Abstract
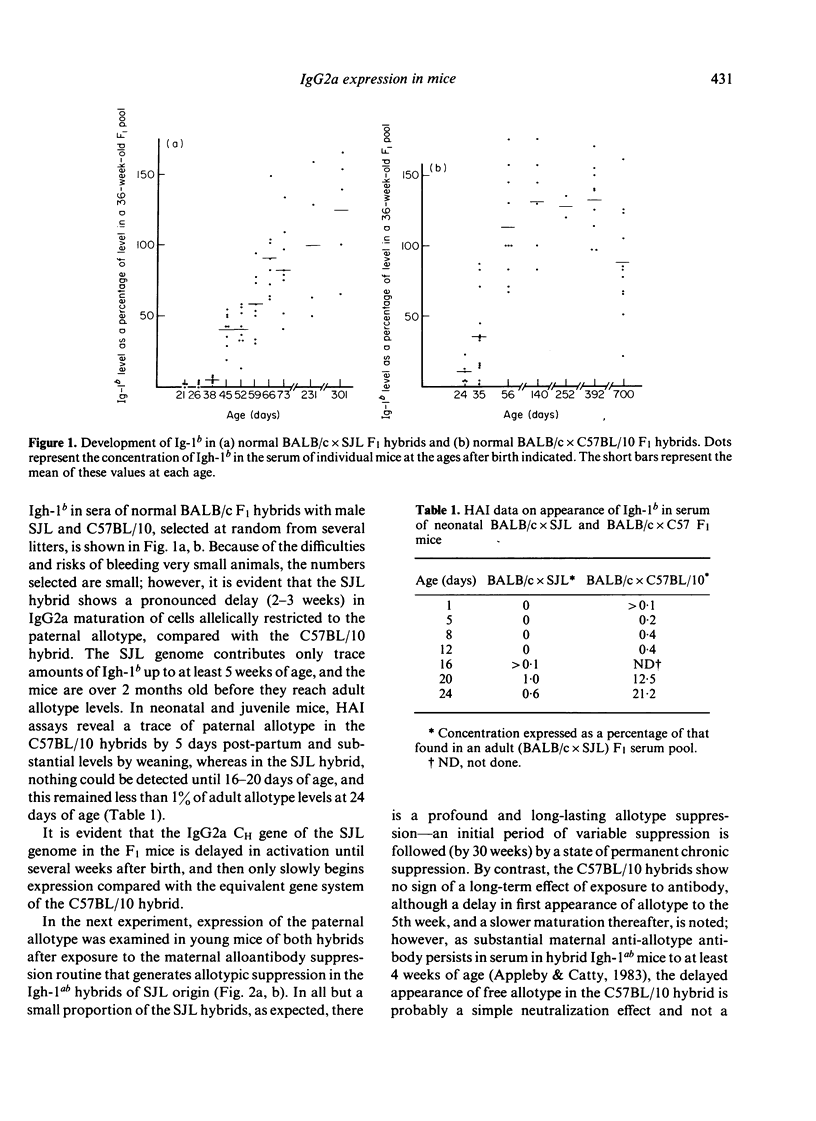
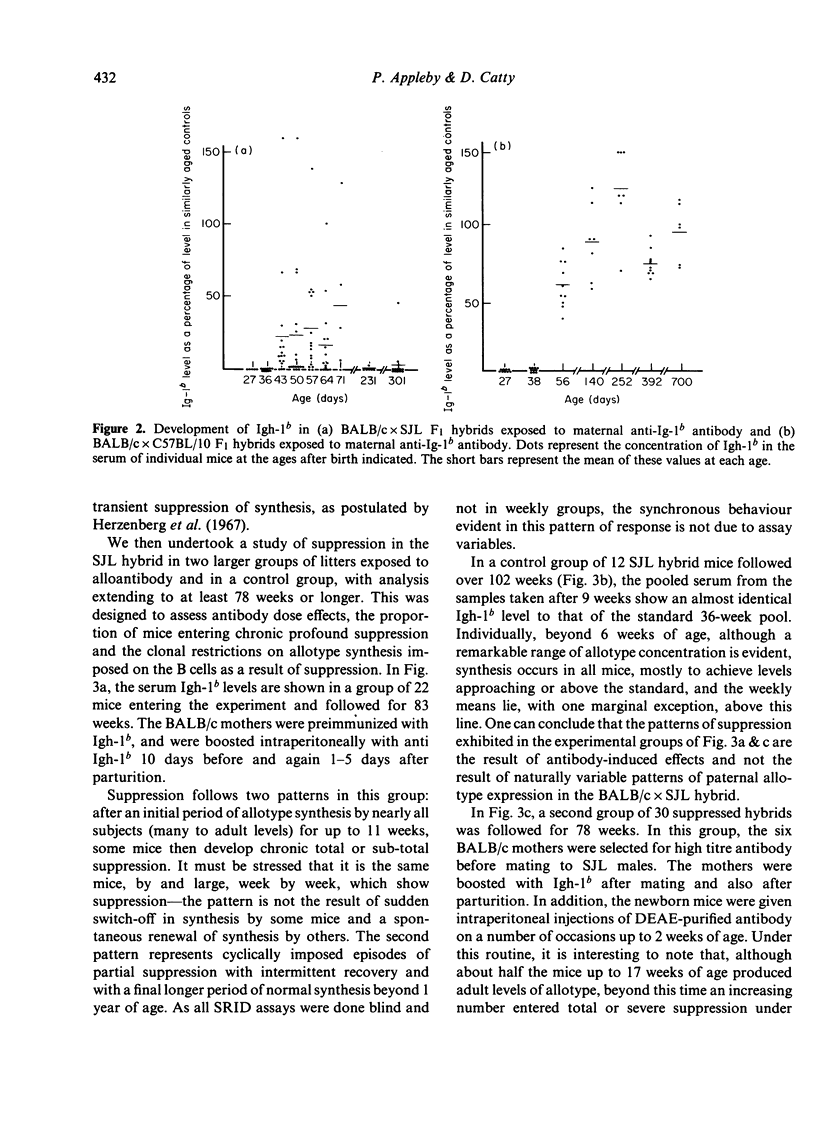
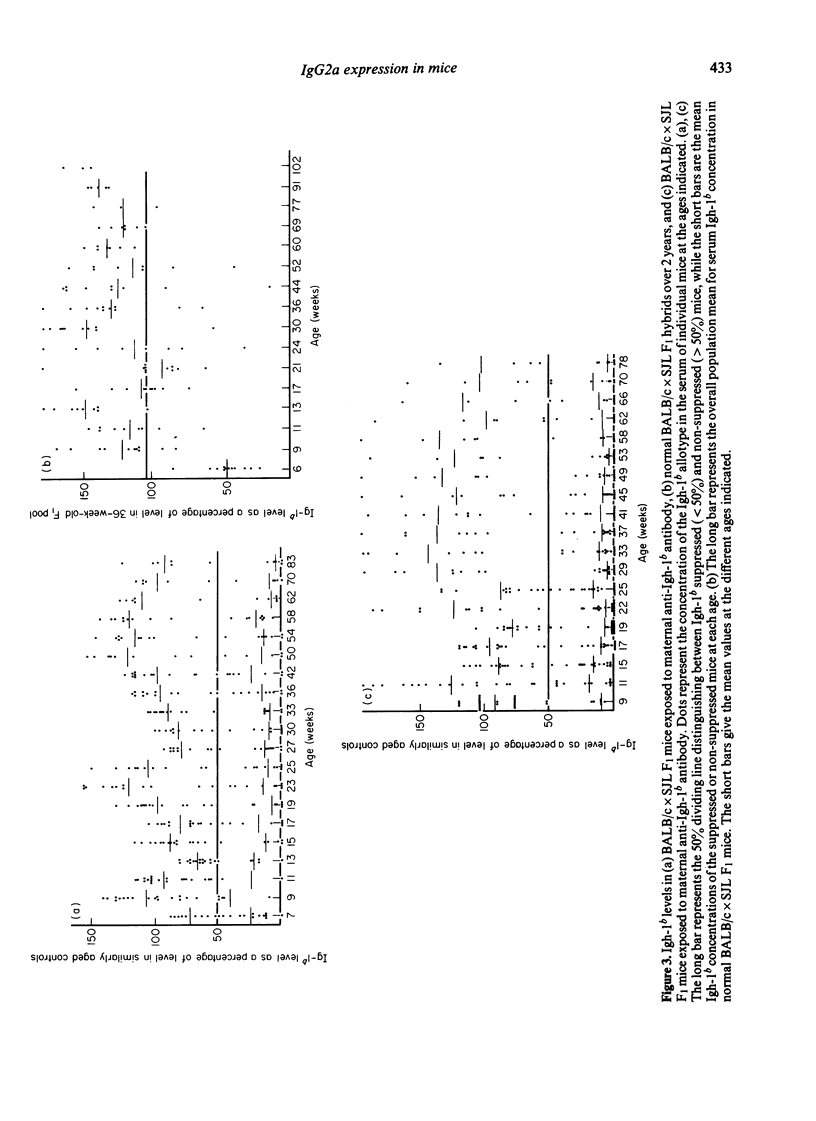
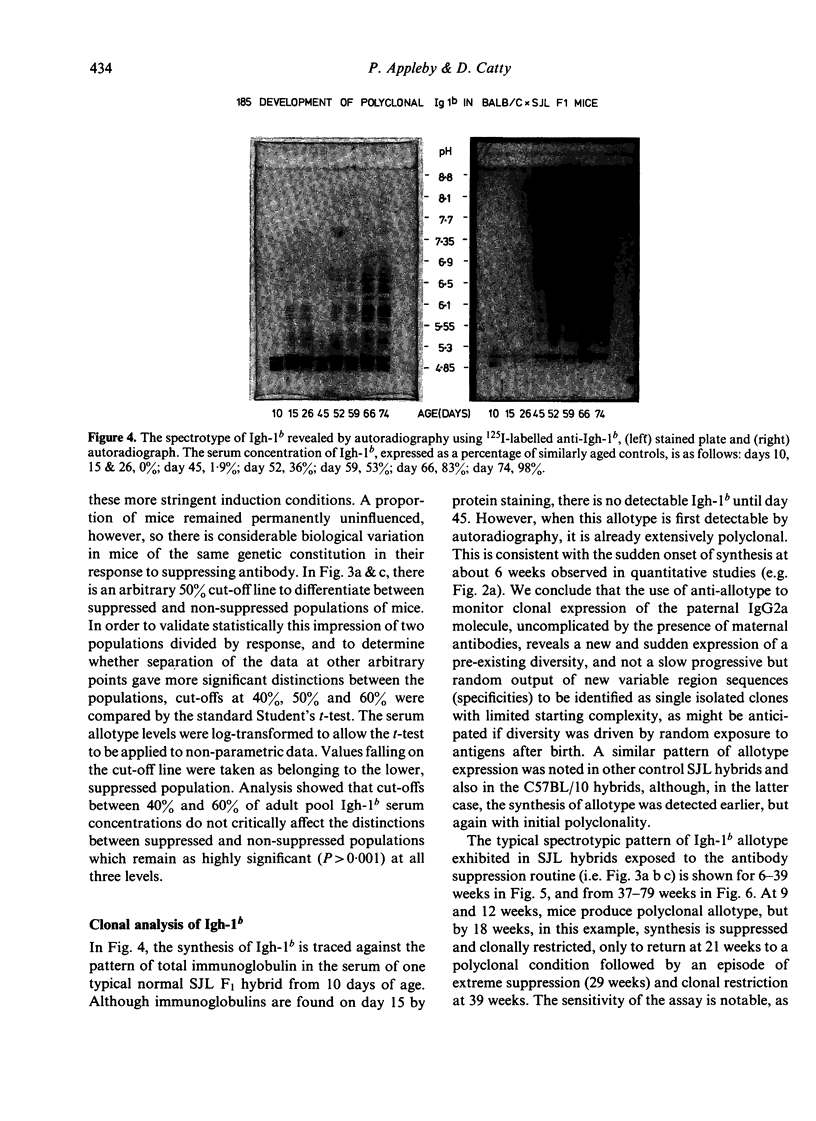
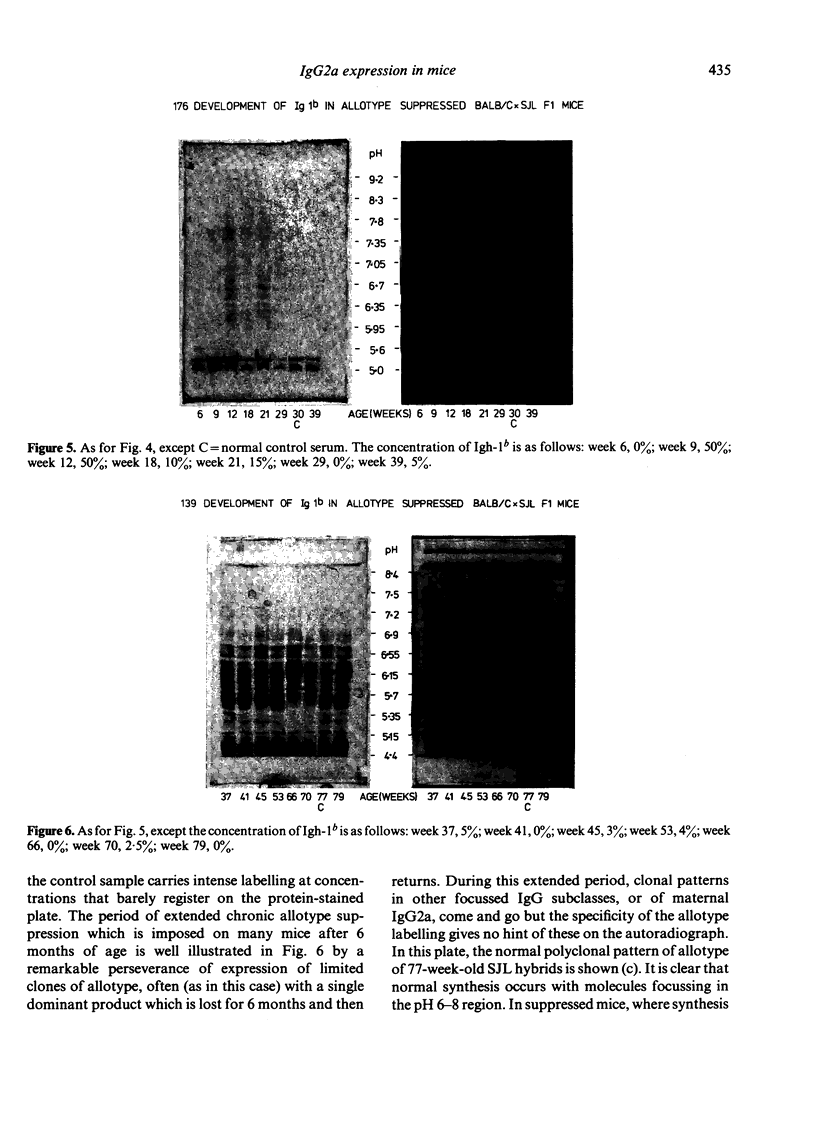
The synthesis and clonal diversity of IgG2a molecules bearing the paternally inherited immunoglobulin allotype have been examined in the offspring of matings between BALB/c mothers (Igh-1a) and SJL or C57BL/10 males (both Igh-1b) using a sensitive quantitative single radial immunodiffusion in gel assay and isoelectric focusing with autoradiography. In normal litters, the first detectable paternally-marked IgG2a is extensively polyclonal in both F1 crosses (i.e. diversity precedes expression); however, there is a delay of 2-3 weeks in the first appearance of the clonally diverse set of molecules when these are coded by the SJL genome, compared with the C57BL/10. Delayed maturation of allelically-excluded Igh-1b-expressing B cells in the (BALB/c X SJL)F1 may explain the unique susceptibility of these offspring to chronic allotype suppression when exposed to maternal anti-Igh-1b antibodies in early life. We find that, although such suppressed mice may begin life with a (delayed) synthesis of polyclonal IgG2a of paternal allele (Igh-1b), the condition of chronic suppression later imposed in the majority of mice is associated with spectrotype (clonal) simplicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby P., Catty D. Spectrotypic analysis of passively acquired and newly synthesised IgG antibodies in the neonatal and young mouse. J Reprod Immunol. 1984 May;6(3):177–186. doi: 10.1016/0165-0378(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Appleby P., Catty D. Transmission of immunoglobulin to foetal and neonatal mice. J Reprod Immunol. 1983 Jul;5(4):203–213. doi: 10.1016/0165-0378(83)90236-x. [DOI] [PubMed] [Google Scholar]
- Black S. J., Herzenberg L. A. B-cell influences on the induction of allotype suppressor T cells. J Exp Med. 1979 Jul 1;150(1):174–183. doi: 10.1084/jem.150.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAY S. Effect of maternal isoantibodies on the quantitative expression of two allelic genes controlling gamma-globulin allotypic specificities. Nature. 1962 Aug 18;195:677–680. doi: 10.1038/195677a0. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Musallam R., Keeler K. D., Dresser D. W. Restricted heterogeneity of antibody synthesized by T-cell deprived mice. Immunology. 1979 Sep;38(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. The chromic chloride method of coupling antigens to erythrocytes: definition of some important parameters. J Immunol Methods. 1976;10(1):61–66. doi: 10.1016/0022-1759(76)90007-7. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Goodlin R. C., Rivera E. C. Immunoglobulin synthesis in mice: suppression by anti-allotype antibody. J Exp Med. 1967 Oct 1;126(4):701–713. doi: 10.1084/jem.126.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Short-term and chronic allotype suppression in mice. Contemp Top Immunobiol. 1974;3:41–75. doi: 10.1007/978-1-4684-3045-5_2. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Okumura K., Cantor H., Sato V. L., Shen F. W., Boyse E. A., Herzenberg L. A. T-cell regulation of antibody responses: demonstration of allotype-specific helper T cells and their specific removal by suppressor T cells. J Exp Med. 1976 Aug 1;144(2):330–344. doi: 10.1084/jem.144.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN R., DRAY S. MATERNAL-FETAL MORTALITY IN MICE WITH ISOANTIBODIES TO PATERNAL GAMMA-GLOBULIN ALLOTYPES. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1069–1074. doi: 10.3181/00379727-116-29454. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Mechanisms of clonal abortion tolerogenesis. I. Response of immature hapten-specific B lymphocytes. J Exp Med. 1978 Nov 1;148(5):1161–1170. doi: 10.1084/jem.148.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. M., Waldmann H. Monogamous T helper cell. Nature. 1977 Aug 18;268(5621):641–642. doi: 10.1038/268641a0. [DOI] [PubMed] [Google Scholar]






