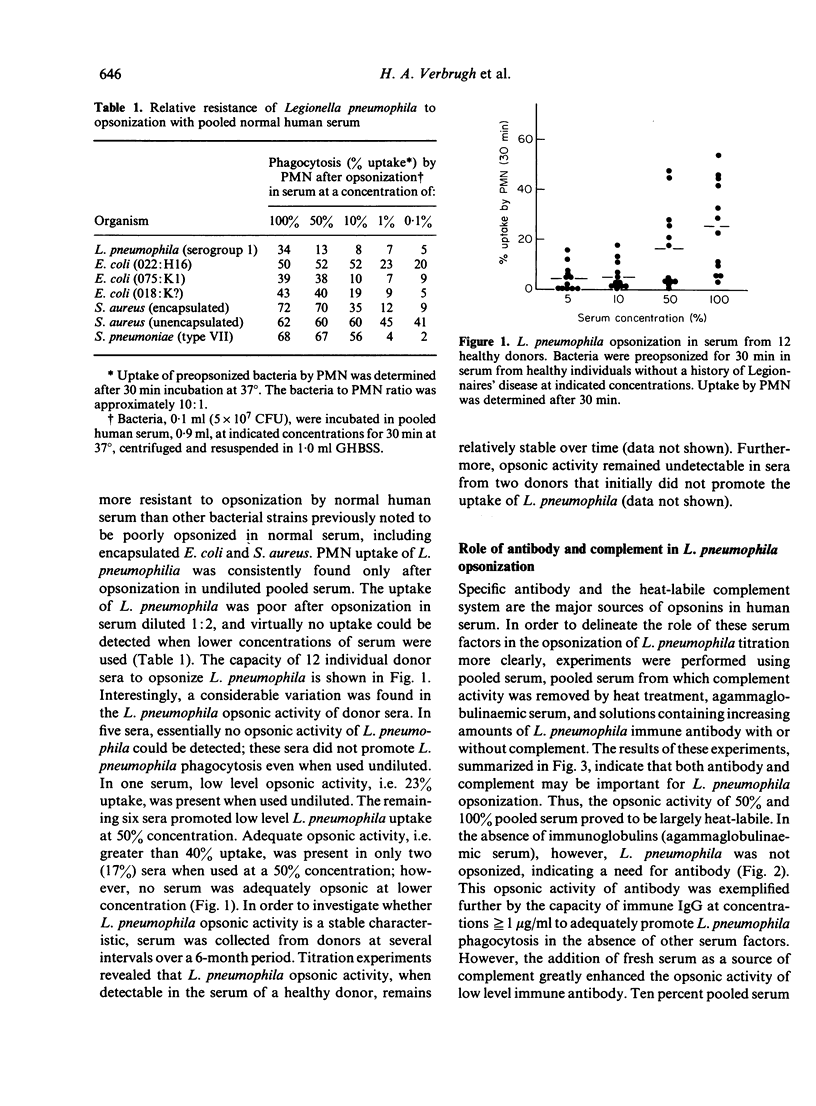
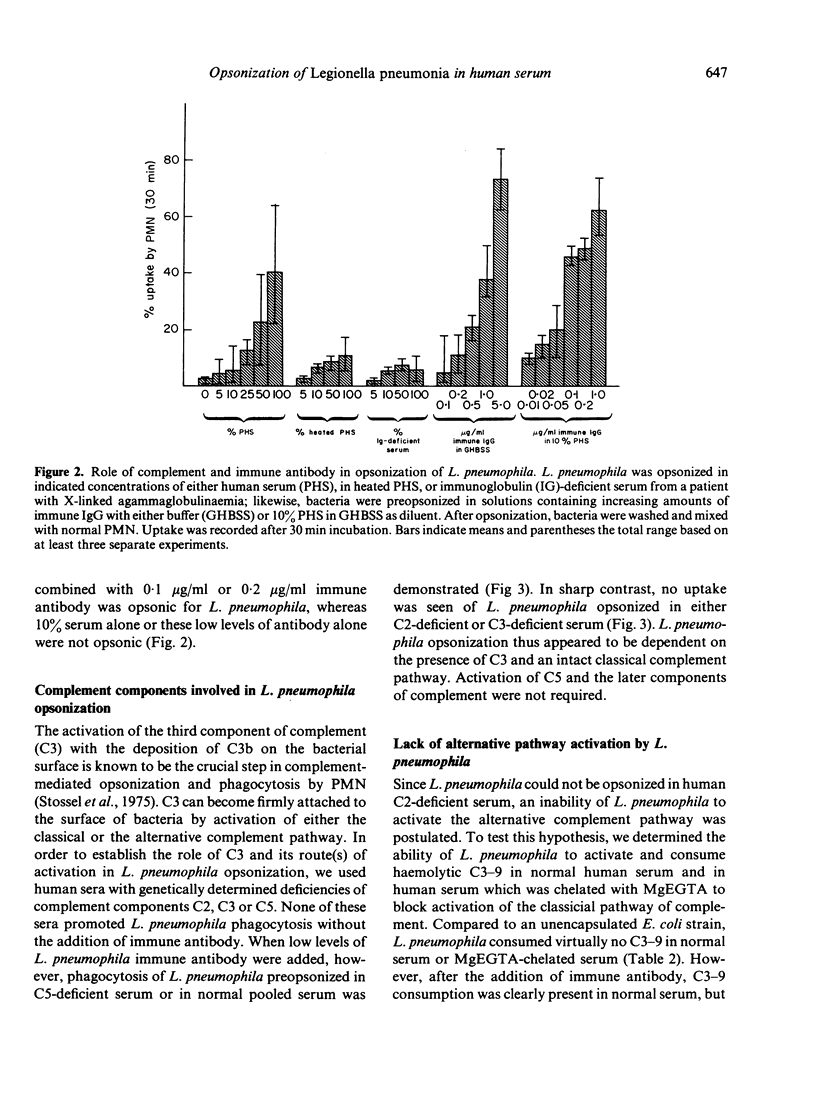
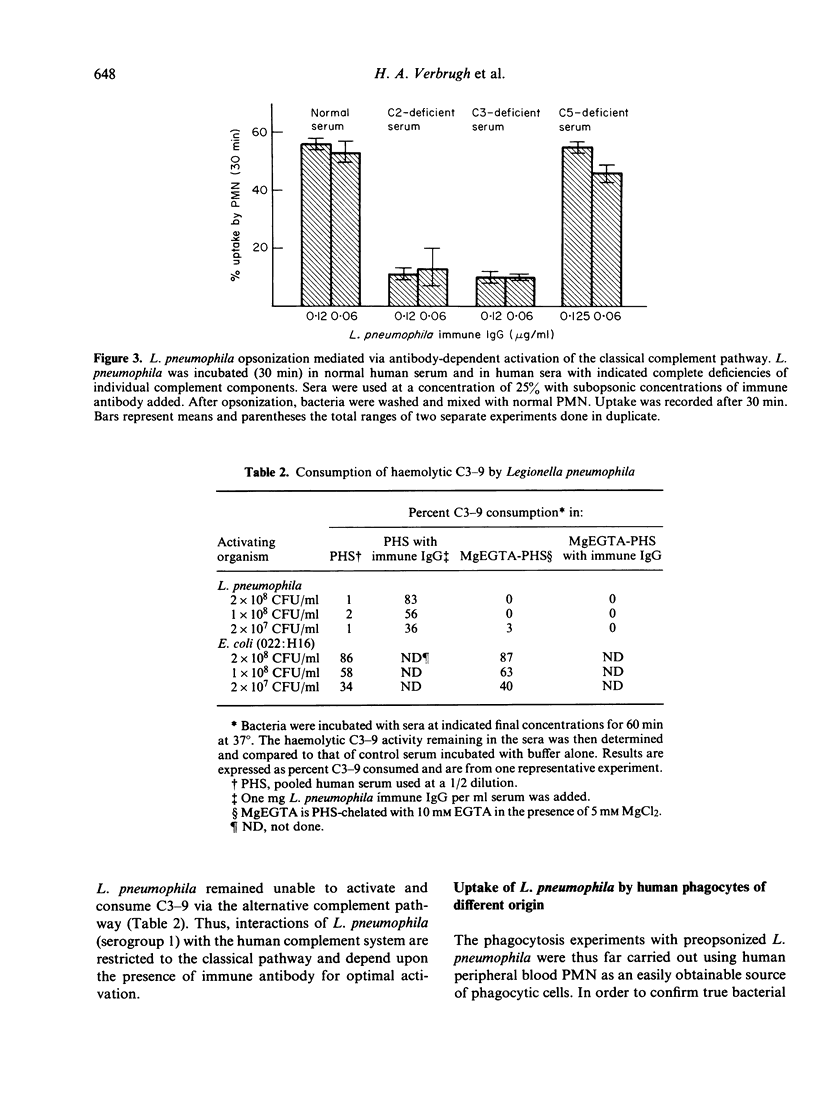
Abstract
Legionella pneumophila has previously been shown to require serum factors for efficient uptake by phagocytic cells. In this investigation, the roles of specific antibody and complement in phagocytosis of L. pneumophila by human polymorphonuclear leucocytes (PMN) and tissue macrophages were determined. Opsonization was assessed by quantitating the uptake of [3H]-labelled Legionellae. Compared to other Gram-negative and to Gram-positive bacterial species, L. pneumophila was highly resistant to the opsonic activity of normal pooled human serum (PHS). Of 12 donor sera tested, only four promoted significant L. pneumophila uptake when used at full strength. Experiments with immune antibody, and with human sera deficient in immunoglobulins, or the complement components C2, C3, or C5, revealed that L. pneumophila opsonization was dependent on antibody-mediated activation of the classical complement pathway; activation of the alternative pathway could not be detected. At high concentrations, immune antibody alone could adequately opsonize L. pneumophila. Human alveolar and peritoneal macrophages required very similar amounts and types of opsonins for L. pneumophila phagocytosis as did human PMN. Heating L. pneumophila to temperatures greater than or equal to 80 degrees abolished its resistance to opsonization by diluted PHS; however, activation of complement via the alternative pathway or via other antibody-independent routes remained undetectable. These studies show that, in addition to immune antibody, the classical pathway of complement plays an important role in the opsonization of L. pneumophila. The limited ability of these bacteria to interact with human complement provides a likely explanation for their resistance to opsonization and may be partly based on heat-sensitive structures on the surface of L. pneumophila.
Full text
PDF


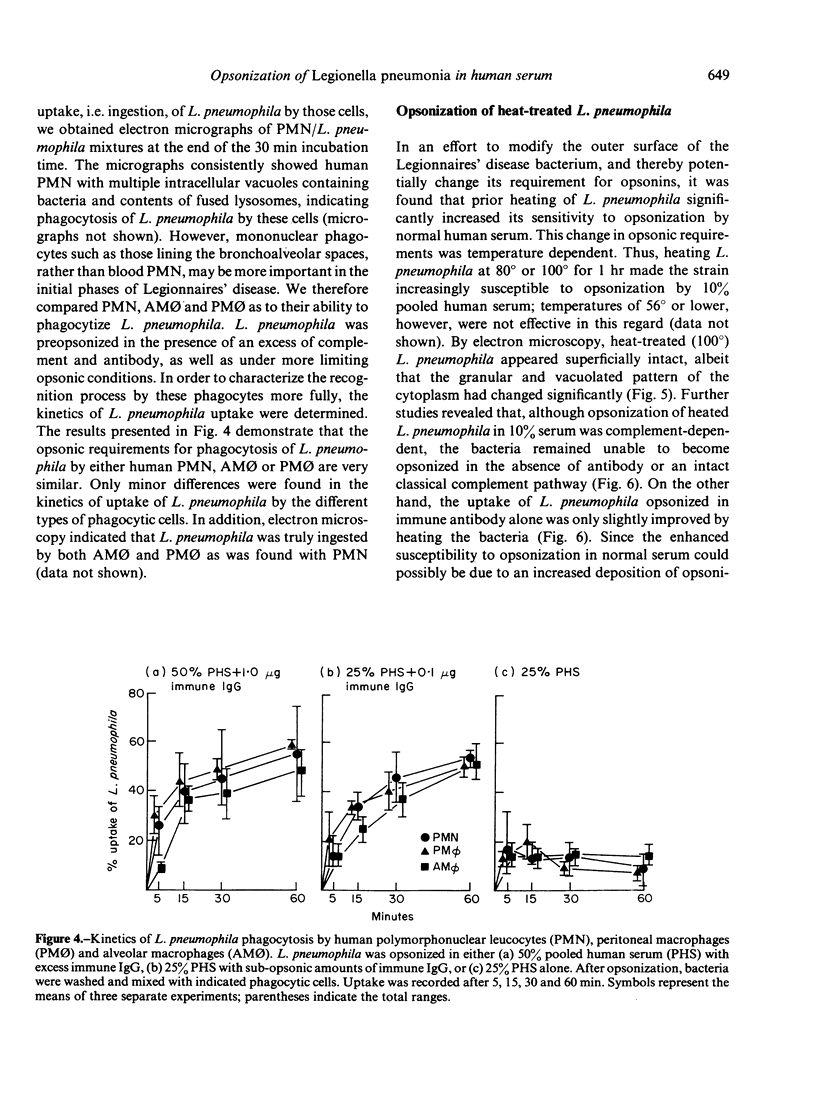
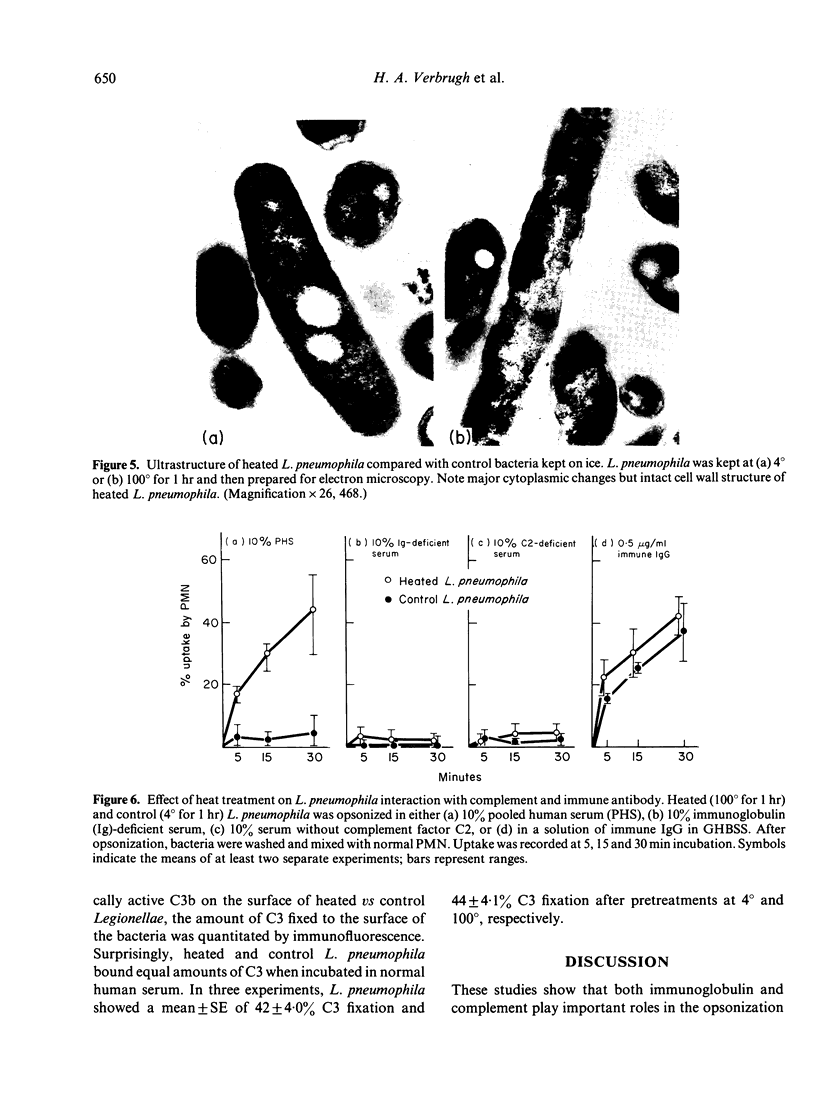



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Edwards M. S., Webb B. J., Kasper D. L. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J Clin Invest. 1982 Feb;69(2):394–404. doi: 10.1172/JCI110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Loos M. Antibody-independent binding of the first component of complement (C1) and its subcomponent C1q to the S and R forms of Salmonella minnesota. Infect Immun. 1981 Mar;31(3):1138–1144. doi: 10.1128/iai.31.3.1138-1144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C. Platelet interaction with bacteria. 3. Ultrastructure. Am J Pathol. 1973 Mar;70(3):449–471. [PMC free article] [PubMed] [Google Scholar]
- Daisy J. A., Benson C. E., McKitrick J., Friedman H. M. Intracellular replication of Legionella pneumophila. J Infect Dis. 1981 Mar;143(3):460–464. doi: 10.1093/infdis/143.3.460. [DOI] [PubMed] [Google Scholar]
- Dierich M. P., Bitter-Suermann D., König W., Hadding U., Galanos C., Rietschel E. T. Analysis of bypass activation of C3 by endotoxic LPS and loss of this potency. Immunology. 1973 Apr;24(4):721–733. [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W., Helms C. M. Ultrastructural localization and protective activity of a high-molecular-weight antigen isolated from Legionella pneumophila. Infect Immun. 1981 Feb;31(2):822–824. doi: 10.1128/iai.31.2.822-824.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Dee T. H., Kim Y., Quie P. G. Alterations in serum opsonic activity and complement levels in pneumococcal disease. Infect Immun. 1980 Sep;29(3):1062–1066. doi: 10.1128/iai.29.3.1062-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979 May;93(5):857–866. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981 Feb 1;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981 Feb 1;153(2):398–406. doi: 10.1084/jem.153.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Callaway C. S., Ewing E. P., Jr Comparison of Legionella pneumophila, L. micdadei, L. bozemanii, and L. dumoffii by transmission electron microscopy. J Clin Microbiol. 1984 Feb;19(2):116–121. doi: 10.1128/jcm.19.2.116-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Friend P. S., Dresner I. G., Yunis E. J., Michael A. F. Inherited deficiency of the second component of complement (C2) with membranoproliferative glomerulonephritis. Am J Med. 1977 May;62(5):765–771. doi: 10.1016/0002-9343(77)90881-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto R. A., Kastello M. D., White J. D., Shirey F. G., McGann V. G., Larson E. W., Hedlund K. W. In vitro interaction between normal cynolmolgus monkey alveolar macrophages and Legionnaires disease bacteria. Infect Immun. 1979 Aug;25(2):761–763. doi: 10.1128/iai.25.2.761-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist-Welsh P., Bjornson A. B. Immunoglobulin-independent utilization of the classical complement pathway in opsonophagocytosis of Escherichia coli by human peripheral leukocytes. J Immunol. 1982 Jun;128(6):2643–2651. [PubMed] [Google Scholar]
- MICHAEL J. G., WHITBY J. L., LANDY M. Studies on natural antibodies to gram-negative bacteria. J Exp Med. 1962 Jan 1;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Hoidal J. R., Nguyen B. Y., Verhoef J., Quie P. G., Peterson P. K. Human alveolar macrophage cytophilic immunoglobulin G-mediated phagocytosis of protein A-positive staphylococci. J Clin Invest. 1982 Jan;69(1):63–74. doi: 10.1172/JCI110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Hoidal J. R., Freiberg M. R., Elliott G. R., Peterson P. K. Peritoneal macrophages and opsonins: antibacterial defense in patients undergoing chronic peritoneal dialysis. J Infect Dis. 1983 Jun;147(6):1018–1029. doi: 10.1093/infdis/147.6.1018. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., Peters R., van Erne M. E., Daha M. R., Peterson P. K., Verhoef J. Opsonic recognition of staphylococci mediated by cell wall peptidoglycan: antibody-independent activation of human complement and opsonic activity of peptidoglycan antibodies. J Immunol. 1980 Mar;124(3):1167–1173. [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., van Erne M. E., Peters R., Peterson P. K., Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979 Dec;26(3):808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wong M. C., Ewing E. P., Jr, Callaway C. S., Peacock W. L., Jr Intracellular multiplication of Legionella pneumophila in cultured human embryonic lung fibroblasts. Infect Immun. 1980 Jun;28(3):1014–1018. doi: 10.1128/iai.28.3.1014-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]