Abstract
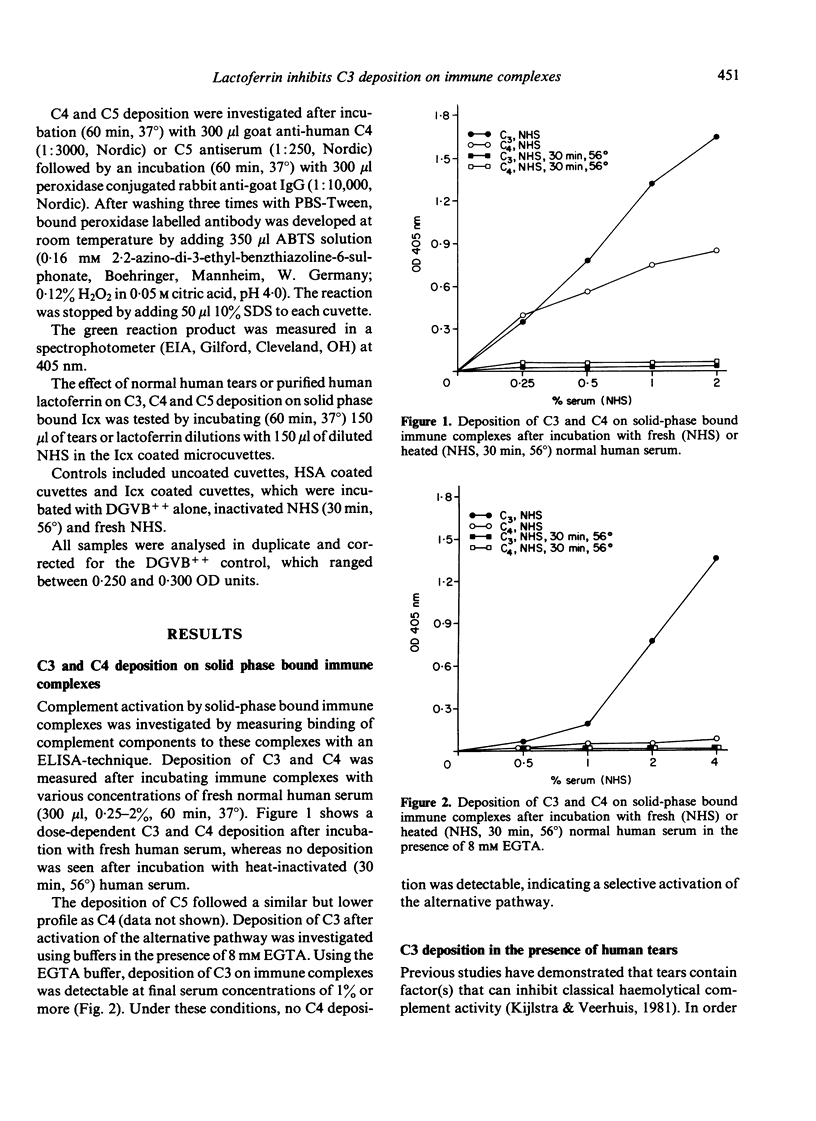
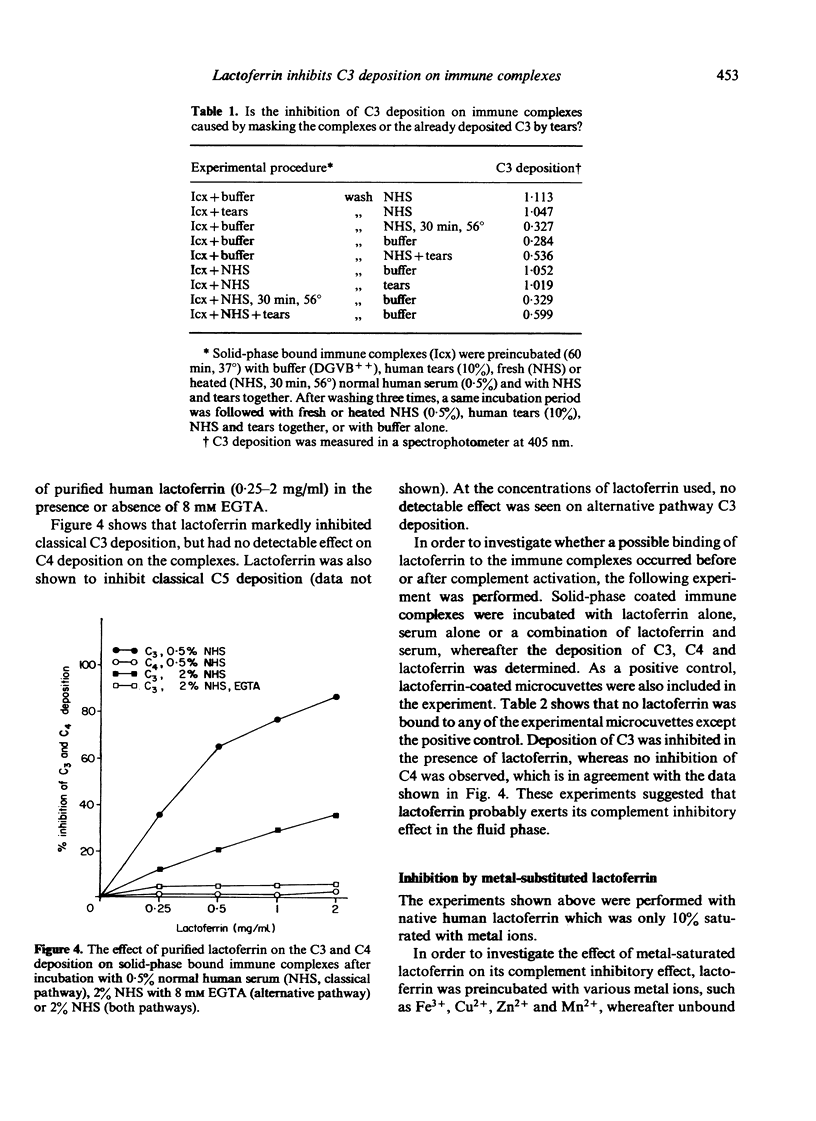
In the study described here, the effect of human tears and purified lactoferrin was investigated on the deposition of complement components on solid-phase bound immune complexes. After incubating immune complexes with fresh normal human serum, the deposition of complement components (C3, C4 and C5) was measured with an ELISA technique. Rabbit antibodies were used as a constituent of the immune complexes, and so both alternative and classical complement pathway activation could be studied. The addition of human tears or purified lactoferrin to this system resulted in the inhibition of classical pathway deposition of C3 and C5, while C4 deposition was not affected. High concentrations of human tears also inhibited alternative pathway C3 deposition on immune complexes, whereas lactoferrin did not detectably affect this pathway. The inhibition of complement activation by tears was not due to a masking of the immune complexes or the already deposited C3. Experiments with purified lactoferrin furthermore showed that lactoferrin did not bind to the complexes, either before or during complement activation. These findings suggest that the complement inhibitory effect is probably taking place in the fluid phase. Saturation of lactoferrin with iron or copper ions resulted in a markedly diminished effect on the capacity of lactoferrin to inhibit complement activation. C4 deposition on immune complexes was not affected by lactoferrin, which suggested that the inhibition of the classical pathway was due to an effect on the classical C3 convertase. The fact that lactoferrin inhibits the classical, but not the alternative C3 convertase, suggests that the effect is probably not mediated through a competition for certain trace metal ions, but may be caused by protein-protein interactions. The findings reported here indicate that lactoferrin may play an important anti-inflammatory role by modulating activation of the complement system. This observation adds a new property to the already described functions of the iron-binding protein lactoferrin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainscough E. W., Brodie A. M., Plowman J. E., Bloor S. J., Loehr J. S., Loehr T. M. Studies on human lactoferrin by electron paramagnetic resonance, fluorescence, and resonance Raman spectroscopy. Biochemistry. 1980 Aug 19;19(17):4072–4079. doi: 10.1021/bi00558a026. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Russell J. E., Champion W. J., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun. 1982 Mar;35(3):792–799. doi: 10.1128/iai.35.3.792-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Boesman-Finkelstein M., Finkelstein R. A. Sequential purification of lactoferrin, lysozyme and secretory immunoglobulin A from human milk. FEBS Lett. 1982 Jul 19;144(1):1–5. doi: 10.1016/0014-5793(82)80556-5. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman A. Association of lactoferrin with other proteins, as demonstrated by changes in electrophoretic mobility. Biochim Biophys Acta. 1971 Dec 28;251(3):380–387. doi: 10.1016/0005-2795(71)90126-7. [DOI] [PubMed] [Google Scholar]
- Kijlstra A., Jeurissen S. H., Koning K. M. Lactoferrin levels in normal human tears. Br J Ophthalmol. 1983 Mar;67(3):199–202. doi: 10.1136/bjo.67.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., Jeurissen S. H. Modulation of classical C3 convertase of complement by tear lactoferrin. Immunology. 1982 Oct;47(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., Veerhuis R. The effect of an anticomplementary factor on normal human tears. Am J Ophthalmol. 1981 Jul;92(1):24–27. doi: 10.1016/s0002-9394(14)75903-3. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier J., Aubert J. P., Loucheux-Lefevre M. H. Comparative circular dichroism studies of iron-free and iron-saturated forms of human serotransferrin and lactortransferrin. FEBS Lett. 1976 Jul 15;66(2):238–242. doi: 10.1016/0014-5793(76)80512-1. [DOI] [PubMed] [Google Scholar]
- Mondino B. J., Zaidman G. W. Hemolytic complement in tears. Ophthalmic Res. 1983;15(4):208–211. doi: 10.1159/000265260. [DOI] [PubMed] [Google Scholar]
- Van Haeringen N. J. Clinical biochemistry of tears. Surv Ophthalmol. 1981 Sep-Oct;26(2):84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R., Kijlstra A. Inhibition of hemolytic complement activity by lactoferrin in tears. Exp Eye Res. 1982 Feb;34(2):257–265. doi: 10.1016/0014-4835(82)90059-8. [DOI] [PubMed] [Google Scholar]



