Abstract
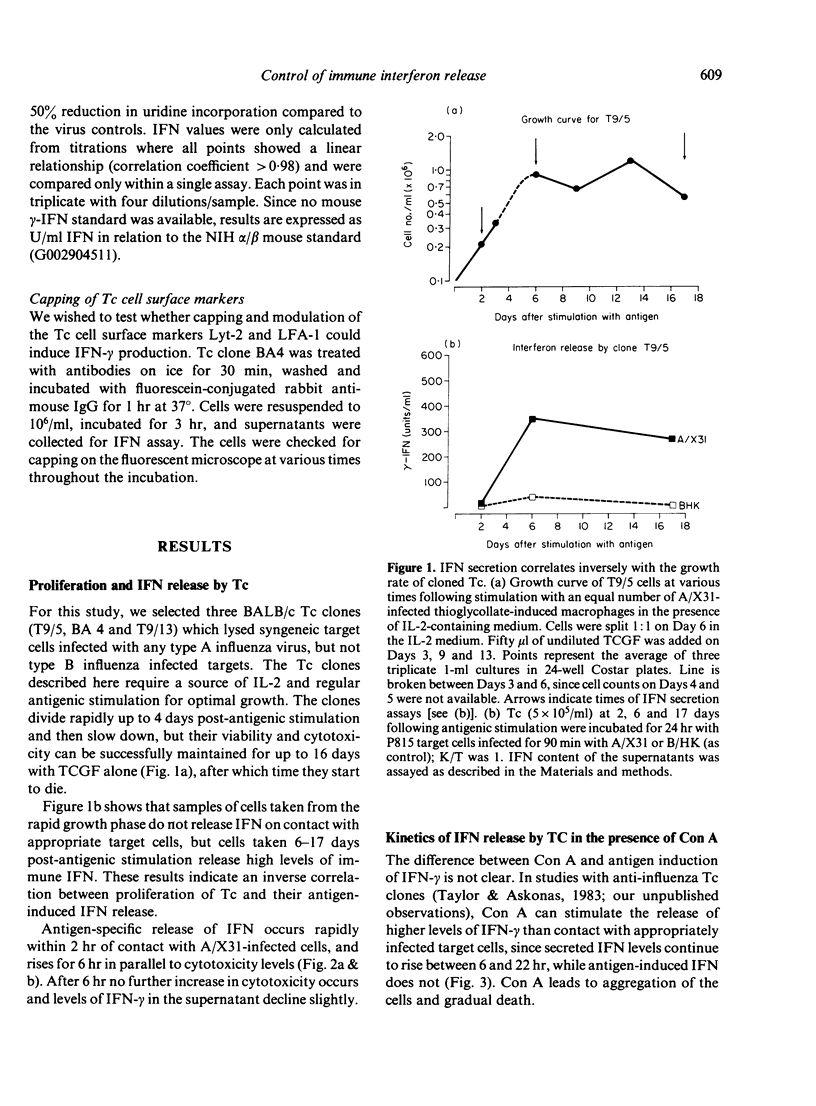
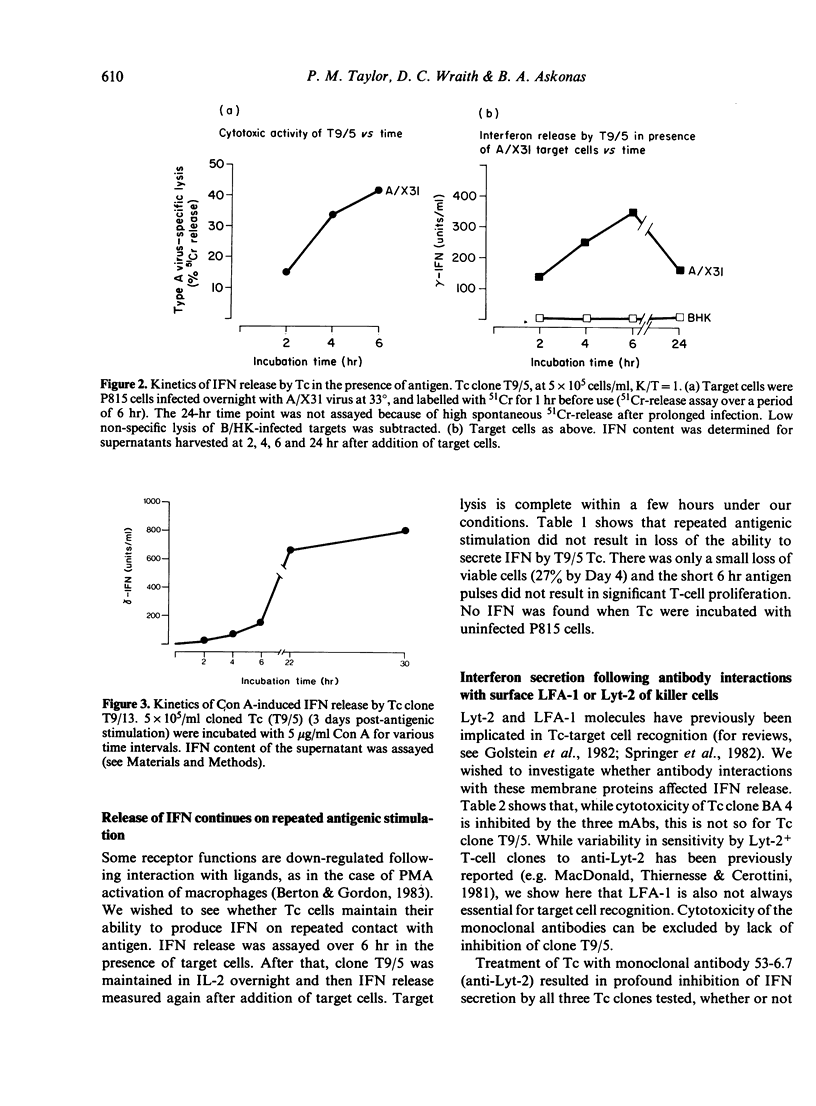
We have studied the release of immune interferon (IFN-gamma) by influenza-specific cytotoxic T-cell (Tc) clones. IFN-gamma release is entirely dependent on specific antigen recognition or mitogen treatment and correlates inversely with the growth rate of the clone, while no differences in cytotoxic activity can be discerned at the different stages of Tc maturation. Although the mitogen Con A provides a more powerful stimulus for IFN release by Tc clones, specific antigen leads to a more rapid secretion, starting within 2 hr of contact with Tc clones and their specific targets. This may be of significance in an infection, providing a quick, but localized, mechanism to prevent viral spread. We also examined whether ligand interactions with T-cell surface glycoproteins Lyt-2 or LFA-1, important in Tc recognition, affected IFN release. Monoclonal antibodies to both Lyt-2 and LFA-1 block specific target cell lysis of Tc clone BA4, but do not affect Tc clone T9/5. This latter finding adds LFA-1 to the list of T-cell surface components which are not always essential for target cell recognition. Antibody to Lyt-2 blocked antigen-induced IFN-gamma release by all Tc clones studied, whilst two monoclonal antibodies to LFA-1 had little or no effect. Thus, the Lyt-2 molecule plays a role in the regulation of IFN secretion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Johnston M. D., Westmacott L. M., Burke D. C. Department of Biological Sciences, University of Warwick, Coventry, CV47AL, England. J Gen Virol. 1974 Dec;25(3):381–390. doi: 10.1099/0022-1317-25-3-381. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Griffin D. B., Band H. A., Beverley P. C. Immune human lymphocytes produce an acid-labile alpha-interferon. J Exp Med. 1983 Mar 1;157(3):1059–1063. doi: 10.1084/jem.157.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Gordon S. Desensitization of macrophages to stimuli which induce secretion of superoxide anion. Down-regulation of receptors for phorbol myristate acetate. Eur J Immunol. 1983 Aug;13(8):620–627. doi: 10.1002/eji.1830130804. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Andrew M. E., Braciale V. L. Heterogeneity and specificity of cloned lines of influenza-virus specific cytotoxic T lymphocytes. J Exp Med. 1981 Apr 1;153(4):910–923. doi: 10.1084/jem.153.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasebrook A. L., Kelso A., MacDonald H. R. Cytolytic T lymphocyte clones that proliferate autonomously to specific alloantigenic stimulation. II. Relationship of the Lyt-2 molecular complex to cytolytic activity, proliferation, and lymphokine secretion. J Immunol. 1983 Apr;130(4):1545–1551. [PubMed] [Google Scholar]
- Golstein P., Goridis C., Schmitt-Verhulst A. M., Hayot B., Pierres A., van Agthoven A., Kaufmann Y., Eshhar Z., Pierres M. Lymphoid cell surface interaction structures detected using cytolysis-inhibiting monoclonal antibodies. Immunol Rev. 1982;68:5–42. doi: 10.1111/j.1600-065x.1982.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Piguet P. F., Vassalli P. Production of interleukin 2, interleukin 3, and interferon by mouse T lymphocyte clones of Lyt-2+ and -2- phenotype. J Immunol. 1984 Apr;132(4):1869–1871. [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Matis L. A. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983 Sep;131(3):1049–1055. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Klein J. R., Raulet D. H., Pasternack M. S., Bevan M. J. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982 Apr 1;155(4):1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer P. H., Echtenacher B., Gemsa D., Hamann U., Hültner L., Kaltmann B., Kees U., Kubelka C., Marcucci F. Immune-interferon (IFN-gamma), macrophage-activating factors (MAFs), and colony-stimulating factors (CSFs) secreted by T cell clones in limiting dilution microcultures, long-term cultures, and by T cell hybridomas. Immunol Rev. 1983;76:5–28. doi: 10.1111/j.1600-065x.1983.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Lu L. Y., Askonas B. A. Cross-reactivity for different type A influenza viruses of a cloned T-killer cell line. Nature. 1980 Nov 13;288(5787):164–165. doi: 10.1038/288164a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Thiernesse N., Cerottini J. C. Inhibition of T cell-mediated cytolysis by monoclonal antibodies directed against Lyt-2: heterogeneity of inhibition at the clonal level. J Immunol. 1981 May;126(5):1671–1675. [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R. H., Yip Y. K., Vilcek J. Immune interferon induction by monoclonal antibody specific for human T cells. Cell Immunol. 1981 Nov 1;64(2):304–311. doi: 10.1016/0008-8749(81)90482-2. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Diversity in the biological properties of anti-influenza cytotoxic T cell clones. Eur J Immunol. 1983 Sep;13(9):707–711. doi: 10.1002/eji.1830130904. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Taylor P. M., Mellor A. L., Askonas B. A. Recognition of Db and Kb gene products by influenza-specific cytotoxic T cells. Immunogenetics. 1983;17(3):283–294. doi: 10.1007/BF00364412. [DOI] [PubMed] [Google Scholar]
- Wraith D. C. Dk-restricted antiinfluenza cytotoxic T-cell clone loses one of its two alloreactivities. Immunogenetics. 1984;20(2):131–139. doi: 10.1007/BF00364485. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Holtkamp B., Askonas B. A. Loss of serological determinants does not affect recognition of H-2Kk target cells by an influenza-specific cytotoxic T cell clone. Eur J Immunol. 1983 Sep;13(9):762–766. doi: 10.1002/eji.1830130912. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Askonas B. A., Millican D., Courtneidge S. A., Skehel J. J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur J Immunol. 1977 Sep;7(9):630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]



