichia coli in young turkeys and chickens. Avian Dis. 36:211-220. [PubMed] [Google Scholar]
- 21.Morley, A. J., and D. K. Thomson. 1984. Swollen-head syndrome in broiler chickens. Avian Dis. 28:238-243. [PubMed] [Google Scholar]
- 22.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 23.Peighambari, S. M., R. J. Julian, J. P. Vaillancourt, and C. L. Gyles. 1995. Escherichia coli cellulitis: experimental infections in broiler chickens. Avian Dis. 39:125-134. [PubMed] [Google Scholar]
- 24.Peighambari, S. M., J. P. Vaillancourt, R. A. Wilson, and C. L. Gyles. 1995. Characteristics of Escherichia coli isolates from avian cellulitis. Avian Dis. 39:116-124. [PubMed] [Google Scholar]
- 25.Pisetsky, D. S., C. Reich, S. D. Crowley, and M. D. Halpern. 1995. Immunological properties of bacterial DNA. Ann. N. Y. Acad. Sci. 772:152-163. [DOI] [PubMed] [Google Scholar]
- 26.Randall, C. J., P. A. Meakins, M. P. Harris, and D. J. Watt. 1984. A new skin disease in broilers? Vet. Rec. 114:246.. [DOI] [PubMed] [Google Scholar]
- 27.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 28.Tokunaga, T., O. Yano, E. Kuramoto, Y. Kimura, T. Yamamoto, T. Kataoka, and S. Yamamoto. 1992. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol. Immunol. 36:55-66. [DOI] [PubMed] [Google Scholar]
- 29.Weeratna, R. D., M. J. McCluskie, Y. Xu, and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, F. G., and J. S. Marshall. 2001. CpG-containing oligodeoxynucleotides induce TNF-alpha and IL-6 production but not degranulation from murine bone marrow-derived mast cells. J. Leukoc. Biol. 69:253-262. [PubMed] [Google Scholar]
- 31.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]