Abstract
Previous studies demonstrated that the innate resistance of mice to Listeria monocytogenes infection by intravenous or intraperitoneal inoculation is regulated principally by the Hc locus on mouse chromosome 2. The A/J and C57BL/6 mouse strains were identified as prototype L. monocytogenes-susceptible and -resistant strains, respectively. In the present study, we compared the relative susceptibilities of A/J and C57BL/6 mice to intragastric (i.g.) inoculation with L. monocytogenes. The results of our study indicate that A/J mice are significantly more susceptible than C57BL/6 mice to an i.g. challenge with L. monocytogenes. This was reflected in the estimated 50% lethal doses for the two strains (106 and 108 CFU for A/J and C57BL/6 mice, respectively) and a more rapid and severe dissemination of the infection to the spleen and liver in A/J mice than in C57BL/6 mice. Histopathological examination of tissues from the infected mice confirmed the greater severity of disease in A/J mice. Clearance of a primary infection enhanced the resistance of both A/J and C57BL/6 mice to reinfection with L. monocytogenes via the gastrointestinal tract. However, the relative difference in susceptibility between the two strains was evident even after immunization. The A/J mouse holds promise as a model for investigating the pathogenesis of gastrointestinal listeriosis because of its ability to develop systemic infection following challenge with numbers of organisms similar to those recovered from some L. monocytogenes-contaminated food products.
Listeria monocytogenes has been widely used as a model for studying the pathogenesis of facultative intracellular pathogens and for investigating the regulation of cellular immunity (9). In addition, L. monocytogenes is an important food-borne pathogen. It is estimated that L. monocytogenes causes approximately 2,500 cases of serious illness and as many as 500 deaths per year in the United States (20, 28). Most of these cases involve fetal infection or infection of adults with some known defect or diminishment in immune function (e.g., immunosuppressive therapy, malignancy, or old age). However, some cases occur in adults with no known predisposing condition, suggesting that there may be considerable genetic variability in the human response to L. monocytogenes infection following ingestion of contaminated food products (20, 28, 32). Because of the risk posed to susceptible individuals, the United States has a very stringent regulation (less than 1 CFU per 25 g of product) for L. monocytogenes in ready-to-eat foods (28). The resulting recalls of L. monocytogenes-contaminated food products result in large costs to the food industry annually.
We know relatively little about the virulence mechanisms that allow L. monocytogenes to cause invasive disease following introduction into the human gastrointestinal (GI) tract nor about the host defense mechanisms that protect against GI listeriosis (15). Murine listeriosis studies have identified some virulence determinants (23, 27, 31) and the importance of certain elements of innate and adaptive immune responses (4, 6, 10, 16, 24, 25, 29) that influence the pathogenesis of systemic listeriosis following inoculation of L. monocytogenes into the GI tract. However, the contributions of these bacterial and host factors to the pathogenesis of local versus systemic infection are incompletely understood. Further progress in this area has been limited in part by the general resistance of mice to listeriosis when inoculated via the GI tract. Although one report describes lethal infection in mice that were inoculated with a relatively low challenge dose via the oral route (30), most reports have described the need to use inocula of 108 CFU or greater to cause systemic infection following introduction of L. monocytogenes into the GI tracts of normal mice (2, 6, 10, 16, 19, 22, 23, 25, 29, 33).
Earlier studies demonstrated that genetic regulation of innate resistance to listeriosis in mice inoculated via the intravenous (i.v.) or intraperitoneal (i.p.) route is controlled chiefly by the Hc locus on chromosome 2 (5, 7, 17). Mouse strains that possess the resistant allele at the Hc locus are far more resistant to an i.v. or i.p. challenge with L. monocytogenes than are mouse strains with the susceptible allele (an approximately 100-fold difference in 50% lethal dose [LD50] or in bacterial burden following an experimental challenge). These studies demonstrated that the C57BL/6 and A/J strains of mice are prototype resistant and susceptible strains of mice when inoculated parenterally (i.v. or i.p. route) with L. monocytogenes (7, 17). There have been no published reports comparing the differing levels of resistance of these strains of mice to GI infection with L. monocytogenes. In the present study, we compared the C57BL/6 and A/J strains of mice for resistance to intragastric (i.g.) inoculation with two strains of L. monocytogenes (Scott A and EGD). The results of this study indicate that the A/J mouse strain is significantly more susceptible than the C57BL/6 strain to infection with L. monocytogenes via the GI tract based on survival rates (the A/J strain was susceptible at an approximately 100-fold-lower LD50), bacterial burdens in the spleen and liver, and histopathology. These findings suggest that the A/J mouse strain may be a useful model for investigating the pathogenesis of GI listeriosis.
MATERIALS AND METHODS
Strains of L. monocytogenes.
Two strains of L. monocytogenes that have been used previously in our laboratory were used in the present study. Strain Scott A (serotype 4b) was originally a clinical isolate obtained from a food-borne disease outbreak (11). Strain EGD (serotype 1/2a) has been widely used for laboratory investigations of molecular pathogenesis, host defense, and cellular immunity in vitro and in vivo (10, 17, 23). We have reported previously that both strains of L. monocytogenes can cause systemic infection following i.g. inoculation of outbred ICR mice, with the severity of infection being greater for strain Scott A-infected mice. (11).
Preparation of L. monocytogenes.
L. monocytogenes was inoculated into brain heart infusion (BHI) broth and incubated overnight with shaking at 37°C. Following this, the bacteria were harvested by centrifugation, resuspended in BHI broth containing 20% glycerol, and stored at −70°C as 1-ml aliquots. Before each experiment, an aliquot was thawed, inoculated into 50 ml of BHI broth, and incubated at 37°C with shaking until mid-log-phase growth was reached. The optical density of the bacterial suspension was read with a spectrophotometer, and the numbers of CFU of L. monocytogenes were extrapolated from a standard growth curve. To prepare the inoculum for the mice, appropriate dilutions were made in sterile phosphate-buffered saline to achieve the desired bacterial concentration. The actual number of CFU in the inoculum was verified by plating on blood agar.
Inoculation of mice.
Female inbred A/J and C57BL/6 mice and outbred ICR mice were obtained (Harlan Sprague-Dawley, Indianapolis, Ind.) at 5 to 6 weeks of age and housed under microisolator caps at the School of Veterinary Medicine animal care facility. The mice were acclimated for 1 to 2 weeks in this facility before being used in an experiment. The mice received food and water ad libitum until 5 h prior to an i.g. inoculation experiment, at which time food was removed from the cage. This was done to prevent the delivery of the listerial inoculum into the stomachs of mice that were engorged with mouse chow, which could lead to aspiration of the inoculum into the lungs. The mice were anesthetized by an i.p. injection of sodium pentobarbital (0.75 to 1 mg per 25 g of mouse body weight). As soon as the mice were mildly sedated, the listerial inoculum was introduced (in a total volume of 0.2 ml) via a 1.5-in.-long, 24-gauge, stainless steel feeding needle attached to a 1-ml syringe.
Recovery of L. monocytogenes from the tissues of infected mice.
At the desired time points, the mice were humanely euthanized by asphyxiation with CO2; this was followed by exsanguination and cervical dislocation. In some experiments, blood was collected into a syringe containing sodium citrate as an anticoagulant. The blood was then serially diluted in sterile saline, plated in duplicate (0.1 ml) on blood agar, and incubated at 37°C to detect bacteremia with L. monocytogenes. Following this, tissues were collected to assess the bacterial burden by plate counts. The skull was opened and a portion of the brain was aseptically removed, and then the abdominal cavity was aseptically opened and portions of the spleen and liver were removed. These tissues were weighed in sterile weigh boats and placed in sterile tissue grinders that contained 1 ml of cold, sterile saline. The tissues were then homogenized, diluted in sterile saline, and plated in duplicate on blood agar. The cecum and its contents were also removed, homogenized in saline as indicated above, and plated on modified Oxford agar. The plates were allowed to dry and then incubated at 37°C for 48 h. The colonies were counted, and the data were expressed as the mean ± standard error of the mean (SEM) log10 CFU of L. monocytogenes per gram of tissue (wet weight).
Histopathology.
At the time of necropsy, portions of the brain, spleen, liver, and terminal ileum were removed, placed in plastic cassettes, and fixed in 10% buffered formalin. Following fixation, the samples were cut into thin sections, mounted on glass slides, and stained with hemotoxylin and eosin and a tissue Gram stain. The slides were then evaluated microscopically by an American College of Veterinary Pathology board-certified pathologist.
Mouse perfusion.
After the mice were euthanized, their body cavities were opened as described previously. With each mouse, the rib cage was carefully cut away to expose the heart, and a 25-gauge butterfly needle attached to a 10-ml syringe containing 0.85% saline with 1% sodium citrate was gently inserted into the left ventricle. Ten milliliters of saline solution was slowly injected into the heart, and the right atrium was clipped with scissors, allowing perfusion to take place over the course of about 5 min. Perfusion was considered complete after the liver had turned pale in color. At this point, the brain, spleen, and liver were removed and processed for bacteriological and histopathological examination as described above.
Statistical analysis.
Data were analyzed by using a repeated-measure analysis of variance with Prism version 3.0 (GraphPad Software, Inc., San Diego, Calif.). If a significant F value was obtained (P < 0.05), then the Tukey-Kramer test was performed to determine whether the means for treatment groups differed from those for the controls. The statistical significance for all comparisons was set at a P of <0.05.
RESULTS
A/J mice are susceptible and C57BL/6 mice are resistant to i.g. inoculation with L. monocytogenes.
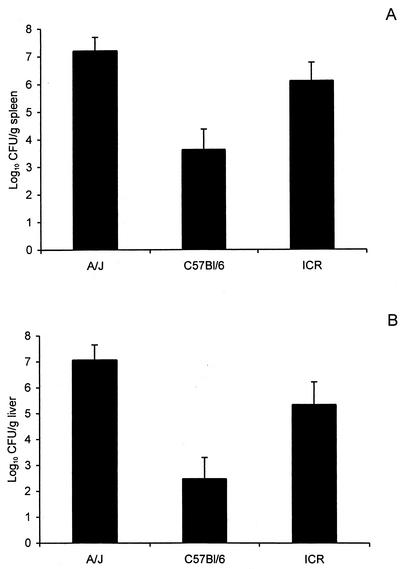
In our first experiments, we compared the levels of resistance of C57BL/6, A/J, and ICR (outbred) mice to an i.g. challenge with 108 CFU of L. monocytogenes strain Scott A. The mice were anesthetized with sodium pentobarbital to facilitate i.g. inoculation and euthanized at 3 days after challenge, a time point that we and others have previously demonstrated represents a peak bacillary burden in the spleen and liver (11, 31). At this high challenge dose, the C57BL/6 mice exhibited significantly greater resistance, as demonstrated by the lower numbers of CFU recovered from the spleen and liver, than did the other two strains of mice (Fig. 1). Although the data in Fig. 1 suggest that the A/J and ICR mice had similar bacterial burdens in the spleen and liver, these numbers do not adequately reflect the more severe and rapid course of infection in the A/J mice. Fifty percent of the A/J mice (in two separate experiments) had succumbed, or were moribund and humanely euthanized, by day 3 after challenge. The mice that died or were euthanized because of their debilitated state were assigned a value of 108 CFU of L. monocytogenes in the spleen and liver. This value was chosen based on previous experiments which indicated that this is the bacterial burden at which mice begin to die due to experimental listeriosis (7, 8). Because the A/J mice succumbed so rapidly (most deaths within 24 to 48 h after challenge), we considered the possibility that dissemination of the infection to the central nervous system had occurred. When we necropsied mice that died of infection or were euthanized because of their debilitated state, we recovered L. monocytogenes from the brains of some of the mice (data not shown).
FIG. 1.
Systemic infection is less severe in C57BL/6 mice than in A/J or ICR mice when inoculated i.g. with L. monocytogenes Scott A. The mice were anesthetized with sodium pentobarbital and then inoculated i.g. with 108 CFU of Scott A. Three days later, the mice were euthanized and the numbers of CFU of listeriae present in the spleens (A) and livers (B) were determined as described in Materials and Methods. The data are given as the mean result ± SEM. for 12 mice per group (two separate experiments with six mice per group). For the C57BL/6 mouse results, P is <0.01 when compared to those for the A/J mice and P is <0.05 when compared to those for the ICR mice, as determined by one-way analysis of variance, followed by the Neuman-Keuls comparison test.
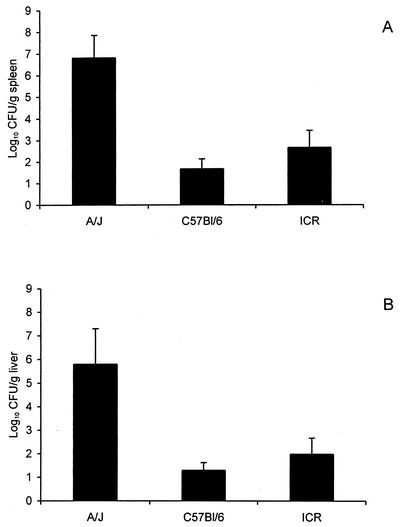
Because the severity of infection in the A/J mice at the 108-CFU challenge dose confounded our ability to quantify the bacterial burden (i.e., death of some of the mice), we repeated this experiment using a challenge dose of approximately 106 CFU (Fig. 2). This challenge dose clearly demonstrated the greater susceptibility of the A/J mouse strain to i.g. inoculation with L. monocytogenes compared to that of the C57BL/6 and ICR mice, which yielded significantly lower numbers of CFU from their spleens and livers. We further confirmed the difference between the A/J and C57BL/6 mouse strains by performing a modified LD50 challenge study which estimated the i.g. challenge LD50s for the A/J and C57BL/6 mouse strains as being approximately 106 and 108 CFU, respectively (Table 1). This 100-fold difference in the susceptibilities of the A/J and C57BL/6 mouse strains to i.g. infection with L. monocytogenes is similar to that in previous reports for mice inoculated via the i.v. or i.p. route (5, 7, 8, 17). We further confirmed the increased susceptibility of the A/J mouse strain by using a second strain (EGD) of L. monocytogenes. All A/J mice (six of six) inoculated with 108 CFU of L. monocytogenes EGD died of disseminated infection within 2 days of challenge, whereas there was only one death among the C57BL/6 or ICR mice (six mice per group).
FIG. 2.
Greater severity of infection in A/J mice than in C57BL/6 or ICR mice inoculated i.g. with 106 CFU of L. monocytogenes Scott A. The mice were euthanized 3 days postinoculation, and the numbers of CFU of listeriae were determined for the spleens (A) and livers (B) as described in Materials and Methods. The data are given as the mean results ± SEM for six mice per group. For the A/J mouse results, P is <0.01 and P is <0.05, for the spleens and livers, respectively, when compared to the results for both C57BL/6 and ICR strains as determined by analysis of variance, followed by the Neuman-Keuls comparison test.
TABLE 1.
Survival of naïve and immunized A/J and C57B1/6 mice following inoculation of L. monocytogenes Scott A
| Mouse type | Challenge dose (CFU) | No. of deaths/no. inoculated
|
|
|---|---|---|---|
| A/J mice | C57B1/6 mice | ||
| Naïvea | 109 | NT | 4/6 |
| 108 | NT | 4/6 | |
| 107 | 6/6 | 1/6 | |
| 106 | 2/6 | NT | |
| 105 | 1/6 | NT | |
| Immunizedb | 1010 | NT | 2/7 |
| 108 | 2/8 | NT | |
Naïve mice were anesthetized with sodium pentobarbital and inoculated i.g. with the indicated dose of L. monocytogenes Scott A. Deaths were recorded for a 10-day period after challenge.
Mice that survived the experimental infection were challenged i.g. 2 weeks later with the indicated dose of L. monocytogenes Scott A, and deaths were recorded for a 7-day period.
Dissemination to internal organs occurs more rapidly in A/J than C57BL/6 mice.
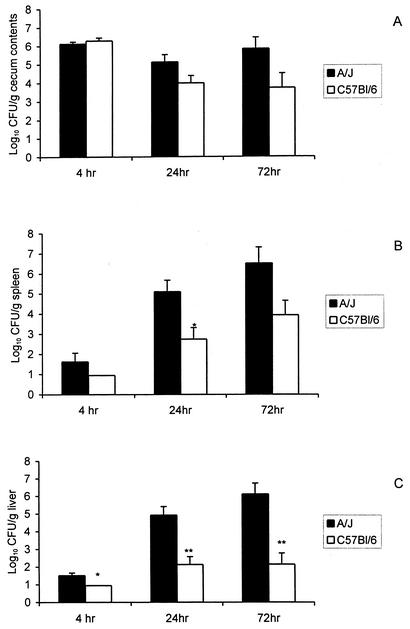
We next examined the temporal course of infection in susceptible A/J and resistant C57BL/6 mice. To minimize the loss of animals due to lethal infection, we inoculated A/J mice and C57BL/6 mice with approximately 0.1 LD50 of L. monocytogenes Scott A (105 and 107 CFU, respectively). Groups of six mice were euthanized at 4, 24, and 72 h after inoculation and the severity of infection was quantified by recovery of viable listeriae from the ceca, spleens, livers, bloodstreams, and brains of infected animals. As reported previously, the number of L. monocytogenes recovered from the cecum was less than the number in the inoculum within 4 h after inoculation (31) and continued to decline with time in the C57BL/6 mice. In contrast, the numbers of CFU in the ceca of A/J mice were relatively stable at 4 to 72 h after challenge, suggesting that perhaps L. monocytogenes was better able to survive in the gut, or invade and multiply within the intestinal mucosa, in the A/J mice than in the C57BL/6 mice (Fig. 3A). Despite the 100-fold difference in the challenge doses, L. monocytogenes was better able to translocate across the GI mucosa and cause systemic infection in the A/J mice than in the C57BL/6 mice. Greater numbers of CFU were recovered from the spleens of A/J mice than from the spleens of the C57BL/6 mice at 24 and 72 h after challenge (Fig. 3B). An even greater difference between the two mouse strains was noted in the numbers of CFU in the liver (Fig. 3C). In keeping with the greater severity of infection in the A/J mice, we detected low-level bacteremias at 4 and 24 h (approximately 102 CFU/ml) and a substantial bacteremia (approximately 105 CFU per ml) in the A/J mice at 72 h (data not shown). In contrast, bacteremia was not observed in the C57BL/6 mice, which is consistent with the relatively low bacterial burdens in the spleens and livers of that mouse strain. We also recovered L. monocytogenes from the brains of A/J mice at 24 and 72 h after infection (approximately 1.5 and 4.0 log10 CFU/g, respectively). Because we did not perfuse the brains before the tissues were homogenized and plated, we could not exclude the possibility that the CFU recovered from the brain homogenates represented listeriae in the bloodstream rather than in the brain parenchyma per se.
FIG. 3.
L. monocytogenes is better able to translocate across the gastrointestinal mucosa and cause systemic infection in A/J mice than in C57BL/6 mice despite a 100-fold difference in the challenge doses. A/J and C57BL/6 mice were anesthetized as previously described and then inoculated i.g. with a 0.1 LD50 challenge of L. monocytogenes Scott A (105 and 107 CFU, respectively). Six mice from each group were euthanized at 4, 24, and 72 h after inoculation, and the numbers of CFU of listeriae present in the ceca (A), spleens (B), and livers (C) were determined as described in Materials and Methods. *, P < 0.05; **, P < 0.005 (as determined by the Mann-Whitney nonparametric test.).
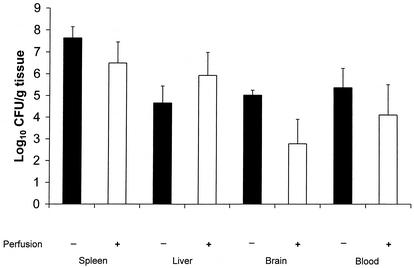
To address the question of whether L. monocytogenes recovered from brain homogenates was due to listeriae in the bloodstream, a subsequent experiment was performed in which groups of A/J mice were infected with 106 CFU of L. monocytogenes (Fig. 4) and euthanized 3 days later. One group of mice was perfused with saline before the spleen, liver, and brain samples were removed, and the other was not. Perfusion slightly reduced the numbers of CFU recovered from the spleen and liver samples, but the effect was not significant. Perfusion reduced the numbers of CFU recovered from the brains below the limits of detection for three of five mice, whereas the remaining two mice still yielded substantial numbers of CFU from homogenates of their brain tissue. These data suggest that in some cases L. monocytogenes may actually invade the brain parenchyma, whereas in other cases L. monocytogenes may be present in the brain vasculature rather than the brain parenchyma.
FIG. 4.
Effects of perfusion on the recovery of L. monocytogenes from A/J mouse tissues. The mice were inoculated i.g. with 106 CFU of L. monocytogenes Scott A. Three days later, some mice were euthanized and their bloodstreams were perfused with saline as described in Materials and Methods. Portions of the brains, spleens, and livers were removed and processed to enumerate viable L. monocytogenes CFU. Other mice were euthanized, and their tissues were removed and processed without perfusion. The results are the means ± SEM. for five mice per group.
Histopathological lesions are more severe in A/J mice than in C57BL/6 mice.
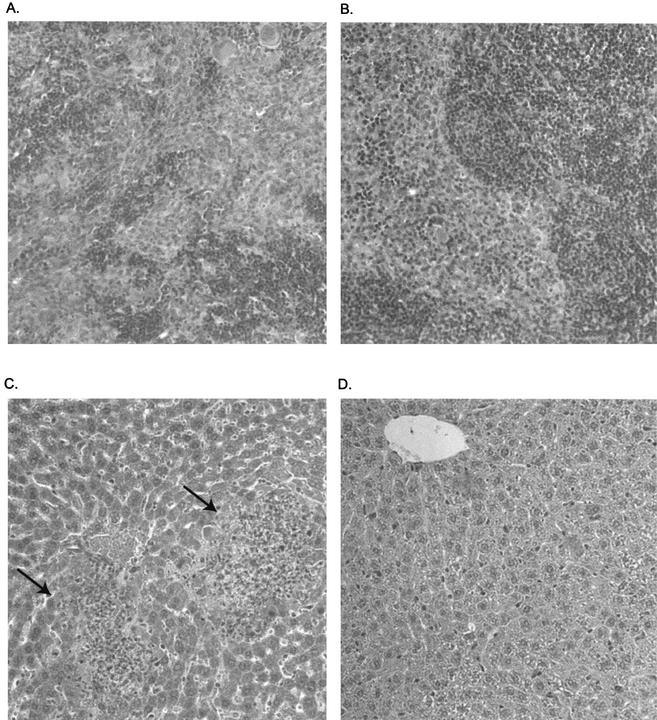
Histopathological examination of tissues taken from the two strains of mice at 3 days after inoculation confirmed the bacteriological data described above (Fig. 3). No inflammatory lesions or tissue bacteria were observed in the ilea or brains of either strain of mice. Lesions were observed in the spleens and livers of both strains of mice but were more prominent and severe in the A/J mice. Four of the six A/J mice exhibited moderate to severe necrotizing hepatitis, with intracellular bacteria being visible within hepatocytes in the lesions (Fig. 5A). These same four mice exhibited mild to moderate suppurative splenitis. Necrotic foci in the spleens of the A/J mice often contained visible bacteria (Fig. 5B). Discrete to coalescing periarteriolar lymphoid sheaths were observed in the same mice; the other two mice exhibited marked lymphoid follicular hyperplasia. In contrast, the histopathological changes in the C57BL/6 mice were less frequent and milder than those observed in the A/J mice. The livers exhibited rare to small numbers of scattered small aggregates of mixed inflammatory cells (Fig. 5C). Few to moderate numbers of periarteriolar lymphoid sheaths were observed in the spleens (Fig. 5D). Bacteria were not observed in the livers and spleens of the C57BL/6 mice, which is consistent with the lower numbers of CFU that were recovered from these mice. Extramedullary hematopoiesis was observed in the spleens of both A/J and C57BL/6 mice.
FIG. 5.
Histopathological damage is greater in the spleens and livers of A/J mice than in those of C57BL/6 mice. The A/J and C57BL/6 mice were anesthetized as previously described and then inoculated i.g. with a 0.1 LD50 challenge of L. monocytogenes Scott A (105 and 107 CFU, respectively). The mice were euthanized at 72 h after challenge, and their tissues were transferred to buffered formalin. Following fixation, thin sections of the tissues were cut, mounted on glass slides, and stained with hemotoxylin and eosin. (A) Disruption of a germinal center in spleen tissue of an A/J mouse, with infiltration of neutrophils and macrophages. (B) Normal-appearing spleen tissue from a C57BL/6 mouse. (C) Liver tissue from an A/J mouse exhibiting necrotic foci (arrows) with infiltration of neutrophils and macrophages. Gram-stained sections frequently revealed the presence of intracellular bacteria in these lesions (data not shown). (D) In contrast to the A/J mouse liver tissue, liver tissue from a C57BL/6 mouse appears relatively normal. Magnification, ×180 (original magnification, ×200).
Prior clearance of a primary infection protects A/J and C57BL/6 mice against a second i.g. challenge with L. monocytogenes.
One of the hallmarks of murine listeriosis is that clearance of a primary infection confers substantial protection against reinfection via the i.v. or i.p. route. We were interested in determining whether clearance of an infection that originated in the GI tract would protect mice against reinfection via the same route. To do so, we took mice that survived the experimental infections used to determine the LD50 in both the A/J and C57BL/6 strains of mice (Table 1) and then reinfected them 2 weeks later via the i.g. route with an inoculum that was 100-fold-higher than the LD50 for the respective strain of naïve mice. As indicated in Table 1, most of the mice that survived the initial infection (i.e., immunized mice) were protected against reinfection with a 100-fold-higher LD50 challenge (108 and 1010 CFU for the A/J and C57BL/6 strains, respectively). Most of the immunized mice (approximately 75%) survived this severe challenge dose, whereas the naïve control mice succumbed to a much lower dose (Table 1).
DISCUSSION
The results of these experiments demonstrate that the A/J mouse strain is relatively susceptible to L. monocytogenes infection via the GI tract. The difference in innate resistance between the A/J and C57BL/6 mouse strains is similar to what has been reported previously for mice infected via the i.v. or i.p. route of infection (7, 8, 17). Perhaps the difference in susceptibilities to GI listeriosis reflects in part the relative effectiveness of L. monocytogenes in invading the GI mucosa in the two strains of mice. There are reports that L. monocytogenes can translocate across the intestinal mucosa through M cells in Peyer's patches (25) and can directly invade intestinal epithelial cells (12, 23, 27). The latter mechanism is thought to reflect the interaction of internalin on the surface of the listeriae with E-cadherin on the intestinal epithelial cells (27). We did not detect lesions or bacteria during microscopic examination of the intestinal mucosa from A/J or C57BL/6 mice (data not shown). Thus, we have no direct evidence for local differences in susceptibility to GI infection with L. monocytogenes between the two mouse strains. It is interesting that greater numbers of listeriae were recovered for a longer period of time from the ceca of A/J mice than from the ceca of C57BL/6 mice (Fig. 3A). Because we homogenized both cecal contents and tissue, we cannot distinguish whether the increased numbers of L. monocytogenes in the ceca of A/J mice reflect a better ability to compete with the intestinal microflora or to adhere to and invade intestinal cells. However, in a subsequent experiment, we did not observe significant reductions in the number of CFU of L. monocytogenes recovered from the ceca of A/J mice that had been perfused with, and whose intestinal tract had been flushed with, saline before the tissue was homogenized, relative to the number in ceca from A/J mice, which were not perfused and flushed (data not shown). This observation suggests that L. monocytogenes recovered from the ceca reflects, at least in part, invasion of the intestinal mucosa. If L. monocytogenes can more efficiently invade or multiply within the intestinal mucosa in A/J mice, the intestinal mucosa could serve as a reservoir of infection that could seed the lymphatics and the bloodstream, leading to the increased bacteremia and bacterial burden in the spleen and liver noted in A/J mice. Although we did not observe inflammatory lesions nor detect gram-positive bacteria within the intestinal mucosa of A/J mice, it is possible that these were diffusely distributed and not present within the sections we examined microscopically.
Alternatively, perhaps the differences in bacterial burden between the A/J and C57BL/6 mice reflect differences in the relative effectiveness of the innate immune response at sites of infection in the two strains of mice. Previous studies have demonstrated that A/J mice are relatively inefficient in mobilizing inflammatory cells, which are critical for host defense against listeriosis (7, 17), following i.v. or i.p. inoculation of L. monocytogenes. It also has been suggested that the anti-Listeria activity of phagocytes from A/J mice is less effective than the activity of phagocytes from C57BL/6 mice (7, 17). A/J mice also initiate a slower and less exuberant production of the inflammatory cytokines that are important for L. monocytogenes resistance (i.e., gamma interferon and others) than do C57BL/6 mice (21). Perhaps in the present study the A/J mice were unable to quickly mobilize the mediators and cells that are required for successful defense of the intestinal mucosa against L. monocytogenes. However, we cannot exclude the possibility that local defenses against L. monocytogenes infection in the intestinal mucosa of A/J and C57BL/6 mice are comparable but their abilities to resist systemic dissemination of the infection are not. Once the listeriae reach the spleen and liver, their multiplication would be expected to be greater in the A/J mice, because of the less effective host defense mechanisms against systemic L. monocytogenes infection in that mouse strain (7, 8,17). Histopathologically, the liver and spleen lesions in the A/J mice were similar to lesions that might be observed in tissues from resistant mice injected parenterally with a large challenge dose of L. monocytogenes or treated in such a way that normal innate defense mechanisms are abolished (6, 10). However, the numbers of listeriae recovered from the spleen and liver were only slightly greater in the A/J mice than in the C57BL/6 mice at 4 h after inoculation. Thus, there does not appear to be a rapid flood of listeriae into the bloodstream and systemic tissues (i.e., spleen and liver) of A/J mice.
The rapid course of infection in the A/J mice challenged with large numbers of L. monocytogenes (death within 1 to 2 days following inoculation of 108 CFU) is somewhat unusual for murine listeriosis and different from the course of infection in more resistant strains of mice (22, 23, 25, 26, 29, 31). This observation led us to speculate that the mice may have succumbed to a fulminant meningoencephalitis, as is reported to occur in immunosuppressed humans and in healthy ruminants (20, 32). Although we recovered substantial numbers of L. monocytogenes from the brain homogenates of some A/J mice that died of listeriosis or were euthanized at 24 to 72 h after challenge (Fig. 3D), histopathological examination failed to reveal inflammatory lesions or the presence of gram-positive bacteria in brain tissue. There are several possible explanations for the contradiction between the recovery of L. monocytogenes from the brain and the absence of visible lesions. It has been reported that lesions occur in distinct regions of the brain in mice with L. monocytogenes central nervous system (CNS) infection (1, 24, 26). Because of the way in which brain tissue was removed and fixed in our experiments, it is possible that we did not obtain the appropriate region of the brain and thus did not detect focal lesions of CNS infection and inflammation. However, we have since modified the method by which the brains are removed, fixed, and prepared for histopathology and still do not observe lesions in the brains or brain stems of A/J mice with significant listerial burdens in their spleens and livers (data not shown). We considered the possibility that the substantial bacteremia exhibited by A/J mice (Fig. 2) could account for the number of CFU recovered from the brains of A/J mice. Perfusion of infected A/J mice reduced the number of CFU recovered from the brain below the background level for some mice, thus suggesting that L. monocytogenes in the bloodstream could contribute to the number of CFU recovered from the brains of A/J mice. Finally, perhaps the timing was not optimal to detect CNS infection. It has been proposed that L. monocytogenes disseminates to the CNS via infected phagocytes, which then adhere to endothelial cells and allow the listeriae to invade these cells, from which they subsequently spread to adjacent cells in the CNS parenchyma (13, 14). This might require a more prolonged infection, as has been suggested by some studies (13, 14, 24, 26), than was used in the present study.
Previous investigations of GI listeriosis in mice have been limited by the relative resistance of the mouse strains that were used. In most instances, very high challenge doses (108 CFU or greater) or experimental impairment of one or more host defense mechanisms was needed to facilitate systemic infection of the mice (2, 6, 10, 16, 18, 22, 25, 29).
The work of Lecuit et al. (23) provided elegant evidence for the importance of internalin on L. monocytogenes, and E-cadherin on intestinal epithelial cells, in the pathogenesis of GI listeriosis. However, the challenge dose of L. monocytogenes required to cause infection in that study (23), even with the optimal internalin-E-cadherin system, was much higher than that needed to cause systemic infection in A/J mice in the present study. This suggests that perhaps other host-pathogen interactions also influence the pathogenesis of listeriosis in the GI tract. The LD50 for an A/J mouse, as estimated in the present study (106 CFU), is within the range of inocula estimated to be present in Listeria-contaminated food products incriminated in human listeriosis outbreaks (20, 32). Although A/J mice exhibit a less exuberant inflammatory response than Listeria-resistant mouse strains and their phagocytes are reported to have somewhat diminished anti-Listeria activity (3, 7,17), they cannot be considered immunodeficient in the usual sense. As a result, the A/J mouse offers an appealing model for investigating the interactions between bacterial virulence mechanisms (and how these are influenced by environmental conditions) and host immune responses that influence the pathogenesis of GI listeriosis.
Acknowledgments
This work was supported by the U.S. Department of Agriculture National Alliance for Food Safety (58-1935-1-128) and by the University of Wisconsin—Madison Food Research Institute.
We thank John Luchansky (Wyndmoor, Pa.) for helpful suggestions during the course of these studies.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Altimira, J., N. Prats, S. Lopez, M. Domingo, V. Briones, L. Dominguez, and A. Marco. 1999. Repeated oral dosing with Listeria monocytogenes in mice as a model of central nervous system listeriosis in man. J. Comp. Pathol. 121:117-125. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boraschi, D., and M. S. Meltzer. 1980. Defective tumoricidal capacity of macrophages from A/J mice. III. Genetic analysis of the macrophage defect. J. Immunol. 124:1050-1053. [PubMed] [Google Scholar]
- 4.Bregenholt, S., P. Berche, F. Brombacher, and J. P. Di Santo. 2001. Conventional alpha beta T cells are sufficient for innate and adaptive immunity against enteric Listeria monocytogenes. J. Immunol. 166:1871-1876. [DOI] [PubMed] [Google Scholar]
- 5.Cheers, C., and I. F. C. McKenzie. 1978. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect. Immun. 19:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan, J. W. 1997. Neutrophils and tumour necrosis factor-alpha are important for controlling early gastrointestinal stages of experimental murine listeriosis. J. Med. Microbiol. 46:239-250. [DOI] [PubMed] [Google Scholar]
- 7.Czuprynski, C. J., B. P. Canono, P. M. Henson, and P. A. Campbell. 1985. Genetically determined resistance to listeriosis is associated with increased accumulation of inflammatory neutrophils and macrophages which have enhanced listericidal activity. Immunology 5:511-518. [PMC free article] [PubMed] [Google Scholar]
- 8.Czuprynski, C. J., and J. F. Brown. 1986. The relative difference in anti-Listeria resistance of C57BL/6 and A/J mice is not eliminated by active immunization or by transfer of Listeria-immune T cells. Immunology 158:437-443. [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., and M. Haak-Frendscho. 1997. Non-specific resistance mechanisms to listeriosis: implications for experimental and naturally occurring infection. Immunol. Rev. 158:47-56. [DOI] [PubMed] [Google Scholar]
- 10.Czuprynski, C. J., C. Theisen, and J. F. Brown. 1996. Treatment with the antigranulocyte monoclonal antibody RB6-8C5 impairs resistance of mice to gastrointestinal infection with Listeria monocytogenes. Infect. Immun. 64:3946-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., N. G. Faith, and H. Steinberg. 2002. Ability of Listeria monocytogenes strain Scott A to cause systemic infection in mice infected by the intragastric route. Appl. Environ. Microbiol. 68:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels, J. J., I. B. Autenrieth, and W. Goebel. 2000. Interaction of Listeria monocytogenes with the intestinal epithelium. FEMS Microbiol. Lett. 190:323-328. [DOI] [PubMed] [Google Scholar]
- 13.Drevets, D. A. 1999. Dissemination of Listeria monocytogenes by infected phagocytes. Infect. Immun. 67:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets, D. A., T. A. Jelinek, and N. E. Freitag. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect. Immun. 69:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farber, J. M., E. Daley, F. Coates, N. Beausoleil, and J. Fournier. 1991. Feeding trials of Listeria monocytogenes with a nonhuman primate model. J. Clin. Microbiol. 29:2606-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontan, E., H. Saklani-Jusforgues, and P. L. Goossens. 2001. Early translocation of acid-adapted Listeria monocytogenes during enteric infection in TNF/LT alpha−/− mice. FEMS Microbiol. Lett. 205:179-183. [DOI] [PubMed] [Google Scholar]
- 17.Gervais, F., M. Stevenson, and E. Skamene. 1984. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J. Immunol. 132:2078-2083. [PubMed] [Google Scholar]
- 18.Golnazarian, C. A., C. W. Donnelly, S. J. Pintauro, and D. B. Howard. 1989. Comparison of infectious dose of Listeria monocytogenes F5817 as determined for normal versus compromised C57BL/6J mice. J. Food Prot. 52:696-701. [DOI] [PubMed] [Google Scholar]
- 19.Goossens, P. L., G. Marchal, and G. Milon. 1988. Early influx of Listeria-reactive T lymphocytes in liver of mice genetically resistant to listeriosis. J. Immunol. 141:2451-2455. [PubMed] [Google Scholar]
- 20.Hurd, S., et al. 1998. Multistate outbreak of listeriosis—United States, 1998. Morb. Mortal Wkly. Rep. 47:1085-1086. [PubMed] [Google Scholar]
- 21.Iizawa, Y., R. D. Wagner, and C. J. Czuprynski. 1993. Analysis of cytokine mRNA expression in Listeria-resistant C57BL/6 and Listeria-susceptible A/J mice during Listeria monocytogenes infection. Infect. Immun. 61:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammerding, A. M., K. A. Glass, A. Gendron-Fitzpatrick, and M. P. Doyle. 1992. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Appl. Environ. Microbiol. 58:3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Guonon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1723. [DOI] [PubMed] [Google Scholar]
- 24.López, S., A. J. Marco, N. Prats, and C. J. Czuprynski. 2000. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infect. Immun. 68:4789-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald, T. T., and P. B. Carter. 1980. Cell-mediated immunity to intestinal infection. Infect. Immun. 28:516-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marco, A. J., N. Prats, J. A. Ramos, V. Briones, M. Blanco, L. Dominguez, and M. Domingo. 1992. A microbiological, histopathological, and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J. Comp. Pathol. 107:1-9. [DOI] [PubMed] [Google Scholar]
- 27.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-Cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 28.Notermans, S., J. Dufrenne, P. Teunis, and T. Chackraborty. 1998. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 61:244-248. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto, M., A. Nakane, and T. Minagawa. 1994. Host resistance to an intragastric infection with Listeria monocytogenes in mice depends on cellular immunity and intestinal bacterial flora. Infect. Immun. 62:3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pine, L., G. B. Malcolm, and B. D. Plikaytis. 1990. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect. Immun. 58:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roll, J. T., and C. J. Czuprynski. 1990. Hemolysin is required for extraintestinal dissemination of Listeria monocytogenes in intragastrically inoculated mice. Infect. Immun. 58:3147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuchat, A., B. Swaminathan, and C. V. Broome. 1991. Epidemiology of human listeriosis. Clin. Microbiol. Rev. 4:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachar, Z., and D. C. Savage. 1979. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect. Immun. 23:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]