Abstract
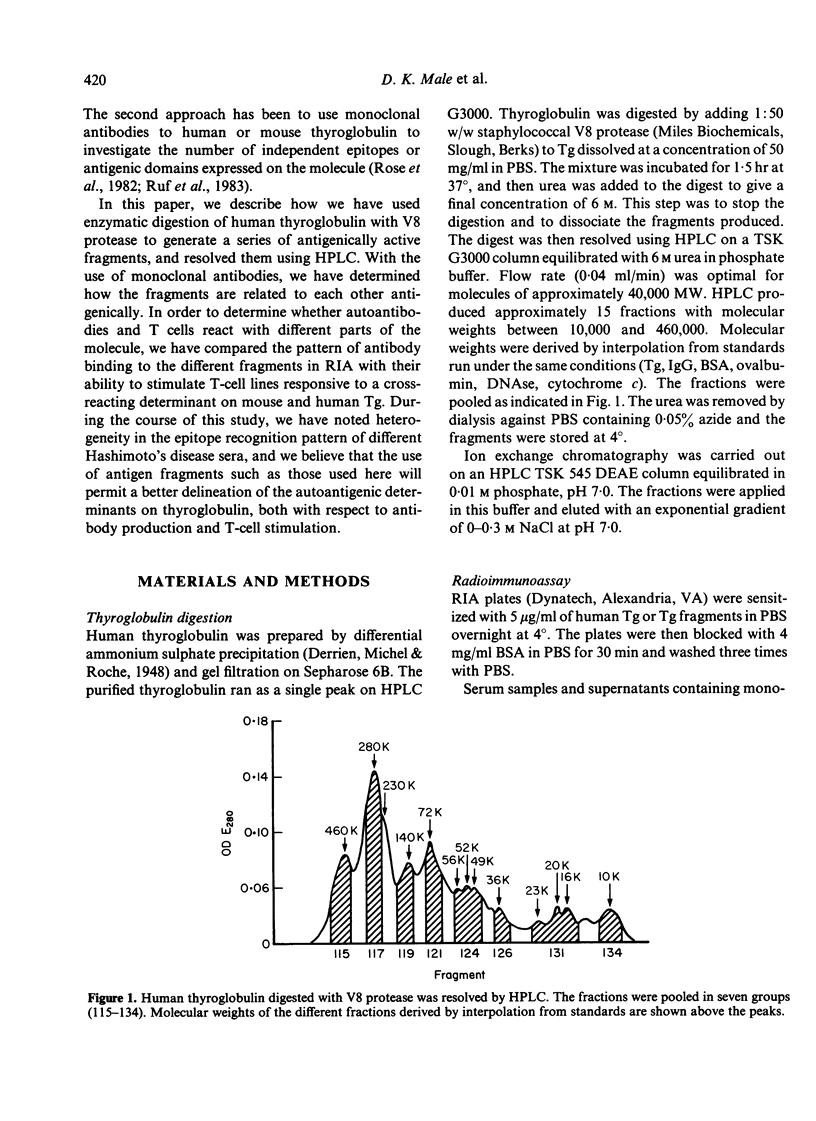
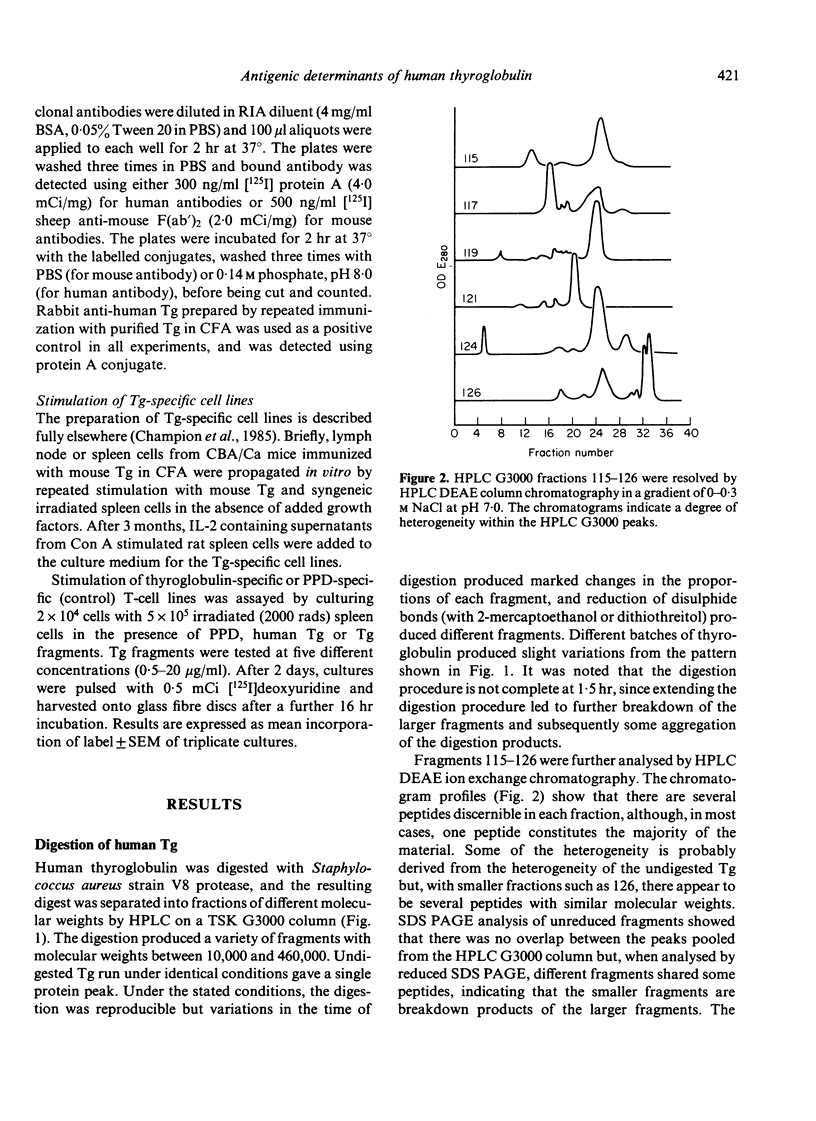
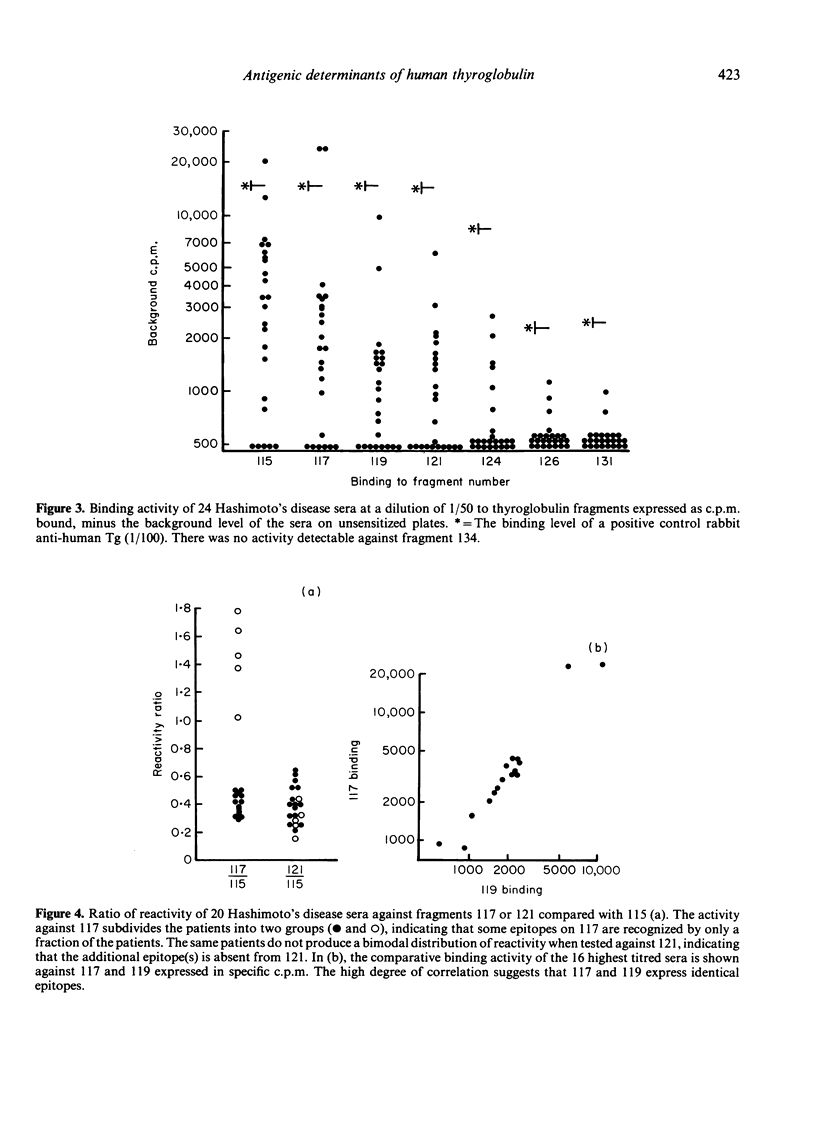
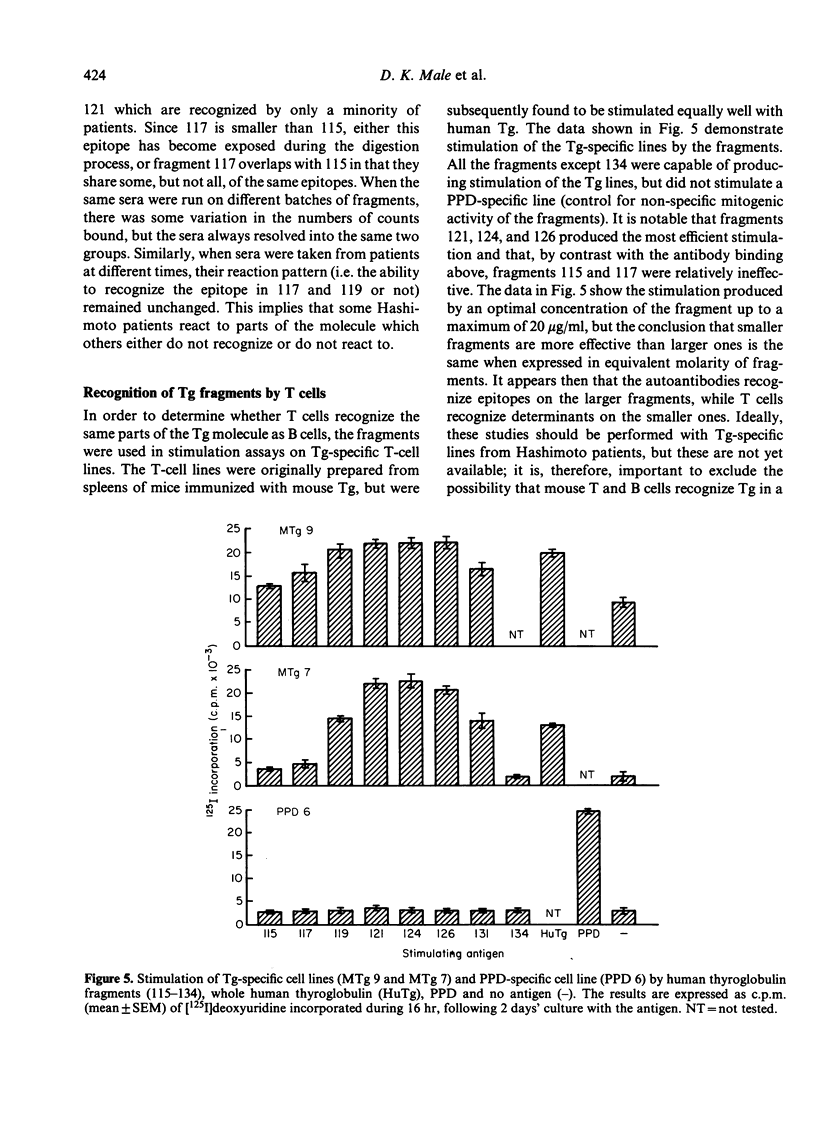
Human thyroglobulin (Tg) was digested with V8 protease and the fragments separated by high performance liquid chromatography (HPLC). The antigenic relationship of the fragments was investigated using mouse monoclonal antibodies to human Tg. The binding of Hashimoto's disease autoantibodies to the fragments was measured by radioimmunoassay. This demonstrated that a minority of the patients recognize an epitope on Tg which others do not. The epitopes identified by the autoantibodies were substantially destroyed in the smaller fragments tested, but these smaller fragments were more efficient stimulators of Tg-specific T-cell lines than the larger fragments which carry the antibody binding determinants. This suggests that the parts of the Tg molecule which stimulate autoimmune B cells differ from those which stimulate T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aihara Y., Sakata S., Nakamura S., Kamikubo K., Tarutani O., Yamada S., Tadokoro I., Okuda K. Genetic control of mouse antibody production to human thyroglobulin. J Immunogenet. 1983 Aug;10(4):325–331. doi: 10.1111/j.1744-313x.1983.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Champion B. R., Varey A. M., Katz D., Cooke A., Roitt I. M. Autoreactive T-cell lines specific for mouse thyroglobulin. Immunology. 1985 Mar;54(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Davoli C., Grimaldi S., Rusca G., Andreoli M., Edelhoch H. The isoelectric focusing of human thyroglobulin. Biochim Biophys Acta. 1982 Jul 26;705(2):243–248. doi: 10.1016/0167-4838(82)90184-4. [DOI] [PubMed] [Google Scholar]
- Mates G. P., Shulman S. Studies on thyroid proteins. 3. Precipitating activity of autoimmune and heteroimmune antisera with human thyroglobulin. Immunochemistry. 1967 Sep;4(5):319–330. doi: 10.1016/0019-2791(67)90115-2. [DOI] [PubMed] [Google Scholar]
- Nye L., Pontes de Carvalho L. C., Roitt I. M. Restrictions in the response to autologous thyroglobulin in the human. Clin Exp Immunol. 1980 Aug;41(2):252–263. [PMC free article] [PubMed] [Google Scholar]
- ROITT I. M., CAMPBELL P. N., DONIACH D. The nature of the thyroid auto-antibodies present in patients with Hashimoto's thyroiditis (lymphadenoid goitre). Biochem J. 1958 Jun;69(2):248–256. doi: 10.1042/bj0690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie D. P., McGregor A. M., Wright J., Weetman A. P., Hall R., Thorpe P. An immunotoxin of ricin A chain conjugated to thyroglobulin selectively suppresses the antithyroglobulin autoantibody response. Lancet. 1983 Dec 10;2(8363):1338–1340. doi: 10.1016/s0140-6736(83)91094-2. [DOI] [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. T and B cell reactivity in experimental autoimmune thyroiditis. Life Sci. 1983 Jan 3;32(1-2):127–138. doi: 10.1016/0024-3205(83)90180-7. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Accavitti M., Pydyn E. F., Leon M. A., Brown R. K. The use of hybridoma antibodies to probe the antigenic determinants of thyroglobulin. Adv Exp Med Biol. 1982;150:23–35. doi: 10.1007/978-1-4684-4331-8_2. [DOI] [PubMed] [Google Scholar]
- Ruf J., Carayon P., Sarles-Philip N., Kourilsky F., Lissitzky S. Specificity of monoclonal antibodies against human thyroglobulin; comparison with autoimmune antibodies. EMBO J. 1983;2(10):1821–1826. doi: 10.1002/j.1460-2075.1983.tb01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salabè G. B., Davoli C., Fontana S. The immunoreaction of auto- and heteroantisera with modified human thyroglobulin. Int Arch Allergy Appl Immunol. 1973;44(2):181–196. doi: 10.1159/000230927. [DOI] [PubMed] [Google Scholar]
- Stylos W. A., Rose N. R. Splitting of human thyroglobulin. II. Enzymatic digestion. Clin Exp Immunol. 1969 Sep;5(3):285–297. [PMC free article] [PubMed] [Google Scholar]
- Stylos W. A., Rose N. R. Splitting of human thyroglobulin. IV. The antigenicity of the pepsin-derived fragments. Clin Exp Immunol. 1977 Feb;27(2):245–253. [PMC free article] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science. 1971 Dec 10;174(4014):1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Cellular basis of the genetic control of immune responsiveness to murine thyroglobulin in mice. Cell Immunol. 1975 May;17(1):106–113. doi: 10.1016/s0008-8749(75)80010-4. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Zaleski M. B. Gene(s) at I-A subregion controls the autoimmune response to thyroglobulin in mice. Immunol Commun. 1981;10(4-5):341–347. doi: 10.3109/08820138109050699. [DOI] [PubMed] [Google Scholar]



